Objective: This study aimed to investigate the effect of high-glucose conditions in the EPCs from whole peripheral and bone marrow of diabetic rats. To determine the expression of critical initiation factor HIF-1α and HIF-1α-induced vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) in high glucose environment. The effect of over expression of HIF-1α to the function of the EPCs in diabetic rats via regulating PI3K/AKT signaling pathway.
Methods: Primary EPCs from whole peripheral and bone marrow of Sprague-Dawley control rats and streptozoctin (STZ)-induced diabetic rats were harvested, isolated and characterized. Cell viability, migration, and tube formation ability were detected by CCK8, Transwell assay and Matrigel-based capillary-like tube formation assay. Gene transcription and protein expression were evaluated by real-time polymerase chain reaction and Western blotting, respectively.
Results: Cell viability, migration, and tube formation ability of EPCs were impaired under high-glucose conditions. Overexpression of HIF-1α alleviated high glucose-induced EPCs dysfunction by promoting the transcription and expression of VEGF and VEGFR in EPCs under high-glucose. Furthermore, high-glucose inhibited PI3K/AKT phosphorylation and PI3K agonist rescued the HIF-1α-VEGF/VEGFR expression of EPCs under high-glucose conditions via activating PI3K/AKT signaling pathway.
Conclusion: These results suggest that the attenuation of high-glucose induced EPCs dysfunction of diabetic rats by HIF-1α overexpression partly requires activating PI3K/AKT signaling pathway, thus providing theoretical basis for the treatment of diabetic vascular neogenesis and vascular injury repair.
EPCs, HIF-1α, VEGF, VEGFR, PI3K/AKT signaling pathway, high-glucose, diabetic rats
Neovascularization is impaired in diabetes mellitus, which leads to the development of peripheral arterial disease and is mainly attributed to the dysfunction of endothelial progenitor cells [1]. Endothelial progenitor cells (EPCs), a subpopulation of adult stem cells, are recruited from bone marrow to the injured vessel to promote endothelial regeneration and neovascularization, playing an important role in angiogenesis [2]. Interestingly, several clinical studies have showed that the number of recruited EPCs is reduced and their function is decreased under diabetic conditions, implying that diabetic EPC dysfunction may contribute to defective angiogenesis and resultant cardiovascular complications in diabetes [3]. Emerging evidence has shown that the reduced number and impaired function of circulating EPCs are involved in vascular complications in diabetes and EPCs transplantation promoted angiogenesis and improved function recovery after hind limb ischemia in diabetic mice [4]. Diabetic EPCs have impaired proliferation, adhesion, and deformability compared with nondiabetic EPCs [5,6]. It is generally accepted that FITC-UEA-1 binding and DiI-ac-LDL uptake are capable of differentiating into endothelial cells and forming vascular structures [7].
The mechanisms of diabetes-induced EPCs deficit are still unclear. Prior reports have showed that hypoxia-inducible factor-1α (HIF-1α) is among possible molecular mechanisms underlying the reduced number and impaired function of EPCs exposed to high glucose conditions [8]. HIF-1α is a critical transcription factor in maintaining oxygen homeostasis in physiological conditions and in regulating the cellular adaptive reaction under hypoxic conditions. Its target genes such as VEGF and VEGFR are involved in many important processes such as angiogenesis, cell proliferation and energy metabolism [9]. EPCs isolated from diabetic individuals have defective migration in response to HIF-1α. In patients with type 1 or type 2 diabetes, the migratory response of EPCs to VEGF binding also is impaired [10]. Fadini GP et al. showed that HIF-1α is a major genetic modifier in coronary artery disease and it protects the heart against ischemic-reperfusion injury in mice [11]. A recent study reported that transplantation of EPCs restored the local blood flow and improved limb function after unilateral hind limb ischemia in diabetic mice by inducing HIF-1α hyperexpression [12]. However, whether the HIF-1α pathway is involved in the mechanisms of EPC therapy in diabetes remains unclear.
PI3K/AKT pathway is one of the typical signal transduction pathways in regulating EPC mobilization from BM to sites of ischaemia and hypoxia [13]. Recently, in type 2 diabetic patients with coronary artery disease (CAD), hyperglycaemia and hyperlipidaemia impaired EPC number, migration, NO bioavailability and NOS activity, which was accompanied by the downregulation of the expressions of CXCR4 and members of PI3 kinase/Akt/eNOS signal cascades [14]. These results provided the evidence for the possible underlying mechanism for hyperglycaemia impaired EPC migration in type 2 diabetic mellitus.
In this study, we investigated whether EPCs function could impair by high-glucose condition in diabetic rats. And if so, whether the HIF-1α pathway is involved in the beneficial effect of EPCs, whether the recuse effect of HIF-1α on EPCs dysfunction requires activating PI3K/AKT signaling pathway.
Animals
All the experimental protocols were approved by the Committee of Ethics of Animal Experiments at Zhejiang University School of Medicine, China. Male Sprague-Dawley rats (Shanghai S&P-Shall Kay Laboratory Animal Co, Ltd, Shanghai, China) aged 7 to 8 weeks were housed in a temperature- and humidity-controlled environment with free access to food and water. The rats were divided into two groups of three animals each. Group1: a sham‐operated control group received the standard laboratory diet (SLD). Group 2: STZ was injected in a dose of 45 mg/kg once and the animals were fed with SLD. 45 mg STZ was weighed and dissolved in 50 mM sodium citrate buffer (pH 4.5) to a final concentration of 45 mg/mL. It was injected intraperitoneally to the animals as to be 1.0 mL/kg. Blood glucose was measured 3 weeks after STZ injection by glucometer and the rats with non-fasting blood glucose (FBG) level of ≥ 11.1 mmol/L were considered as diabetic.
Isolation, culture, and identification of EPCs
All healthy rats were euthanized by cervical dislocation, and the femur and tibia were harvested aseptically for isolation of EPCs. 20ml peripheral blood and cell suspension of bone marrow was prepared, and mononuclear cells were isolated using the separation medium. After being washed two times with phosphate-buffered saline, the cells were resuspended in the complete medium containing 10% fetal bovine serum (FBS; Gibco Laboratories, Gaithersburg, Md), 10 mg/L basic fibroblast growth factor (TBD Science, Tianjin, China), and 10 mg/L vascular endothelial growth factor (VEGF; TBD Science). The cells were then plated into a 25 cm2 flask at a density of 5X105 cells/mL and cultured in humid air with 5% CO2 at 37°C. The medium was replaced every 4 days, and nonadherent cells were removed. The culture was maintained for 2 weeks, and the cells were cultured for subsequent experiments. The phenotype of EPCs was evaluated by double positive for FITC-UEA-1 binding (green fluorescence) and DiI-ac-LDL (red fluorescence) uptake by using fluorescence microscope. EPCs cultured in normal glucose medium contain 5mM glucose (NG) and high glucose medium contain 22mM glucose (HG).
Recombinant adenovirus
First-generation adenovirus was regulated by the cytomegalovirus promoter using an open reading frame (ORF) Shuttling system (Vigene Biosciences, Inc.). Briefly, rat HIF-1α cDNA (NM_024359) (Vigene Biosciences, Inc.) was excised from the pENTR vector and inserted into a pAD-ORF transfer vector. The recombinant adenoviral constructs were transfected into 293 cells, in order to generate a recombinant adenovirus with a high Ad-HIF-1α titer [1X1010 plaque-forming unit (pfu)/ml]. Recombinant adenoviruses can express exogenous gene fragments and can infect dividing and non-dividing cells in a wide range of hosts. The present study obtained Ad-HIF-1α through a customized adenovirus service (by Vigene Biosciences, Inc.). In the present study, Ad-control refers to an empty adenovirus that was used as a control. To detect infection efficiency, all adenovirus vectors were labeled with flag. In addition, the effects of adenovirus infection were verified using western blot analysis, in order to confirm that Ad-HIF-1α was highly expressed.
Cell viability assay
Cell viability was quantified with CCK-8 method. Briefly, EPCs were plated in flat-bottomed 96-well microplates at 1X104 cells/well and incubated in Endothelial Basal Medium-2 (EBM-2, Lonza, Walkersville, Md) medium containing 2% FBS for 24 hours (6 wells per group). Then the culture medium was changed to EBM-2 medium containing 10% FBS and cultured for an additional 6 hours. Cells were finally exposed to high glucose medium contain 22mM glucose for 24 hours. Control cells were without any treatment. CCK-8 (10 μL/well) was added to the wells at the end of the experiment. After incubation at 37°C for 48 hours, the absorbance of each well was determined using a microplate reader at 450 nm. The degree of EPCs proliferation was determined as the percentage of absorbance of treated EPCs to that of control EPCs.
Migratory activity of EPCs was investigated by Transwell assay (Corning Inc, Corning, NY; 8-mmpore size filters). After treatment of EPCs with high glucose or HIF-1α overexpressed adenovirus, a fraction of 5X104 cells in 100 mL non-FBS EBM-2 was seeded into the upper chamber; 500 mL culture medium containing 20% FBS was placed in the lower chamber. The EPCs were allowed to migrate for 24 hours at 37°C in the tissue culture incubator. Transwell membranes were stained with 0.1% crystal violet for 30 minutes, and the migrated cells were examined using an inverted fluorescence microscope (IX51; Olympus, Tokyo, Japan). All experiments were done in triplicate with cells counted in three random fields of view.
Matrigel-based capillary-like tube formation assay
The effect of high glucose or overexpression on capillary-like tube formation capacity of EPCs was investigated using the capillary tube formation assay angiogenesis assay kit (ECM625; Merck Millipore, Billerica, Mass) in vitro. Briefly, after treatment of EPCs with high glucose for 24 hours, a fraction of 1.0×104 cells per well was seeded on a Matrigel-precoated 96-well plate (Corning Inc, Corning, NY). Tube formation was observed under an inverted light microscope (Olympus IX51). Three independent fields were measured for each well, and the average values of the tubes were analyzed by WimTube Quantitative Image Analysis (Wimasis).
Semi-quantitative RT-PCR
Total RNAs in EPCs were extracted using TRIzol reagent (Invitrogen, Carlsbad, Calif) according to the manufacturer’s instruction. Reverse transcription was carried out with 0.5 μg total RNA using the PrimerScript™ RT reagent kit (Takara, Shiga, Japan). After incubation for 1 h at 42°C, the reactions were terminated by heating at 70°C for 15 min. 5′ primer (5′-ACCATGCCCCAGA TTCAAGA-3′) and 3′ primer (5′-TAGGGAGCCAGCATTTCCAA-3′) to HIF-1α; 5′ primer (5′-CACGATGGAGGGGCCGGACTCATC-3′) and 3′ primer (5′-TAAAGACCTCTATGCCAACACAGT-3′) to β-actin were used for PCR amplification (30 cycles, 94°C, 30s; 56°C, 30s; 72°C, 1 min) with 2xEasy Taq superMix (Transgen Biotech, China). PCR products were separated and analyzed on agarose gels, with the bands of the HIF-1α being confirmed by DNA sequencing.
Real-time polymerase chain reaction (PCR)
Total RNAs in EPCs were extracted using TRIzol reagent (Invitrogen, Carlsbad, Calif). Complementary DNAs were generated using the PrimeScript 1st Strand cDNAs Synthesis kit (D6110A; Takara Bio, Mountain View, Calif). All real-time PCR assays were carried out using SYBR Green Realtime PCR Master Mix (QPK-201, QPK-201T; Toyobo, Osaka, Japan). The amplification parameters were 95°C for 30 sec followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec. The measurement was conducted in triplicate. Data analysis was performed using the 2−ΔΔct method. HIF-1α forward primer was 5′-CCATTCCTCATCCATCAA-3′, reverse primer was 5′-CCATCAACTCAGT AATCCT-3′. VEGF forward primer was 5′-GCCCTGAGTCAAGAGGACAG-3′, the reverse primer was 5′-CAGGCTCCTGATTCTTCCAG-3′. VEGFR forward primer was 5′-TTTGGTAGCGGGATGAA-3′, the reverse primer was 5′-ATGGGATTGGTGAGGATGA-3′. GAPDH forward primer was 5′-AGACAGCCGCATCTTCTTGT-3′, the reverse primer was 5′-CTTGCCGTGGGTAGAGTCAT-3′.
Western blotting
Briefly, EPCs were lysed using protein extraction buffer containing 100 mM phenylmethanesulfonyl fluoride. The proteins from the lysates were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were then transferred onto a polyvinylidene difluoride membrane and probed with primary antibodies against HIF-1α (1:1000, Life Technologies), VEGF (1:1000, Life Technologies), VEGFR (1:1000, Life Technologies), PI3K (1:1000, Santa Cruz Biotechnology), p-PI3K (1:1000, Santa Cruz Biotechnology), AKT (1:1000, Santa Cruz Biotechnology), p-AKT (1:1000, Santa Cruz Biotechnology), GAPDH (1:3000, Santa Cruz Biotechnology). Expression of proteins of interest was visualized with an enhanced chemiluminescence detection kit (Millipore).
Statistical analysis
All results were expressed as mean ± SD. Data were analyzed by SPSS 18.0 for both parametric and nonparametric comparisons. A probability value of p < 0.05 was considered as statistical significant.
The isolation, establishment and characterization of EPCs
In this study, two different types of blood sources, whole peripheral blood (PB-EPC) and bone marrow (BM-EPC), were harvested for the establishment of EPCs. EPCs were isolated from whole peripheral blood and bone marrow of STZ-induced diabetic SD rats and control SD rats. EPCs were isolated, cultured, and identified according to the method described in the previous study by Andreas Brandl [15]. After using Percoll density gradient centrifugation and two week’s cultivation, the phenotype of EPCs was evaluated as adherent cells by double positive for FITC-UEA-1 binding (green fluorescence) and DiI-ac-LDL (red fluorescence) uptake by using fluorescence microscope (Figure1A). Isolated cells were small round cells after cultured less than 24 hours, exhibited a spindle-shaped morphology after cultured for 4~7 days, formed cordlike structures after 10 days (Figure1B). A total of 76.5%±9.19% PB-EPC adherent cells of control rat and 47.3%±13.65% PB-EPC adherent cells of diebatic rat showed uptake of FITC-UEA-1 binding and DiI-ac-LDL uptake after 10 days of culture in our study, differences were accepted to be statistically significant at p<0.05. Meanwhile, a total of 68.3%±11.50% BM-EPC adherent cells of control rat and 43.3%±10.40% BM-EPC adherent cells of diebatic rat showed uptake of FITC-UEA-1 binding and DiI-ac-LDL uptake after 10 days of culture, p<0.05. EPCs from diabetic rats were significantly less than control rats, both in whole peripheral blood and bone marrow (Figure1C).
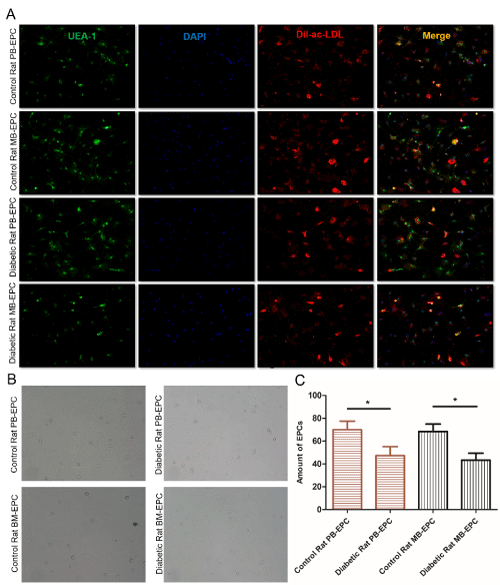
Figure 1. Establishment and phenotypic characterization of cultured EPCs. (A) Control or diabetic rat PB-EPCs and BM-EPCs were double positive for both FITC-UEA-1 binding (green fluorescence) and DiI-ac-LDL (red fluorescence) uptake under fluorescence microscope, the markers of EPCs. Nuclear counterstaining was performed using DAPI (blue). Merge for their overlay. Scale bars are 200um. (B) Morphology of EPCs after cultured 10 days. EPCs from buffy coats formed colonies with a typical cobblestone-like cell morphology on day 10 after seeding. Scale bars are 200um. (C) EPCs were counted manually using the ImageJ cell counter, and the number of EPCs per image were converted to number of cells per cm2. *p<0.05
High-glucose impairs the viability, migration and capaillary-like tube formation of EPCs
Angiogenesis is initiated from endothelial cell activation, followed by cell proliferation, migration and capaillary-like tube formation. To investigate the role of high-glucose in EPCs function, cell viability and cell migration, as well as tube formation assays were performed on above four different types of EPCs using normal glucose medium (NG, 5mM glucose) compared to high glucose medium (HG, 22mM glucose). As shown in Figure 2, the amount of EPCs evaluated by CCK8 cell viability assay was decreased compared with diabetic rats and control rats, as well as in high glucose medium compared to normal glucose medium, both in whole peripheral blood and bone marrow, all p<0.05 (Figure 2A). Since angiogenesis is dependent on the ability of the cells to properly migrate, we were interested in reviewing the effect of high-glucose on EPCs migration. To this end, migratory activity of EPCs was investigated by Transwell assay. As expected, high-glucose reduced EPCs migration significantly compared with diabetic rats and control rats, as well as in high glucose medium compared to normal glucose medium, both in whole peripheral blood and bone marrow (Figure 2B and 2C). Then we investigated high-glucose’s inhibitory effect on the tube formation ability of EPCs using matrigel-based tubule formation assay. EPCs seeded on Matrigel-coated plate, formed robust tubular-like structures and high-glucose inhibited EPCs tube formation, as evidenced by a significant reduction in mean tube number, segment length, and number of junctions (Figure 2D). Altogether, the above data demonstrated that high-glucose dramatically inhibits EPCs tube-formation, indicating a possible suppressive effect on neo-angiogenesis of EPCs.
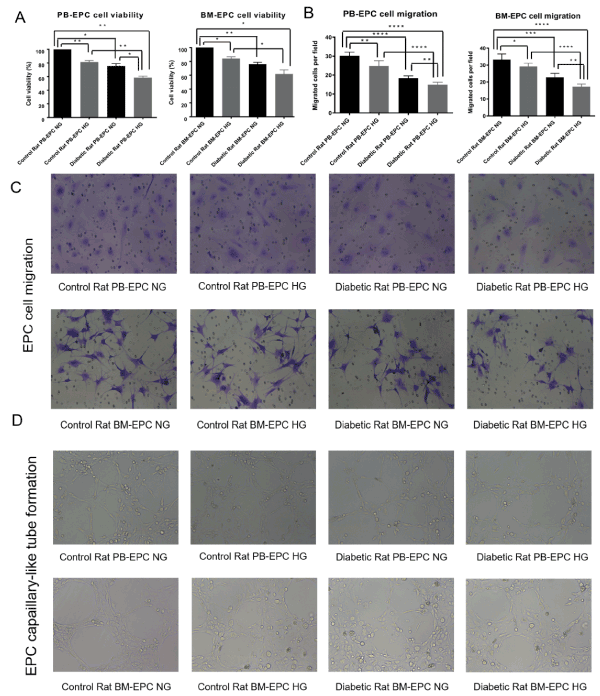
Figure 2. The viability, migration and tube formation of EPCs. (A) EPCs were plated on 96-well plates and cultured with normal glucose medium or high glucose medium. 48 h later, cell viability was assessed by CCK8 assay, *p<0.05, **p<0.01. (B), (C) Migration capacity in EPCs treated with high glucose. EPCs were seeded onto the upper chamber and allowed to migrate for 24 h, migratory cells from the bottom chamber were stained with 0.1% crystal violet for 30 minutes and examined using an inverted fluorescence microscope(C). EPCs were counted manually using the ImageJ cell counter, and the number of EPCs per image were converted to number of cells per cm2. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001(B). (D) Tube formation capacity in EPCs treated with high glucose. EPCs were plated on matrigel-coated plates in HG medium or NG medium. Tube formation was evaluated after 24h by light microscopy
High-glucose down-regulates HIF-1α, VEGF and VEGFR mRNA or protein level of EPCs
Because angiogenesis is controlled by transcriptional factor hypoxia-inducible factor-1α (HIF-1α) and its target gene vascular endothelial growth factor (VEGF). To determine the role of high-glucose in EPCs, we detected the mRNA expression and protein level of HIF-1α after high-glucose medium cultivation by semi-quantitative RT-PCR (sqRT-PCR), quantitative real-time RT-PCR (RT-qPCR) and Western Blotting, as well as the protein levels of its target VEGF and VEGFR after high-glucose medium cultivation by Western Blotting. High-glucose induced an obvious decrease of HIF-1α mRNA expression by sqRT-PCR (Figure 3A) and RT-qPCR assay (Figure 3B). Consistently, the protein level of HIF-1α was also significantly down-regulated in EPCs cultured in high-glucose medium or isolated from diabetic rats, both in whole peripheral blood and bone marrow (Figure 3C and 3D). Meanwhile, the protein levels of VEGF and its receptor VEGFR, which were among the known target genes of HIF-1α, were also detected by Western Blotting. The results showed that the protein levels of VEGF and VEGFR were notably reduced in high-glucose medium or isolated from diabetic rats (Figure 3E and 3F). These data suggested that inhibition of HIF-1α and its target genes VEGF/VEGFR may aggravate EPC dysfunction induced by high-glucose.
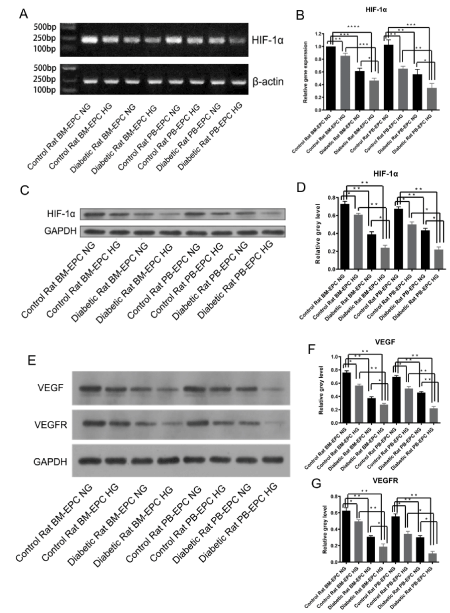
Figure 3. The mRNA or protein levels of HIF-1α, VEGF and VEGFR reduced by high glucose. (A) High-glucose down-regulated HIF-1α mRNA expression by sqRT-PCR assay, β-actin was used as a loading control. (B) High-glucose down-regulated HIF-1α mRNA expression by RT-qPCR assay. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. (C) Representative western blotting analysis of HIF-1α treated with NG or HG medium, GAPDH was used as a loading control. (D) The stoichiometric relationship of the western blotting analysis of HIF-1α, *p<0.05, **p<0.01. (E) Representative western blotting analysis of VEGF and VEGFR treated with NG or HG medium, GAPDH was used as a loading control. (F) The stoichiometric relationship of the western blotting analysis of VEGF, *p<0.05, **p<0.01. (G) The stoichiometric relationship of the western blotting analysis of VEGFR, *p<0.05, **p<0.01
Overexpression of HIF-1α ameliorates the impairment of cell viability, migration and capaillary-like tube formation ability of EPCs induced by high-glucose
The above results indicated that high glucose is associated with reduced production of HIF-1α, VEGF and VEGFR, established inducer of neovascularization in EPCs. To investigate whether the negative impact of high-glucose on EPCs function can be partly rescued by HIF-1α, recombinant adenoviral particles containing the HIF-1α ORF under the control of a constitutive CMV promoter were created, packaged and identified by Vigene Biosciences. After adenovirus infection for 48h, cell viability and cell migration, as well as tube formation assays were performed again as described above on BM-EPCs under normal glucose medium or high glucose medium. As shown in Figure 4, the mRNA expression (Figure 4A) and protein level (Figure 4F) of HIF-1α was up-regulated by adenovirus infection and restored by high-glucose treatment. High-glucose induced BM-EPCs cell viability (Figure 4B) and cell migration (Figure 4C and 4D) inhibition were alleviated by HIF-1α overexpression. Consistently, the impairment of the tube formation ability of BM-EPCs under high-glucose environment was ameliorates by HIF-1α overexpression (Figure 4E). Furthermore, Western Blotting and RT-qPCR assay showed that HIF-1α overexpression rescued the decreased protein levels (Figure 4F) and mRNA expression (Figure 4G) of VEGF and VEGFR by high-glucose treatment. Altogether, the above data demonstrated that overexpression of HIF-1α ameliorates the impairment of cell viability, migration and capaillary-like tube formation ability of EPCs induced by high-glucose. These results suggested a high glucose-induced HIF-1α down-regulation, thus HIF-1α-mediated VEGF/VEGFR inhibition may be partially responsible for neovascularization in diabetic rat EPCs.
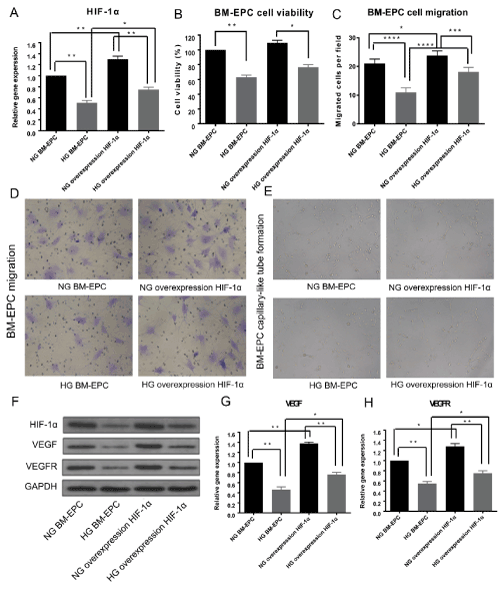
Figure 4. Overexpression of HIF-1α ameliorates the function of BM-EPCs. (A) BM-EPCs were infected with Ad-HIF-1α for 48h to up-regulated the expression of HIF-1α. The mRNA expression of HIF-1α after adenovirus infection cultured with normal glucose medium or high glucose medium was assessed by RT-qPCR assay. (B) BM-EPCs viability was assessed by CCK8 assay after Ad-HIF-1α infection cultured with normal glucose medium or high glucose medium. (C) Migration capacity in BM-EPCs treated with Ad-HIF-1α with or without high-glucose. BM-EPCs were counted manually using the ImageJ cell counter. (D) Representative migratory cells observed over time under the microscope. (E) Tube formation capacity in BM-EPCs infected with Ad-HIF-1α with or without high glucose. BM-EPCs were plated on matrigel-coated plates and evaluated by light microscopy. (F) Representative western blotting analysis of HIF-1α, VEGF and VEGFR after Ad-HIF-1α infection cultured with normal glucose medium or high glucose medium, GAPDH was used as a loading control. (G, H) The mRNA expression of VEGF(G) and VEGFR(H) after Ad-HIF-1α infection cultured with normal glucose medium or high glucose medium was assessed by RT-qPCR assay, *p<0.05, **p<0.01
High-glucose exposure decreased HIF-1α expression in EPCs of diabetic rats via blocking PI3K/AKT signaling pathway
The major downstream effectors of the HIF-1α-VEGF/VEGFR pathway include phosphatidylinositol-3 kinase (PI3K) and protein kinase B (PKB/AKT) [16]. Given the role of HIF-1α and high glucose on EPCs dysfunction, we tested the hypothesis that HIF-1α may alleviate the negative effect on EPCs function of high-glucose by the PI3K/AKT signaling pathway. We investigated the PI3K/AKT protein levels and phosphorylation-PI3K/phosphorylation-AKT (p-PI3K/p-AKT) activation with or without high-glucose on BM-EPCs by Western Blotting. The results showed that both PI3K and AKT phosphorylation were inhibited by high-glucose treatment, the same as HIF-1α (Figure 5A and 5B). Subsequently, to determine whether the recuse effect of HIF-1α on EPCs dysfunction partly requires activating PI3K/AKT signaling pathway, we detected the protein level of HIF-1α in the presence and absence of PI3K agonist (Wortmannin) with or without high-glucose on BM-EPCs. The results showed that PI3K agonist regain the protein levels of p-PI3K and p-AKT, which obviously decreased by high-glucose. Meanwhile, the downregulated expression of HIF-1α induced by high-glucose was rescued by PI3K agonist treatment (Figure 5C and 5D). These data suggested that the attenuation of high-glucose induced diabetic rats EPCs dysfunction by HIF-1α activation partly requires activating PI3K/AKT signaling pathway.
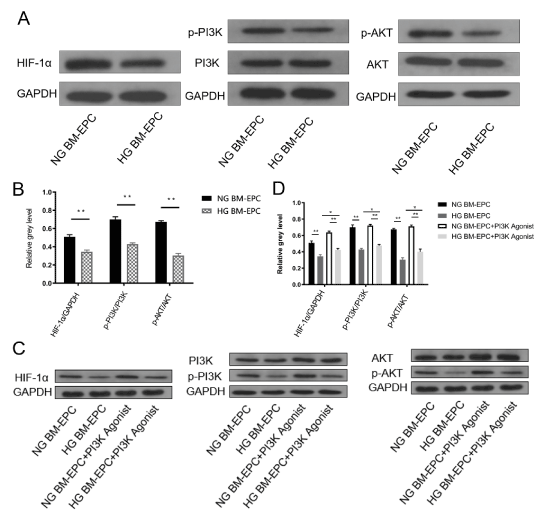
Figure 5. High-glucose decreased HIF-1α expression in BM-EPCs via regulating PI3K/AKT signaling pathway. (A) Representative western blotting analysis of HIF-1α, PI3K, AKT, p-PI3K and p-AKT by high-glucose treatment, GAPDH was used as a loading control. (B) The stoichiometric relationship between the protein expression of HIF-1α and GAPDH (HIF-1α/ GAPDH), between the p-PI3K and the protein expression of PI3K (p-PI3K/PI3K), between p-AKT and the protein expression of AKT (p-AKT/AKT) in BM-EPCs with or without high-glucose by western blotting analysis, **p<0.01. (C) Representative western blotting analysis of HIF-1α, PI3K, AKT, p-PI3K and p-AKT by PI3K agonist treatment with or without high-glucose by western blotting, GAPDH was used as a loading control. (D) The stoichiometric relationship of HIF-1α/GAPDH, p-PI3K/PI3K and p-AKT/AKT in BM-EPCs by PI3K agonist treatment with or without high-glucose by western blotting analysis, *p<0.05, **p<0.01
Vascular complications contribute significantly to morbidity and mortality of diabetes mellitus. The primary cause of vascular complications in diabetes mellitus is hyperglycaemia, associated with endothelial dysfunction and impaired neovascularization. Circulating EPCs was shown to play important roles in vascular repair and promoting neovascularization [3,17]. In the present study, we demonstrated the impaired effects of high-glucose on the cell viability, cell migration and tube formation ability of EPCs isolated from diabetic rats. Assays and data exhibited that high-glucose condition inhibits the expression of HIF-1α and its target genes VEGF and VEGFR. Furthermore, HIF-1α overexpression ameliorated EPCs dysfunction by activating PI3K/AKT signaling pathway, indicating defective EPCs may be amenable to pharmacological manipulation and restoration of the cells’ function by PI3K agonist.
The contribution of EPCs to vascular dysfunction in diabetes remains controversial. There are profound differences in endothelial cell behavior in peripheral vascular complications in which there is poor vessel growth and in microvascular complications of the eye in which excess and aberrant vascularization leads to blindness. This disparity partially may be explained by differences in subpopulations of EPCs in the circulation that participate in repair of the defective vascular beds [18]. There are numerous studies demonstrating that diabetic EPCs do not repair vascular injury using both type 1 and type 2 diabetic rodent models [19,20]. In fact, endothelial dysfunction has long been viewed as the first event for the development of atherosclerosis and clinical events [21]. Therefore, the maintenance of an intact endothelial layer and improving its function are essential for keeping vessels healthy. There is increasing evidence that neovascularization has two cell sources. One is from the proliferation and migration of the fully differentiated endothelial cells (ECs). The other is from the homing of the circulating endothelial progenitor cells (EPCs) to sites of endothelial disruption and incorporating into nascent endothelium [22]. It has been estimated in animal models that EPCs contribute to 25% of ECs in newly formed vessels [23].
Hypoxia is an important pathophysiological stress generated during blood vessel damages, associated with differential expression of specific genes capable of enhancing cell and tissue adaptation to low oxygen tension, including bFGF, VEGF, IL-8, and others [24]. Hypoxia-induced expression of these genes is regulated by the activation of specific transcription factors HIF-1, The availability of HIF-1 is mainly determined by HIF-1α that is regulated in an oxygen-sensitive manner [25]. Sang Hun Lee et al. reported that hypoxia can prevent proliferative senescence, increase proliferation capacity and lifespan, and maintain the stem cell properties of EPCs through HIF-1α-induced TWIST expression [26]. Our results demonstrated that high-glucose condition reduced the HIF-1α mRNA and protein expression and overexpression of HIF-1α by adenovirus restored the function of EPCs. HIF-1α overexpression also attenuated the VEGF and VEGFR protein level reduced by high-glucose treatment, thus rescued the cell viability, cell migration and tube formation ability of EPCs.
The results of various studies have shown that high-glucose impair EPC function by exerting a deleterious effect on the PI3K/AKT signaling pathway [27]. Recently, it was demonstrated that hyperglycemia-induced impairment of early EPCs could be restored via the modulation of p38 mitogen activated protein kinase (MAPK) [28] and Akt/FoxO1 signaling [29]. Zheng H et al. demonstrated that stimulation of the CXCR4 by SDF1α activates the PI3K/AKT/eNOS signaling pathway to trigger EPC migration and neovascularization [13]. Similar with these previous studies, we reported that high-glucose down-regulated PI3K/AKT protein expression and phosphorylation. The agonist of PI3K showed a recuse effect of EPCs dysfunction by high-glucose, as well as regain HIF-1α-VEGF/VEGFR protein levels. Christoph Gensch et al. reported that the PPAR-gamma agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells [30]. On the basis of the experiments presented here, we want to reveal that the attenuation of high-glucose induced diabetic rats EPCs dysfunction by HIF-1α overexpression partly requires activating PI3K/AKT signaling pathway.
In conclusion, hyperglycemia severely impairs EPCs viability, migration and tube formation. Our results indicate that the underlying mechanism for this impaired EPC function is linked to the HIF-1α-VEGF/VEGFR-PI3K/AKT signaling pathway. We hope to provide a new strategy of PI3K agonist that could potentially benefit patients with vascular diseases of diabetes mellitus. Here, we want to point out that this study did not determine the rescue effects of HIF-1α overexpression or PI3K agonist in vivo, in-depth studies deserve future investigation.
We thank the staff of the Department of Endocrinology, the Affiliated Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University for their valuable input and support throughout this study.
This project was supported by grants from the Zhejiang Provincial Natural Science Foundation of China (LY16H070002).
The authors have declared that no conflict of interest exists.
- Jude EB, Oyibo SO, Chalmers N, Boulton AJ (2001) Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome.Diabetes Care24: 1433-1437. [Crossref]
- Saito H, Yamamoto Y, Yamamoto H (2012) Diabetes alters subsets of endothelial progenitor cells that reside in blood, bone marrow, and spleen. Am J Physiol Cell Physiol 302: C892-901.
- Kang H, Ma X, Liu J, Fan Y (2017) High glucose-induced endothelial progenitor cell dysfunction.Diab Vasc Dis Res14: 381-394. [Crossref]
- Megherbi SE, Milan C, Minier D, Couvreur G, Osseby GV, et al. (2003) Association between diabetes and stroke subtype on survival and functional outcome 3 months after stroke: data from the European BIOMED Stroke Project.Stroke34: 688-694. [Crossref]
- Petrelli A, Di FR, Carvello M, Gatti F, Secchi A, et al. (2012) Strategies to reverse endothelial progenitor cell dysfunction in diabetes. Exp Diabetes Res 2012: 471823.
- Kim KA, Shin YJ, Kim JH, Lee H, Noh SY, et al. (2012) Dysfunction of endothelial progenitor cells under diabetic conditions and its underlying mechanisms.Arch Pharm Res35: 223-234. [Crossref]
- Hong SH, Jang HH, Lee SR, Lee KH, Woo JS, et al. (2015) Impact of lysophosphatidylcholine on survival and function of UEA-1(+)acLDL (+) endothelial progenitor cells in patients with coronary artery disease.Heart Vessels30: 115-125. [Crossref]
- Geng J, Wang L, Qu M (2017) Endothelial progenitor cells transplantation attenuated blood-brain barrier damage after ischemia in diabetic mice via HIF-1a. Stem Cell Res Ther 8: 163.
- Villalvilla A, Fernandez-Durango R (2010) [Endothelial progenitor cells: their possible potential in cell therapy for ischemic retina].Arch Soc Esp Oftalmol85: 291-293. [Crossref]
- Wang C, Wang Q, Gao W, Zhang Z, Lou Y, et al. (2018) Highly efficient local delivery of endothelial progenitor cells significantly potentiates angiogenesis and full-thickness wound healing.Acta Biomater69: 156-169. [Crossref]
- Fadini GP, Sartore S, Schiavon M, Albiero M, Baesso I, et al. (2006) Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats.Diabetologia49: 3075-3084. [Crossref]
- Sunkari VG, Lind F, Botusan IR, Kashif A, Liu ZJ, et al. (2015) Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice.Wound Repair Regen23: 98-103. [Crossref]
- Zheng H, Fu G, Dai T, Huang H (2007) Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway.J Cardiovasc Pharmacol50: 274-280. [Crossref]
- Hamed S, Brenner B, Abassi Z, Aharon A, Daoud D, et al. (2010) Hyperglycemia and oxidized-LDL exert a deleterious effect on endothelial progenitor cell migration in type 2 diabetes mellitus.Thromb Res126: 166-174. [Crossref]
- Brandl A, Yuan Q, Boos AM, Beier JP, Arkudas A, et al. (2014) A novel early precursor cell population from rat bone marrow promotes angiogenesis in vitro.BMC Cell Biol15: 12. [Crossref]
- George AL, Rajoria S, Suriano R, Mittleman A, Tiwari RK (2012) Hypoxia and estrogen are functionally equivalent in breast cancer-endothelial cell interdependence.Mol Cancer11: 80. [Crossref]
- Kim HK, Kim YJ, Kim JT, Kwon CH, Kim YK, et al. (2008) Alterations in the proangiogenic functions of adipose tissue-derived stromal cells isolated from diabetic rats.Stem Cells Dev17: 669-680. [Crossref]
- Tan K, Lessieur E, Cutler A, Nerone P, Vasanji A, et al. (2010) Impaired function of circulating CD34(+) CD45(-) cells in patients with proliferative diabetic retinopathy.Exp Eye Res91: 229-237. [Crossref]
- Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, et al. (2002) Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures.Circulation106: 2781-2786. [Crossref]
- Jiang S, Walker L, Afentoulis M, Anderson DA, Jauron-Mills L, et al. (2004) Transplanted human bone marrow contributes to vascular endothelium.Proc Natl Acad Sci U S A101: 16891-16896. [Crossref]
- Kinlay S, Libby P, Ganz P (2001) Endothelial function and coronary artery disease.Curr Opin Lipidol12: 383-389. [Crossref]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis.Science275: 964-967. [Crossref]
- Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, et al. (2002) Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo.Exp Hematol30: 967-972. [Crossref]
- Cummins EP, Taylor CT (2005) Hypoxia-responsive transcription factors.Pflugers Arch450: 363-371. [Crossref]
- Shweiki D, Itin A, Soffer D, Keshet E (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis.Nature359: 843-845. [Crossref]
- Lee SH, Lee JH, Yoo SY, Hur J, Kim HS, et al. (2013) Hypoxia inhibits cellular senescence to restore the therapeutic potential of old human endothelial progenitor cells via the hypoxia-inducible factor-1α-TWIST-p21 axis.Arterioscler Thromb Vasc Biol33: 2407-2414. [Crossref]
- Chen YH, Lin SJ, Lin FY (2007) High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes 56: 1559-1568.
- Seeger FH, Haendeler J, Walter DH, Rochwalsky U, Reinhold J, et al. (2005) p38 mitogen-activated protein kinase downregulates endothelial progenitor cells.Circulation111: 1184-1191. [Crossref]
- Marchetti V, Menghini R, Rizza S, Vivanti A, Feccia T, et al. (2006) Benfotiamine counteracts glucose toxicity effects on endothelial progenitor cell differentiation via Akt/FoxO signaling.Diabetes55: 2231-2237. [Crossref]
- Gensch C, Clever YP, Werner C, Hanhoun M, Böhm M, et al. (2007) The PPAR-gamma agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells.Atherosclerosis192: 67-74. [Crossref]





