Heart failure (HF) with reduced ejection fraction (HFrEF) is a clinical syndrome in which pathological myocardial stress or injury leads to cardiac inability to perfuse the body’s vital organs at rest or during exertion, usually documented by left ventricular ejection fraction ≤ 40% on echocardiography. HFrEF constitutes about a half of all HF hospitalizations and remains the most common discharge diagnosis in older adults. Despite major advancements in the treatment of HFrEF and concomitant improvement in both outcomes and survival, nonspecific symptoms continue to make diagnosis difficult as well as HFrEF has the most ominous prognosis relative to HF with mid-range and preserved ejection fraction. Even upon diagnosis, predicting which patients are at a greater risk of death or major cardiovascular events remains a clinical challenge. In a critical appraisal of published evidence on HFrEF, this review purposes to provide a comprehensive understanding of the clinical status and management of HFrEF.
heart failure with reduced ejection fraction, left ventricular failure, lefty ventricular systolic failure
Heart failure (HF) with reduced ejection fraction (HFrEF) is a complex and progressive clinical syndrome characterized by unacceptably high rates of post-discharge mortality, hospital length of stay, re-hospitalization, compromised functional capacity, reduced quality of life and substantial caregiver burden [1-3]. HFrEF accounts for about half of all the reported cases of HF and its prevalence continues to increase principally attributed to major therapeutic advances (improved survival rates) and an increasingly ageing population [4]. In older adults, HFrEF often represents the final pathway of many cardiovascular diseases [5]. In the effort to improve the management of HFrEF, the majority of recent studies have shifted focus from pathophysiology, prognosis and diagnosis to researching novel clinical and non-clinical care-based management strategies [6-11]. However, many non-specific symptoms (of limited diagnostic value) continue to make diagnosis difficult, post-diagnosis identification of patients at an elevated risk of death or cardiovascular events challenging and prognosis ominous [12]. Further, despite post hoc analysis of hospitalized heart failure registries revealing significant progress in the diagnosis and treatment of HFrEF, high mortality rates of 15% to 20% during the first year of diagnosis and rising to 40% to 50% within five years of diagnosis have remained relatively consistent over recent decades [5]. Such reports suggest HFrEF continues to represents a significant clinical problem even for the best performing healthcare systems. Thus, in a critical appraisal of published literature and smeta-analyses of 3D echocardiography assessment of LV systolic function, and natriuretic (NP)-guided HF therapy, this review seeks to aggregate current scholarly and practitioner evidence on epidemiology, etiopathophysiology, diagnosis and clinical management to advance both the understanding and management of HFrEF.
Definition
Previously, HFrEF was defined as a condition in which the heart fails to discharge its contents adequately [13] or a pathophysiological state in which an abnormality of cardiac function caused the failure of the heart to pump blood at a rate commensurate with the requirements of the metabolizing tissues [14]. Although these definitions accurately described the principal pathologic mechanism of HFrEF, they were difficult to apply in a clinical setting [15]. Today, most clinical trials and practice rely on or cite the definitions of the European Society of Cardiology (ESC) HF guidelines [16] or the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) Task Force on the management of HF [17].
The 2016 ESC guidelines define HF as a clinical syndrome in which patients exhibit typical symptoms (peripheral edema, fatigue, and dyspnea) and signs (elevated jugular venous pressure, pulmonary crepitation and displaced apex beat) caused by structural and/or functional cardiac abnormality resulting into reduced cardiac output and/or elevated intra-cardiac pressures at rest or during stress [16]. The 2013 ACCF/AHA guidelines define HF as a complex clinical syndrome resulting from any structural or functional impairment of ventricular filling or ejection of blood with clinical manifestations of dyspnea, fatigue, limited exertional tolerance and fluid retention leading to splanchnic congestion and/or peripheral edema [17]. In both definitions, the diagnostic hallmark of HFrEF is left-ventricular ejection fraction (LVEF) equal to or less than 40% [16,17].
Taken the two definitions together, HFrEF is a clinical syndrome characterized by the cardinal triad of edema, fatigue and dyspnea resulting from an impairment of ventricular ejection of blood usually documented by LVEF of 40% or less. Although HFrEF is sometimes referred to as systolic dysfunction, it is not entirely accurate since some HFrEF patients may develop subtle diastolic dysfunction [16]. About one in every four females and one in every six males with HFrEF may show echocardiographic signs of an impaired diastolic function [4].
Classification: HFrEF is a progressive syndrome that worsens overtime resulting in a general decline in health and/or function. Several classification systems have been developed to assist in the objective determination of HFrEF stage based on symptomatic severity and/or the presence or absence of structural heart disease. The two most frequently cited classification systems are the ACCF/AHA stages of HF [18] and the New York Heart Association (NYHA) functional classification [19]. The ACCF/AHA stages of HF focused on disease development and progression, and could even be useful in describing both individuals and populations (Table1).
Table 1. The ACCF/AHA stages of heart failure
HF Stage
|
Description
|
Example
|
A |
High risk, no structural heart disease and/or symptoms |
Hypertension, coronary artery disease (CAD), diabetes mellitus (DM) |
B |
Structural heart disease no signs and/or symptoms |
LV hypertrophy, asymptomatic left ventricular systolic dysfunction |
C |
Structural heart disease, prior or current symptoms |
Dyspnea or fatigue due to HF |
D |
Structural heart disease, refractory HF |
Patients with end stage heart failure |
Adapted from Hunt, et al. 2009 Focused update of the ACC/AHA 2005 HF Guidelines [18]
The ACCF/AHA classifies HF into four progressive and inviolate stages of HF (A-D) – once a patient has progressed to a higher stage, regression to an earlier stage is not observed. In the initial Stage A, patients are at a high risk of developing HFrEF but lack any demonstrable structural heart disease or symptoms. This stage includes patients with hypertension, CAD and DM. In Stage B, patients have demonstrable structural heart disease in the absence of signs or symptoms. This stage includes patients with LV hypertrophy and asymptomatic LV systolic dysfunction. In Stage C, patients have demonstrable structural disease and previous or current HF symptoms. This stage includes patients with HF-related dyspnea and fatigue. In the final Stage D, patients have structural heart disease and refractory to HF therapy. This stage includes patients with end stage HF [18]. The basis of the ACCF/AHA classification are abnormalities of cardiac structure and symptoms, quantified from a lower to a higher stage using a 5-year reduction in survival and an increase in the levels of plasma natriuretic peptides [20]. The four stages also provide guidance in therapeutic interventions: modifying risk factors (Stage A), treating structural heart disease (Stage B), and reducing morbidity and mortality (Stages C and D) [18]. The NYHA functional classification on the other hand focuses on exercise (exertional) capacity and symptomatic status of HFrEF (Table 2).
Table 2. The NYHA functional classification
NYHA Class
|
Description (based on physical activity and symptoms)
|
I |
No limitation of physical activity. Ordinary physical activity does not cause symptoms of HF |
II |
Slight limitation of physical activity. Comfortable at rest, but ordinary physical activity results in symptoms of HF |
III |
Marked limitation of physical activity. Comfortable at rest, but less than ordinary activity causes symptoms of HF |
IV |
Unable to carry on any physical activity without symptoms of HF, or symptoms of HF at rest |
Adapted from the Criteria Committee of the New York Heart Association [19]
The NYHA functional classification estimates symptomatic severity in those with structural heart disease (relating to Stages C and D in ACCF/AHA HF Stages). It is a subjective assessment, in which functional class can change frequently over short periods. Its main determinant is functional ability. In NYHA I, patients show no limitation in physical activities or symptoms. In NYHA II, patients exhibit HF symptoms during ordinary physical activities but not at rest. In NYHA III, patients have marked limitation of physical activities and ordinary activities may induce HF symptoms. In NYHA IV, patients are unable to carry any activities without inducing HF symptoms, which may also occur at rest [19]. It has reproducibility and validity challenges but remains an independent predictor of mortality in HFrEF patients [21,22] and widely adopted in clinical practice as a tool for determining eligibility of patients to receive certain health services [21].
Epidemiology: The precise estimate of HFrEF incidence and trends in the global population are scarce and at most unreliable. Most of the literature on HFrEF epidemiology is derived from high-income developed nations where the incidence may be plateauing or even decreasing [23]. On the other hand, while epidemiological data from North America and Europe estimates the prevalence of HF at 1% to 2% [24], the exact prevalence of HFrEF remains poorly understood. Most epidemiological studies and clinical registries that have included the HFrEF phenotype report high variability of its prevalence. The variation may be attributed to dissimilar definitions of HFrEF (different cut-off thresholds for LVEF), clinical setting (primary care, ambulatory care or hospital) and population characteristics (age, gender and history of myocardial infarction) used in individual studies [13,14]. In addition, epidemiological data on HFrEF prevalence from developing nations are conspicuously lacking [16]. Despite the variation, population-based studies conducted between 1997 and 2010 involving older patients (≥ 60 years) report prevalence rates of between 2.4% to 4.8%, with higher rates for males (range = 3.3% to 7.2%) than females (range = 1.5% to 4.1%) (Table 3).
Table 3. Reported prevalence of HFrEF in patients ≥ 60 Year
1st Author [Ref #]
|
Period
|
LVEF Cut-off
|
All Patients
|
Male
|
Female
|
Raymond et al. [25] |
1997-2000 |
40% |
4.0 |
5.5 |
2.9 |
Abhayaratna et al. [26] |
2002-2003 |
50% |
2.5 |
NR |
NR |
Tiller et al. [4] |
2002-2004 |
50% |
5.8 |
7.2 |
4.1 |
Mureddu et al. [27] |
2007-2010 |
50% |
2.4 |
3.3 |
1.4 |
The combined HFrEF prevalence in the four studies is 3.3%, range: 2.4% to 5.8% [28]. Besides gender, the prevalence of HFrEF has a positive correlation with age. Individuals aged 50 years or below are unlikely to have HFrEF but in those aged above > 50years, the prevalence and incidence increases progressively. One in six older adults > 65 years presenting to a primary care with exertional dyspnea will have unrecognized HF [16]. Increasing incidence of HF with age might explain the high prevalence rates in Raymond et al. [24] and Tiller et al. [4] studies, which recruited much older patients.
Risk factors and prognosis: Risk factors are individual characteristics, attributes or co-occurring diseases that may precipitate or aggravate the natural cause of HFrEF. The most frequent independent risk factors are cardiac conditions such as coronary artery disease, myocardial infarction and hypertension; extra cardiac conditions such as diabetes mellitus, chronic kidney disease and anemia; and patient characteristics such as the male gender and older age (Table 4).
Table 4. Independent risk factors for heart failure with reduced ejection fraction
Categories
|
Specific Conditions/Characteristics
|
Cardiac conditions |
Coronary artery disease [4,28-32], arterial hypertension [4,17,29], myocardial infarction [4,28,30-32] |
Extra-cardiac conditions |
Diabetes mellitus [17,33], impaired kidney function [34, 35,36], anemia [33,34] |
Patient Characteristics |
Older age [4,16,17,28,33,37], male gender [4,16,17,28,33,37], and race [17,28,29]. |
Coronary artery disease and hypertension have been identified as the major risk factors for the incidence and accelerated progression of HFrEF [4,28,29]. These two risk factors frequently co-exist in HFrEF patients each conferring an additive effect on the onset or progression of LV remodeling and HFrEF [29]. Coronary artery disease with antecedent myocardial infarction is also a major risk factor for the development of HFrEF [31]. Extra cardiac conditions such as diabetes mellitus, impaired kidney function and anemia may also increase the risk of HFrEF but not as intense as in HF patients with preserved ejection fraction [17,33-37]. While CAD, hypertension and myocardial infarction increase the risk of HFrEF, the effect vary based on age, gender and race [28]. Older age and male gender impose an independent risk on the likelihood of developing HFrEF associated with increased prevalence and burden of CAD in older adults [4,17].
HFrEF shares many risk factors (although at varying degrees) with HF with mid-range ejection fraction (HFmEF) and HF with preserved ejection fraction (HFpEF) but has the most ominous prognosis irrespective of age, gender and etiology [38,39]. Knowledge of HFrEF prognosis for morbidity, disability and mortality is important to guide the most appropriate type and timing of therapy [16]. The ESC HF guidelines list many prognostic factors of mortality and hospitalization in HFrEF patients (Table 5).
Table 5. Prognostic predictors of death and hospitalization in left heart failure
Predictors of an Ominous Prognosis
|
Description of Specific Predictors of an Ominous Prognosis (Death and/or Hospitalization)
|
Patient demographics |
Older age, male sex, low socio-economic status. |
Clinical markers |
NYHA III-IV, limited exercise tolerance, reduced functional state or poor quality of life,
pulmonary congestion, splanchnic congestion, peripheral edema, jugular venous dilation and hepatomegaly,
high resting heart rate and frailty |
Severity of ventricular dysfunction |
Depressed LVEF, LV dilatation, severe diastolic LV dysfunction,
elevated LV filling pressure, mitral regurgitation,
aortic stenosis, LV hypertrophy, left atrial dilatation,
RV dysfunction, pulmonary hypertension, and ventricular dysynchrony, |
Biomarkers (for cardiac damage/dysfunction, neurohormonal activation and/or inflammation) |
Low sodium, high natriuretic peptides, high plasma renin activity,
high aldosterone and catecholamine, high endothelin-1, high adrenomedullin, and high vasopressin |
Cardiovascular co-morbidities |
Atrial fibrillation (AF), ventricular arrhythmia,
non-revascularizable coronary artery disease, previous stroke/ transient ischemic attack,
peripheral artery disease. |
Extra-cardiac co-morbidities |
Diabetes, anemia, iron deficiency, chronic obstructive pulmonary disease,
renal failure, liver dysfunction, sleep apnea,
cognitive impairment, and depression. |
Clinical events |
Non-adherence with treatment, HF-related hospitalization, aborted cardiac arrest and implantable cardioverter defibrillator shocks. |
HF: Heart Failure; LV: Left Ventricular; LVEF: Left Ventricular Ejection Fraction; NYHA: New York Heart Association. Adapted from the 2012 ESC Guidelines for diagnosis and treatment of acute/chronic heart failure [40]
Despite the poor prognosis, advances in pharmacotherapy (beta-blockers, angiotensin-converting enzyme-inhibitors/angiotensin receptor blockers [ACE-I/ARB]) and device therapies (implantable cardioverter-defibrillator [ICD] and cardiac resynchronization therapy [CRT]) have significantly improved prognosis of mortality and hospitalization rates relative to HFpEF [41]. If HFrEF therapies are included in prognostic studies comparing HFrEF, HFmEF and HFpEF, prognosis of HFrEF may be lower because of greater number evidence-based therapies with proven clinical efficacy in the reduction of mortality and hospitalization relative to HFmEF and HFpEF phenotypes [41,42].
Etiology and pathophysiology
Etiology: Overall, etiology of HF is diverse both within and among world regions. There is a lack of a single classification system for the causes of HF, with much overlap between HFrEF, HFmEF and HFpEF phenotypes. Several different pathologies (cardiac and extra-cardiac) could conspire to cause HF, and the identification of such pathologies should form part of the diagnostic work-up of HF phenotypes [16]. The ESC HF guidelines list pathological myocardial injury (coronary artery disease, cardiomyopathies, viral infection and toxins), abnormal loading conditions (arterial hypertension and valvular diseases) and arrhythmias (tachyarrhythmias and bradyarrhythmias) as the principal etiology of HFrEF (Table 6).
Table 6. Principal etiologies of heart failure with reduced ejection fraction
Etiology
|
Conditions
|
Description
|
Effect on Cardiac Function/Structure
|
Myocardial injury |
Coronary artery disease |
Blockages in your coronary arteries that limit blood flow to your heart muscle |
It weakens or damages the myocardium and impairs its ability to eject blood. |
|
Cardiomyopathy |
A progressive myocardial disorder |
Weakens the myocardium impairing contractility and decreasing stroke volume. |
|
Viral myocarditis |
Viral infection of the myocardium |
Causes inflammation in the myocardium affecting its ability to contract and eject blood. |
|
Toxins |
Alcohol, chemotherapy agents and radiation |
Continued exposure may affect the myocardium and impairs its ability to eject blood. |
Abnormal loading conditions |
Arterial hypertension |
Elevates arterial pressure |
Increases cardiac workload to eject blood against increased pressure, which weakens the myocardium |
|
Aortic stenosis |
Narrows the opening of aortic valve and impairs blood flow |
Increases cardiac workload to eject blood through the narrowed valve, weakening the myocardium. |
|
Mitral regurgitation |
Improper closure of the mitral valve, leading to leakage on left side of the heart |
Increases blood volume leading to dilatation and weakened myocardium. |
Arrhythmias |
Tachyarrhythmias, bradyarrhythmias |
Causes irregular heart rhythm |
Irregular rhythm decreases cardiac pumping effectiveness |
Pathophysiology: The functional hallmark of HF is the inability of cardiac performance to meet the metabolic demands of body tissues. Three key factors conspire to impair cardiac performance: (a) increased preload (or volume overload); (b) the loss of intrinsic contractility; and (c) increased afterload (or pressure overload), which cause a reduction in stroke volume [43]. Preload (also known as LV end-diastolic pressure) refers to the degree of ventricular stretch at the end of diastole. It is reflected by blood volume filling the ventricle prior to contraction and thus changes in blood volume causes alterations in both preload and stroke volume. [43,44] Afterload refers to the pressure the ventricles have to work against when ejecting blood generated by pulmonary and systemic circulations. A rise in peripheral vascular resistance increases ventricular stroke work [45]. Contractility refers to ventricular force used to eject blood usually affected by myocardial injury (cardiomyocyte necrosis) [43]. While all the three factors may occur in HFrEF, the loss of contractility is the cardinal pathologic process resulting from ventricular remodeling and neurohormonal changes through the activation of renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system (SNS) [46].
Activation of renin-angiotensin-aldosterone system: Cardiac inability to function effectively as a pump activates the renin-angiotensin-aldosterone system (RAAS). Reduced blood flow due to decreased cardiac output stimulates the kidney to secrete renin, which participates in the conversion of angiotensin I into active hormone angiotensin II angiotensin-converting enzyme (ACE) found in the lungs [43]. Angiotensin II, a potent vasoconstrictive peptide, exert a deleterious effect to cardiac tissues by causing blood vessels to narrow, resulting into elevated blood pressure and cardiomyocyte hypertrophy. Angiotensin II also stimulates the release of aldosterone hormone, which causes sodium and water retention in turn increasing blood volume and cardiac output [43]. Increased blood volume by aldosterone and vasoconstriction by angiotensin II leads to additional cardiomyocyte damage and fibrosis to occur [44]. Another hormone causing water retention and vasoconstriction is arginine vasopressin (an anti-diuretic hormone [ADH]), which is released because of atrial stretching and decreased cardiac output. Atrial overstretching due to increased volume overload increases preload and decreases cardiac output [43]. Vasoconstriction and water retention causes an increase in afterload (pressure overload) leading to increased cardiac workload and consequently an increase in metabolic demands. When compensatory mechanisms of cardiomyocytes cannot keep up with metabolic demands, LV remodeling and hypertrophy occurs, perpetuating the cycle of HF [44].
Activation of sympathetic nervous system: Decreased blood pressure and cardiac output also activates the sympathetic nervous system (SNS). Activation of the SNS stimulates the release of noradrenaline norepinephrine, which in turn triggers beta-receptors to increase the heart rate, strength and rapidity of contractions [45]. Increased heart rate, the LV begins to hypertrophy and collagen depositions occur causing pathologic LV remodeling [43]. Left ventricular remodeling conveys a substantial negative effect on cardiac contractility. Mechanical stimulation of the myocardium is the key process leading to ventricular hypertrophy. Myocardial fibrosis on the other hand, may be the result of the activation of neurohormonal compensatory mechanisms such as cytokines and hormones [46]. Overtime, compensatory mechanisms can turn toxic to the cardiomyocytes and render them unable to response to the body’s attempt to compensate [43]. The activation of the RAAS and the SNS neurohormonal systems are linked to the development of HF symptoms and poor prognosis over time leading to diminished quality of life, decreased functional capacity, re-hospitalization, and premature death secondary to pump failure or ventricular arrhythmias. As such, the interruption of RAAS and SNS neurohormonal response is the basis of much of the current treatment of HFrEF [43,46].
Left ventricular remodeling: Whereas neuro-hormonal activation contributes to depressed LV function (LVEF), the key pathophysiologic mechanism of depressed LVEF is ventricular remodeling, referring to a process in which mechanical, neurohormonal and genetic factors regulate the size, shape and function of the ventricles. Ventricular remodeling is a normal physiological and adaptive process but may also be pathological, occurring in the setting of cardiac conditions such as acute myocardial infarction, hypertension, cardiomyopathy and valvular heart disease [46]. During ischemia or post-infarction, three major cell death modalities – apoptosis, necrosis and autophagy – occur in cardiomyocytes [47]. Upon increased cardiomyocyte death, inflammatory reaction occurs, which is a prerequisite for healing and scar formation. However, chronic inflammation can become pathologic. In the later stages of HF, increased loading conditions and chronic inflammation may induce compensatory changes such as LV hypertrophy and dilatation as adaptive processes to LV remodeling to offset increasing load, attenuate progressive LV dilatation and stabilize LV contractility [46,48].
Damaged myocardium produces reactive oxygen species (ROS) causing stress, inflammation and cardiomyocyte necrosis and leading to the release of pro-inflammatory cytokines interleukin-1 (IL-1) and tumor necrosis factor - alpha (TNF-α) [49]. These molecules exacerbate LV failure by increasing leukocyte attraction, inflammatory response and endothelial destruction. In turn endothelial destruction leads to the reduction of vasodilator nitric oxide causing vasoconstriction [48]. Vasoconstriction continues to stimulate neurohormonal and inflammatory response proliferating the cycle of myocardial injury [44]. If left untreated, LV remodeling progressively worsens over time characterized with increasing LV dilatation and declining LVEF [13,41]. In addition to ventricular remodeling, limited cardiac reserve in HFrEF patients depends on several other mechanisms including atrial contraction, synchronized LV/RV contraction and ventricular interdependence. Events that effect any of these mechanisms such as atrial fibrillation, conduction abnormalities or additional hemodynamic load could lead to acute decompensation [46].
Clinical presentation and diagnosis
Clinical presentation: Patients with HF usually present with the classic triad of symptoms – edema, fatigue, and dyspnea. Other typical symptoms may include orthopnea, paroxysmal nocturnal dyspnea, reduced exercise tolerance, and increased time to recover from exercise. Less typical signs include nocturnal cough, wheezing, bloated feeling, loss of appetite, confusion (especially the elderly), depression, palpitations, dizziness, syncope and bendopnea [12,16]. However, symptoms are non-specific and non-sensitive, and therefore are less useful in discriminating HFrEF from HFmEF or HFpEF. In addition, atypical presentation should be considered when evaluating obese patients and older adults because of potentially different etiology, clinical presentation and outcome compared to the general population [50-53]. Typical signs of HFrEF include elevated-jugular venous pressure, gallop rhythm, hepatojugular reflux, and laterally displaced apical impulse. Less typical symptoms include weight gain, cachexia (tissue wasting), cardiac murmur, peripheral edema (ankle, sacral, scrotal), pulmonary rales, tachycardia, irregular pulse, tachypnea, hepatomegaly, ascites, cold extremities, oliguria and narrow pulse pressure [16]. The assessment of signs and symptoms is clinically significant to suggest the likelihood of HFrEF as well as to monitor response to therapy and stability overtime. Persistent symptoms despite treatment often suggest the need for additional therapy and worsening of symptoms often suggest serious development and the need for prompt medical attention [16].
Diagnosis
Diagnosis work-up: Diagnosis of HFrEF is made when symptoms and physical signs of congestion and decreased tissue perfusion are documented in the setting of systolic and/or diastolic dysfunction. The 2016 ESC, the 2013 AHA and the 2012 Canadian HF guidelines recommend diagnostic work-up for the assessment of HFrEF should follow the general heart failure algorithm but distinguished using echocardiography-defined LVEF < 40%. The diagnostic work-up include assessment of clinical history and physical examination, laboratory examination, and echocardiography assessment of LV structure and function. If echocardiography is inconclusive, additional tests imaging tests such as radionuclide, cardiac catheterization, magnetic resonance imaging, computed tomography (CT), scan and endomyocardial biopsy, and cardiopulmonary exercise testing may be considered to confirm diagnosis (Figure 1).
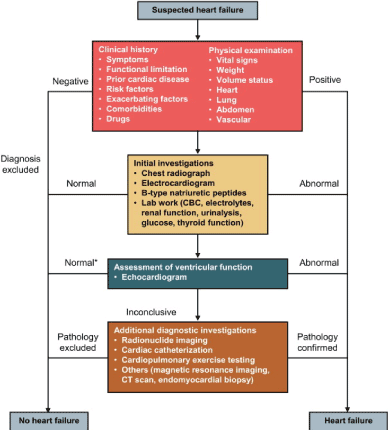
Figure 1. Algorithm for diagnosis of heart failure
Diagnosis of HFrEF begins with the assessment of clinical history and physical examination to determine risk factors, and signs and symptoms. If all the findings are normal HFrEF is excluded otherwise clinical investigations (chest radiography, ECG and/or natriuretic peptide assessment) should be considered. If one of the findings is abnormal, echocardiography should be done to confirm diagnosis. If the findings remain unclear or inconclusive, additional diagnostic tests should be considered such as radionuclide imaging, cardiac catheterization, cardiopulmonary exercise, magnetic resonance imaging, CT scan or endomyocardial biopsy) to confirm diagnosis
History/physical examination: The 2013 ACCF/AHA HF guidelines recommends the initial step in the diagnosis of HFrEF is the assessment of the history and physical examination to determine typical HF signs and symptoms, and diseases that can cause HF or contribute to its progression. [17]. Table 7 summarizes medical history and physical examination and their suggestive diagnostic clues. Clinical history should include assessment of symptoms, functional limitation, prior cardiac diseases, presence of risk factors, exacerbating factors, comorbidities, and previous or current drugs. Physical examination should include assessment of vital signs, weight, volume status, heart, lung, abdomen and vascular [12].
Table 7. Medical history and physical examination and their suggestive diagnostic clues
Assessment
|
Signs/Symptoms
|
Suggestive Diagnostic Clues
|
Detailed history |
Clues suggesting HF etiology |
Genetic etiology as familial cardiomyopathy. |
|
Duration of illness or symptoms |
Recent onset of HF |
|
Anorexia, early satiety |
Gastrointestinal abnormalities |
|
Rapid weight gain |
Volume overload |
|
Palpitations, syncope |
Paroxysmal AF or ventricular tachycardia. |
|
Transient ischemic attack |
Thromboembolism |
|
Peripheral edema/ascites |
Volume overload |
|
Disordered nocturnal breathing or sleep problems |
Pulmonary hypertension due to sleep apnea |
Physical Examination |
Body mass index/weight loss |
Obesity a contributory factor cachexia suggests poor prognosis |
|
Blood pressure in supine/upright |
Hypertension or hypotension |
|
Jugular pressure at rest |
Congestion |
|
Third heart sound and murmurs |
Valvular disease and adverse prognosis |
|
Hepatomegaly/ascites |
Markers of volume overload |
|
Peripheral edema |
Peripheral rather than cardiac causes |
|
Cold extremities |
Inadequate cardiac output |
Adapted from 2012 ACCF/AHA Heart Failure Guidelines
Laboratory tests: If patient history and physical examination suggests abnormal signs and symptoms related to HF, 12-lead electrocardiogram (ECG) should be performed. Although ECG increases the diagnostic likelihood for HFrEF, it has very low specificity [37,50,54,55]. Usually, ECG is used to determine abnormalities in heart rhythm, heart rate, QRS duration and morphology [12]. Abnormal ECG increases the likelihood of HFrEF diagnosis or provide information on possible etiologies such as myocardial infarction as well as indication for therapy such as anticoagulation for atrial fibrillation and pacing for bradycardia. Completely normal ECG has a high sensitivity and useful to rule out HFrEF [16].
The assessment of the levels of serum natriuretic peptides (NPs) is recommended in non-acute settings especially when echocardiography is not immediately available. Normal serum NPs levels indicates a very low likelihood of HFrEF (Table 8).
Table 8. Threshold for natriuretic peptides for exclusion of HFrEF diagnosis
Serum Natriuretic Peptides
|
Non-Acute Setting
|
Acute Setting
|
B-type natriuretic peptide (BNP) |
< 35 pg/mL |
< 100 pg/mL |
N-terminal pro-BNP |
< 125 pg/mL, |
< 300 pg/mL |
Mid-regional pro A-type natriuretic peptide (MR-proANP) |
-- |
< 120 pmol/L |
Threshold values (upper limit for normal serum NPs) are lower in non-acute setting relative to acute setting. The threshold values have low positive predictive values in non-acute and acute settings (44% to 67%) but with high negative predictive values (94% to 98%) [56-59]. Thus, the use of serum NPs is recommended for the exclusion of HFrEF rather than to establish diagnosis [12,16]. Other potential cardiac and extras cardiac causes of elevated NPs such as atrial fibrillation, obesity, age and renal failure should be considered during interpretation. In obese patients, NPs maybe disproportionally low [60].
Echocardiography: Echocardiography should be considered in all suspected HFrEF patients with abnormal signs and symptoms, and/or abnormal ECG and elevated levels of serum NPs [16,17]. Echocardiograph is useful for the assessment of cardiac structure and function to quantify LV function for establishing diagnosis, prognostic stratification and planning for treatment [12]. It provides immediate information on cardiac chamber volumes, ventricular systolic function, LV wall thickness, valve function and pulmonary hypertension [61-67]. The LVEF cut off values of 40% or less confirms the diagnosis of HFrEF. Other abnormalities include LV hypertrophy and dilatation [16].
Other Tests: If echocardiography evaluation is inconclusive or uncertain such as suboptimal images or an unusual cause of HFrEF is suspected, additional tests should be considered to confirm diagnosis. The 2016 ESC recommends cardiac magnetic resonance (CMR) imaging for assessing myocardial structure and function in patients with poor acoustic window and complex congenital heart diseases and in cases of suspected myocarditis, amyloidosis, myocarditis, and sarcoidosis. In addition, CMR with late gadolinium enhancement may be considered in patients with dilated cardiomyopathy to distinguish ischemic and non-ischemic myocardial injury. Invasive coronary angiography is recommended in patients with a history of symptomatic ventricular arrhythmia or aborted cardiac arrest. It may also be considered in patients with HF and intermediate-to-high probability of CAD, or angina pectoris deemed candidates for coronary revascularization. Cardiac CT may be considered in HF patients with intermediate to high probability of CAD or with equivocal non-invasive stress tests to exclude coronary stenosis. Chest radiography (X-ray) is recommended in HF patients to determine or exclude alternative pulmonary or other diseases contributing to dyspnea or identify pulmonary congestion/edema more helpful in suspected HF patients in the acute setting. Non-invasive imaging re-assessment of myocardial structure and function should be considered in patients with worsening symptoms, patients receiving maximal dose of pharmacotherapy as a bridge before the decision on device implantation and to patients exposed to therapies with the potential to injure the myocardium [16].
Meta-Analysis of diagnosis methods: The mainstay of the diagnosis of HFrEF is the assessment of LV systolic dysfunction defined using LVEF < 40% [12,16,17]. The conventional two-dimensional echocardiography (2DE) is the most widely used imaging technique for assessing LV size and dysfunction in clinical practice because of its wide availability, portability, inexpensive, radiation free and timely [17]. However, concerns have been raised on its reliability, reproducibility and accuracy in the measurement of LVEF and volumes due to errors of foreshortening, narrow echocardiograph windows, poor endocardial definition and assumptions on LV shape [68]. On the other hand, because of the ability of the CMR imaging modality to visualize the entire heart in multiple planes and has excellent endocardial definition, it is considered the gold standard for assessing LV volumes and ejection fraction, but due to limited availability and great expense, it is impractical for widespread use [69,70]. Some echocardiography laboratories have begun using three-dimensional echocardiography (3DE) to assess LV volumes and ejection fraction. However, it is not clear whether 3DE provides superior diagnostic accuracy compared to 2DE in the assessment of LV volumes and ejection fraction. The present meta-analysis seeks to combine current real-time (or live) 3DE published literature to evaluate its diagnostic performance relative to CNR and its utility relative to the conventional 2DE in the assessment of LVEF and end diastolic volumes in HFrEF patients.
Search strategy and inclusion criteria: Published studies evaluating 2DE, real-time 3DE (RT3DE) and CMR on the assessment of LV structure and function were searched in PubMed, EMBASE, Clinical Trials, the Cochrane Central Register of Controlled, National Institutes of Health ClinicalTrials.gov, and UpToDate Online. Supplementary checks were conducted on citations of all studies that met the inclusion criteria and relevant review articles. Grey literature, databases and conference proceedings were also searched to obtain a comprehensive list of included studies. Variations and combinations of the following key search terms were used: two-dimensional echocardiography, real-time/live three-dimensional echocardiography, cardiac magnetic resonance imaging and ventricular systolic function, ejection fraction, end diastolic volume. The inclusion criteria were, the studies (a) included patients with reduced ejection fraction; (b) compared RT3DE, 2DE and/or CMR for the assessment of LV function; (b) provided correlation between RT3DE and 2DE with CMR on estimates of LVEF and LV end-diastolic volumes, and Bland-Altman values. The exclusion criteria were studies including animal models, did not use CMR as a reference standard, and studies with only abstracts. There was no restriction on publication year or language. Two reviewers independently assessed qualifying studies against the inclusion criteria and any disagreements resolved through consensus. Bothe reviewers independently abstracted data relevant to the study topic and did quality assessment using the Quality Assessment of Diagnostic Accuracy of Studies (QUADAS) criteria. The abstracted data included first author, patient sample and mean age and clinical outcomes (Bland-Altman values, and correlation of RT3DE and 2DE with CMR on LV end-diastolic volume (LVEDV) and ejection fraction (EF) (Tables 9 and 10).
Table 9. Summary of patient characteristics in included studies
|
Jenkins et al. [71] |
Gutiérrez-Chico et al [72] |
Jacobs et al. [73] |
Jenkins et al. [74] |
Jenkins et al. [75]19 |
Bicudo et al. [76] |
Chukwu et al. [77] |
Marsan et al. [78] |
Year |
2004 |
2005 |
2005 |
2006 |
2007 |
2008 |
2008 |
2011 |
Sample |
50 |
35 |
50 |
30 |
110 |
20 |
69 |
52 |
Age |
64(8) |
61(17) |
58(19) |
66(7) |
63(11) |
32(15) |
66(5) |
62(10) |
Table 10. Summary of correlation and band altman in included studies
1st Author
|
RT3D Echocardiography
|
2D Echocardiography
|
[Ref #]
|
r LVEDV
|
r LVEF
|
BA LVEDV
|
BA LVEF
|
r LVEDV
|
r LVEF
|
BA LVEDV
|
BA LVEF
|
Jenkins et al. [71] |
0.98 |
0.92 |
-4(29) |
0(7) |
0.92 |
0.66 |
-54(33) |
-1(13) |
Gutiérrez-Chico et al [72] |
0.99 |
0.98 |
-13(34) |
NR |
0.78 |
0.92 |
-25(13) |
-21(11) |
Jacobs et al. [73] |
0.96 |
0.93 |
-14(34) |
-1(13) |
0.89 |
0.86 |
-23(58) |
0.8(19) |
Jenkins et al. [74] |
0.86 |
0.81 |
-15(28) |
-1(8) |
0.68 |
0.70 |
-70(39) |
-3(11) |
Jenkins et al. [75] |
0.89 |
0.92 |
-15(31) |
-1(7) |
0.72 |
0.65 |
-57(40) |
-5(12) |
Bicudo et al. [76] |
0.94 |
0.83 |
-4(16) |
-2(11) |
0.91 |
0.73 |
1(23) |
-1(14) |
Chukwu et al. [77] |
0.99 |
0.79 |
-1(10) |
2(8) |
0.50 |
0.23 |
-38(51) |
9(16) |
Marsan et al. [78] |
0.97 |
0.97 |
-22(40) |
1(5) |
0.94 |
0.96 |
-41(49) |
1(5) |
2D: Two-dimensional; BA: Bland-Altman; LVEDV: Left Ventricular End-Diastolic Volume; LVEF: Left Ventricular Ejection Fraction; r: Correlation; RT3DE: Real-time three-dimensional echocardiography.
Study characteristics and outcomes: The online search and screening of bibliographies yielded 102 potential studies. After exclusion based on title and keywords, and screening against the inclusion/exclusion criteria, 94 studies were excluded because they contained abstracts only, did not provide Bland-Altman values, evaluated 2DE/RT3DE strain, did not include CMR, reported LV mass only, included children or reported right ventricular function and/or mass. Ultimately, eight (8) studies [71-78] were include comprising of 416 patients with reduced LV ejection fraction (mean age = 59 years; range – 32 to 66) with a majority being male (71%). Reasons for referral to cardiac imaging included various cardiac condition including a history of myocardial infarction, coronary artery disease (CAD), dilated cardiomyopathy (DCM), ischemic/non-ischemic cardiomyopathy, congenital heart diseases and valvular disease, which suggest LV systolic dysfunction. Analysis of RT3DE required < 2 minutes per patient. Considering cardiac magnetic resonance (CMR) imaging as the reference standard for the assessment of LV systolic function (LVEDV and LVEF), RT3DE showed an excellent correlation with CMR for LVEDV (0.95) and LVEF (0.89) with slight underestimation of volumes (-11) and ejection fraction (-0.29%). On the other hand, 2DE had a relatively lower correlation with CMR for LVEDV (0.79) and LVEF (0.71) and with higher underestimation of volumes (-38.38) and ejection fraction (-2.53). The findings reveal that RT3DE is suitable for clinical use because it demonstrates superior performance (accuracy and precision) in the assessment of LVEDV and LVEF compared to 2DE.
Discussion of outcomes: Accurate quantification of LV volumes and ejection fraction is of critical importance to the practice of cardiology. Measures of LVEDV and LVEF are useful to inform prognosis in most patients with cardiac diseases, determine treatment decisions for a variety of therapies and provides eligibility criteria in clinical trials [79,79-81]. Current clinical guidelines, the Canadian Cardiovascular Society [12], ESC [16], and the ACCF/AHA [17] recommend the basis of a variety of therapeutic decision should be volumetric and LEVF measures in various patient cohorts including HF, valvular disease and cardiomyopathies. Although the most ubiquitous imaging technique in clinical practice to assess LVEDV and LVEF is the 2DE, its accuracy and reproducibility have significant limitations due to foreshortening errors and reliance on geometric models that may less accurate in diseased hearts [82-85]. Whereas CMR is the gold standard for assessing LVEDV and LVEF, high cost and limited availability problematizes its use in routine clinical practice [69,60]. Efforts to improve diagnosis and prognosis in HF patients has motivated research interest into the validity and benefits of newer cardiac imaging modalities, including 3DE, whose utility in assessing LV structure and function has already been embraced by some echocardiography clinics. The present meta-analysis sought to compare the accuracy of RT3DE compared to CMR in assessing LV volumes and ejection fraction.
The present analyasis finds RT3DE has a stronger correlation to CMR in assessing both LV volumes and ejection fraction compared to 2DE. While correlation analysis indicate a strong relationship, Bland-Altman analysis reveals RT3DE has a lower underestimation of volumes and better agreement with CMR compared to 2DE. Two studies [71,73] also compared the reproducibility of 3DE with 2DE based on intra-observed and inter-observer agreement or variability. For all parameters (LVEDV and EF), Jenkins et al. [71] reported higher inter-observer agreement with 3DE (LEVDV: r = 0.95; LEVF: r = 0.88) compared to 2DE (LEVDV: r = 0.76; LEVF: r = 0.61) and intra-observer agreement with RT3DE (LEVDV: r = 0.98; LEVF: r = 0.97) compared to 2DE (LEVDV: r = 0.90; LEVF: r = 0.61). Jacobs et al. [73] reported lower intra-observer variability with RT3DE vs. 2DE (LVEDV: 7.9% vs. 23%) and lower inter-observer variability (11% vs. 26%), with 3DE having an excellent correlation with CMR values (r = 094 and 0.98) [73]. The findings indicate RT3DE has superior reproducibility (lower re-test variability) compared to 2DE in the assessment of LV volumes and ejection fraction. Higher observer agreement is particularly important in clinical practice with a variety of reader and sonographers interpreting imaging results for patients undergoing serial examination to determine disease or therapeutic progress.
The superiority of 3DE to 2DE in the assessment and reproducibility of LV volumes and ejection fraction has been reported in previous clinical trials and review articles [86-89]. The superiority is associated with the RT3DE modality using recently developed matrix array echocardiographic probes to visualize the entire heart in ≤ 8 beats per minute providing volumes with minimal post-processing [90]. In the U.S., some echocardiography clinical have already embraced the RT3DE modality for routine clinical practice [91]. However, if RT3DE is not fully automated, it will remain time-consuming and resource-intensive with sub-optimal intra-observer and inter-observer agreement, which may undermine its implementation in routine clinical practice. There is need for additional developments in both hardware and software including fully automated knowledge-based algorithm for assessing and quantifying ventricular volumes and ejection fraction [88]. In the present medical era, where new imaging technology may escalate medical costs, RT3DE require thorough evaluation prior to any recommendations to widespread use.
Clinical management
Pharmacotherapy: Pharmacologic therapy remains the cornerstone of clinical management of HFrEF [16,17,92,93]. Diagnostic algorithm for pharmacologic support includes the main validated HF medication – angiotensin-converting enzyme-inhibitors (ACE-I)/Angiotensin Receptor Blockers (ARBs), beta-blockers, mineralocorticoid receptor antagonists (MRA), digoxin, ivabradine, arterial vasodilator (Figure 2).
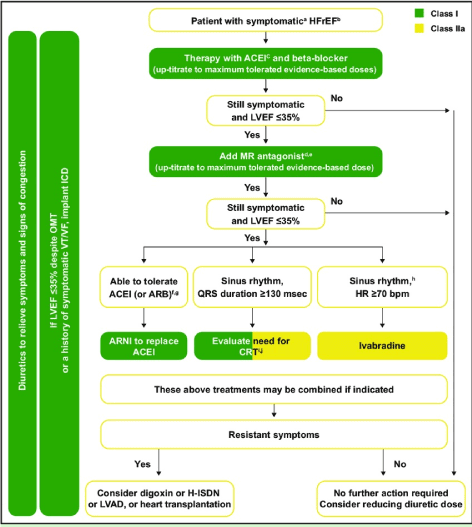
Figure 2. 2016 ESC recommended pharmacologic management for symptomatic HFrEF
Frontline medical therapy for HFrEF are ACE-I and beta-blocker, and diuretis for symptom relief. If still symptomatic and LVEF ≤35%, MR antagonist should be added. If still symptomatic and LVEF ≤35%, ARNI should replace ACE-I, Ivabradine or cardiac resynchronization therapy should be considered. If still asymptomatic, digoxin, LV assist device or heart transplantation should be considered [16]
Angiotensin-converting enzyme inhibitors: The efficacy of ACE-I in reducing mortality in HFrEF patients and in patients with myocardial infarction complicated by reduced ejection fraction [94-98]. ACE-I are the initial indications for HFrEF unless contraindicated or not tolerated in asymptomatic patient. For optimal outcomes, ACE-I should be titrated up to the maximum tolerated doses to achieve adequate inhibition of the RAAS system. Sub-optimal doses reported in a majority of patients in clinical practice may not achieve the maximal medication benefit [99]. ACE-I are also indicated in patients with asymptomatic LV systolic dysfunction to reduce the risk of developing HF or HF-related hospitalization or mortality [16]. In patients contraindicated to ACE-I, ARBs should be considered. ARBs have been demonstrated to be non-inferior to ACE-I in patients with HFrEF or with MI patients complicated by reduced ejection fraction [99-101].
Beta-blockers: Beta-blockers such as bisoprolol, metoprolol and carvedilol have been demonstrated to reduce mortality and morbidity in symptomatic HFrEF patients despite treatment with ACE-I and/or diuretic [102-105]. Beta-blockers are also recommended in patients with a history of MI and asymptomatic LV systolic dysfunction. There is increasing consensus that beta-blockers may complement ACE-I, and thus, a dual therapy of beta-blocker is frequently used as the initial pharmacotherapy in HFrEF patients [12,16]. Initiation of beta-blockers in stable patients should begin at a low dose and gradually up=titrated to the maximum tolerated doses. In patients admitted with acute HF, beta-blockers should be cautiously initiated in hospital setting after stabilizing the patient [12].
Mineralocorticoid receptor antagonists: Mineralocorticoid receptor antagonists (MRAs) such as spironolactone and eplerinone are recommended in all symptomatic HFrEF patients despite the use of ACE-I and beta-blockers to reduce mortality and HF hospitalization [106,107]. However, caution should be observed when MRAs are given to patients with renal dysfunction and those with elevated serum potassium levels (> 5.0 mmol/L) with regular assessment of serum potassium concentration and renal function [16].
Diuretics: Diuretics are indicated in HFrEF patients to reduce signs and symptoms for congestion. Although their effect on mortality and morbidity have not been conclusively investigated, a meta-analysis reports loop and thiazide diuretics in chronic HF patients reduces the risk of death and worsening [108] and improves exercise function [108,109]. Loop diuretics produce a more intense and shorter diuresis effect than thiazides. Loop diuretics and thiazides act synergistically and their combination may be considered in the treatment of edema but with caution of frequent adverse effects. Diuretics dosage should be adjusted according to individual needs over time. The use of diuretics may be temporarily discontinued in euvolemic or hypovolemic patients [17].
If channel antagonist: Ivabradine, an If channel antagonist slows heart rate and indicated for HFrEF patients in sinus rhythm and a resting heart rate of ≥ 70 beats per minute who have a contra-indication or intolerant to beta-blocker. Ivabradine should be used together with ACE-I/ARBs or MRA/ARB. Ivabradine has been shown to reduce composite clinical endpoint of mortality and re-hospitalization in symptomatic HFrEF patients with a heart rate of ≥ 70 beats per minute [16].
Digoxin: Digoxin may be considered in symptomatic HFrEF patients in sinus rhythm despite treatment with ACE-I/ARBs, beta-blocker and MRAs. The aim of using digoxin is to reduce the risk of hospitalization [110].
Non-pharmacological therapy: In HFrEF patients intolerant or non-responsive to pharmacotherapy, device therapy using OCD or CRT should be considered to prevent sudden cardiac death [12,16].
Implantable cardioverter-defibrillator: Device therapy using implantable cardioverter-defibrillator (ICD) is recommended for secondary prevention of sudden cardiac death and all-cause mortality. It is indicated in HFrEF patients who have recovered from ventricular arrhythmias causing hemodynamic instability and expected to survive for more than one year. ICD is also recommended for the primary prevention of sudden death and all-cause mortality in symptomatic HFrEF patients, NYHA functional class II-III despite over three months of optimal medical therapy and expected to survive more than one year and have ischemic heart disease or dilated cardiomyopathy. However, ICD is not recommended for HFrEF patients in NYHA functional class IV with severe symptoms refractory to optimal medical therapy [16].
Cardiac resynchronization therapy: Cardiac resynchronization therapy (CRT) is another validated device therapy for HFrEF. It is recommended for symptomatic HFrEF patients in sinus rhythm with QRS duration ≥ 150 msec and with or without LBBB QRS morphology despite optima medical therapy to improve symptoms and reduce mortality and morbidity. CRT is also indicated with HFrEF patients who have received conventional pacemaker or ICD and develop worsening symptoms despite optimal medical therapy, and have a high proportion of RV pacing considered for upgrade to CRT. However, CRT is contra-indicated in patients with QRS < 130 msec [16].
Lifestyle modification: Effective clinical management of HFrEF should consider all available patient and clinical factors [16,17,92]. It should include both clinical management strategies (pharmacotherapy and non-pharmacotherapy) and non-clinical strategies (self-management and lifestyle modification). Non-clinical strategies require patient and family education on the disease to improve self-management. Lifestyle changes is an import part of self-management, which should include smoking cessation, decreased alcohol intake, and increase exercise [92]. Diet modification including low sodium and fluid intake, and daily monitoring of weight [45].
Meta-analysis of clinical management: Measurement of serum natriuretic peptides (BNP and NTpro-BNP) have an established role in the diagnosis of HFrEF. Although they are non-specific for the diagnosis of HFrEF, they have high sensitivity (excellent negative predictive values) for the exclusion of HFrEF diagnosis as well as in the appropriate selection of patients for echocardiography assessment of LV function [56-59]. They also provide valuable prognostic information for HFrEF progression and therapeutic response, HFrEF patients with reducing NPs levels may suggest better prognosis and vice-versa [57,58]. Despite the diagnostic and prognostic value of serum NPs, several published clinical trials investigating changes in serum concentration of NPs in guiding treatment in HFrEF patients have not established conclusively its clinical benefits. The present systematic review and meta-analysis sought to aggregate current research evidence to determine the overall effect of BNP-guided therapy in HFrEF patients on mortality and hospitalization.
Search strategy and inclusion criteria: The search for relevant published studies was conducted in PubMed, EMBASE, the Cochrane Controlled Clinical Trials Register Database, and the ClinicalTrials.gov. Variations and combination of the key terms used in the search were B-type natriuretic peptide, N-terminal pro-BNP, heart failure, and therapy. Complementary manual search of citations of the retrieved studies were screened to obtain a comprehensive list of included studies. Studies were included if they satisfied the following criteria: (a) enrolled patients with HFrEF; (b) randomized patients based on a strategy of titrating medication based on the concertation of serum NPs compared to a control group; and (c) reported all-cause mortality. There was no restriction on the publication language and year. Two reviewed independently reviewed qualifying studies against the inclusion criteria using a hierarchical strategy: title, abstract and full-text screening. Both reviewers also abstracted data relevant data from the included studies and resolved any discrepancy by consensus. The included studies were assessed for patient characteristics, usual care and BNP-guide groups, clinical outcomes (all-cause mortality and survival free of hospitalization (Table 11).
Table 11. Summary of studies on natriuretic peptide-guided therapy
Author |
Study Year |
Patient Size |
NP-Guided |
Control |
NP |
Follow-up |
All-cause Mortality |
Hospitalization |
|
|
NP |
C |
Age (yrs.) |
Male (%) |
Age (yrs.) |
Male (%) |
|
|
NP |
C |
NP |
C |
Felker et al. [9] |
2017 |
446 |
448 |
62 |
69 |
64 |
67 |
NT-proBNP |
15 |
12 |
13 |
33 |
32 |
Beck‐da‐Silva et al. [111] |
2005 |
21 |
20 |
64.5 |
33 |
65.5 |
35 |
BNP |
3 |
9.5 |
30 |
4.7 |
10 |
Jourdain et al. [112] |
2007 |
110 |
110 |
65 |
59 |
66 |
56 |
BNP |
15 |
2.7 |
8.2 |
20 |
44 |
Pfisterer et al. [113] |
2009 |
251 |
248 |
76 |
68 |
77 |
63 |
NT-proBNP |
18 |
8 |
9 |
59 |
60 |
Eurlings et al. [114] |
2010 |
174 |
171 |
72 |
55 |
73 |
31 |
NT-proBNP |
24 |
25 |
33 |
NR |
NR |
Berger et al. [115] |
2010 |
92 |
20 |
70 |
40 |
71 |
30 |
BNP |
12 |
28 |
40 |
22 |
39 |
Persson et al. [116] |
2010 |
126 |
124 |
78 |
76 |
77 |
66 |
NT-proBNP |
NR |
3 |
4 |
NR |
NR |
BNP: B-Type Natriuretic Peptide; NT-proBNP: N-terminal pro-BNP
Study characteristics and outcomes: Seven (7) studies meeting the inclusion criteria were included in this meta-analysis [9,111-116]. The studies compared NP-guided therapy with usual care or symptom guided therapy. The seven studies recruited a combined population of 2,261 patient undergoing HF therapy (medical and/or device therapy) constituting 1,120 in the NP-guided therapy group with (mean age = 70 years; make 57%) and 1,141 in the control group (mean age = 71 years; male 50%). Two natriuretic peptides (NP) used to guide therapy were BNP [11,112,115] and NTpro-BNP [9,113,114,116]. Therapy guided by NPs were medication (beta-blocker, ACE-I/ARB, neprilysin inhibitor, mineralocorticoid antagonist) and device therapies (implantable cardioverter-defibrillator [ICD] and cardiac resynchronization therapy [CRT]). Patients were followed for a mean of 14.5 months. NP-guided therapy has better clinical outcomes in reducing hospitalization (27.7%) and HF related mortality (12.6%) when compared to usual care, hospitalization (37%) and HF-related mortality (19.6%). The findings reveal NP-guided therapy has superior effect in protecting against HF-mortality and hospitalization compared to usual care.
Discussion of outcomes: Despite therapeutic advances, approximately 30% of HFrEF patients get re-admitted within two to three months after HF hospitalization and about 10% die within this period [117]. Attributable to such poor discharge outcomes may be sub-optimal care linked to poor treatment compliance and inadequate markers for follow-up [118]. Biomarkers play a significant role in medical practice and in cardiovascular care in particular. Natriuretic peptides have been demonstrated as powerful diagnostic and prognostic tools. However, the extent to which NPS could be used to guide titration of medical therapy for HFrEF patients remains uncertain. The primary finding in the present meta-analysis reveals utility of serial assessment of NPs to aid of titration of medical therapy conveys a superior effect compared to the usual HF care or symptom-guided care in the reduction of HF-related hospitalization and mortality in HF patients with reduced ejection fraction (<40%).
Besides the reduction of HF mortality and hospitalization, individual studies provide additional important findings. Beck‐da‐Silva et al. [111] report that BNP-guided therapy significantly improves LVEF by 8.1±10.7% and a trend towards improvement of quality of life scores and NYHA functional class. The study also reported the effect on age on BNP-guided therapy, where patients aged 60-74 years benefited with hospitalization-free survival whereas patients aged ≥ 70 years did not. Jourdain et al. [112] report BNP-guided therapy might confer better clinical outcomes because of frequent changes of all types of medication used, and higher dosage of beta-blockers compared to usual care.
The beneficial outcomes of NP-guided therapy in protecting against HF-hospitalization and mortality in HFrEF patients have been demonstrated elsewhere. In an earlier meta-analysis, Felker et al. [120] finds NP-guided heart failure therapy, incorporating serial measurements of BNP and/or NT-proBNP significantly reduces all-cause mortality compared to usual care patients. In another earlier meta-analysis, Porapakkham et al. [121] supports the findings that NP-guided therapy reduces all-cause and cardiovascular-related mortality with more noticeable effect in HF patients aged < 75 years. However, the effect of NP-guided therapy on the reduction of all-cause hospitalization and hospitalization-free survival was not significant [121]. The inconsistent findings suggests the need for large-scale clinical trials with long-term follow-up to establish the value of NP-guided therapy in reducing HF-related hospitalization and mortality in HFrEF patients.
Heart failure (HF) with reduced ejection fraction (HFrEF) is the originally described HF phenotype defined by left ventricular ejection fraction (LVEF) < 40% on echocardiography. It accounts for approximately half of all reported hospitalized HF cases. Its main etiology is cardiac and extra cardiac conditions that lead to myocardial injury, abnormal loading conditions and pathologic ventricular arrhythmias. The key pathophysiologic perturbations of HFrEF include activation of neurohormonal systems (sympathetic nervous system and renin-angiotensin-aldosterone system), which may precipitate or aggravate cardiomyocyte necrosis leading to myocardial injury and remodeling, and ultimately impairing left ventricular systolic function. Typical clinical symptoms and signs include edema, fatigue, dyspnea, reduced exercise tolerance and orthopnea elevated-jugular venous pressure, gallop rhythm, hepatojugular reflux, and a laterally displaced apical impulse. Symptoms are non-specific and non-sensitive, and obese and elderly patients may present with atypical symptoms. Diagnosis work-up begins with the assessment of clinical history and physical examination followed by laboratory tests (electrocardiography assessment and evaluation of the concentration of serum natriuretic peptides). Patients with at least one abnormal finding in physical examination or laboratory assessment should be considered for echocardiography to assess LV systolic function. In case of inconclusive echocardiography findings, further imaging tests such as cardiac magnetic resonance or endomyocardial biopsy should be considered to confirm diagnosis. The mainstay method of clinical management is pharmacotherapy; however, if intolerant or non-responsive, device therapy (implantable cardioverter-defibrillator or cardiac resynchronization therapy) should be considered. Lifestyle modification therapy – increased exercise, smoking cessation, minimal alcohol intake, and low dietary intake of sodium and fluids – is a recommended complementary therapy to slow down the progression of HFrEF.
- Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, et al. (2014) The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63: 1123-1133. [Crossref]
- Vadlamani L, Anderson K, Kumar S (2016) Using technology to reduce readmission rates for congestive heart failure in high risk patients. J Am Coll Cardiol 67: 1410. [Crossref]
- Reynolds K, Butler MG, Kimes TM, Rosales AG, Chan W, et al. (2015) Relation of acute heart failure hospital length of stay to subsequent readmission and all-cause mortality. Am J Cardiol 116: 400-405. [Crossref]
- Tiller D, Russ M, Greiser KH, Nuding S, Ebelt H, et al. (2013) Prevalence of symptomatic heart failure with reduced and with normal ejection fraction in an elderly general population–the CARLA study. PLoS One 8: e59225. [Crossref]
- Crişan S, Petrescu L, Lazăr MA, Văcărescu C, Nicola AR, et a;. (2018) Reduced ejection fraction heart failure–new data from multicenter studies and national registries regarding general and elderly populations: hopes and disappointments. Clin Interv Aging 13: 651-656. [Crossref]
- Abraham WT, Zile MR, Weaver FA, Butter C, Ducharme A, et al. (2015) Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. JACC Heart Fail 3: 487-496. [Crossref]
- Lewis GD, Semigran MJ, Givertz MM, Malhotra R, Anstrom KJ, et al. (2016) Oral iron therapy for heart failure with reduced ejection fraction: design and rationale for oral iron repletion effects on oxygen uptake in heart failure. Circ-Heart Fail 9: e000345. [Crossref]
- Gottlieb SS, Harris K, Todd J, Estis J, Christenson RH, et al. (2015) Prognostic significance of active and modified forms of endothelin 1 in patients with heart failure with reduced ejection fraction. Clin Biochem 48: 292-296. [Crossref]
- Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, et al. (2017) Effect of natriuretic peptide–guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. Jama 318: 713-720. [Crossref]
- Del Trigo M, Bergeron S, Bernier M, Amat-Santos IJ, Puri R, et al. (2016) Campelo-Parada F, Altisent OA, Regueiro A, Eigler N, Rozenfeld E, Pibarot P. Unidirectional left-to-right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: a safety and proof-of-principle cohort study. The Lancet 387: 1290-1297. [Crossref]
- Komajda M, Cowie MR, Tavazzi L, Ponikowski P, Anker SD, et al. (2017) Physicians' guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail 19: 1414-1423. [Crossref]
- McKelvie RS, Moe GW, Ezekowitz JA, Heckman GA, Costigan J, et al. (2013) The 2012 Canadian Cardiovascular Society heart failure management guidelines update: focus on acute and chronic heart failure. Can J Cardiol 29: 168-181. [Crossref]
- Friedberg CK (1950) Diseases of the Heart. Academic Medicine. 25: 159. [Crossref]
- Braunwald, E. (1980). Clinical manifestations of heart failure. In Braunwald E, (Eds). Heart disease: a textbook of cardiovascular medicine (1st Ed). Philadelphia: WB Saunders.
- Chatterjee K, Massie B (2007) Systolic and diastolic heart failure: differences and similarities. J Card Fail 13: 569-576. [Crossref]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, et al. (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37: 2129-2200. [Crossref]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, et al. (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62: e147-239. [Crossref].
- Hunt SA, Abraham WT, Chin MH (2009) American Heart Association 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 53: e1-e90. [Crossref]
- The Criteria Committee of the New York Heart Association. (1994). Nomenclature and criteria for diagnosis of diseases of the heart and great vessels (9th ED.) Boston, Ma: Little & Brown.
- Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, et al. (2007) Prevalence and prognostic significance of heart failure stages: application of the ACC/AHA heart failure staging criteria in the community. Circ 115: 1563-1570. [Crossref]
- Goldman L, Hashimoto B, Cook EF, Loscalzo A (1981) Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circ 64: 1227-1234. [Crossref]
- Madsen BK, Hansen JF, Stokholm KH, Brons J, Husum D, et al. (1994) Chrome congestive heart failure: Description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J 15: 303-310. [Crossref]
- Ziaeian B, Fonarow GC (2016) Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol 13: 368-378. [Crossref]
- Bui AL, Horwich TB, Fonarow GC (2011) Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol 8: 30-41. [Crossref]
- Raymond I, Pedersen F, Steensgaard-Hansen F, Green A, Busch-Sorensen M, et al. (2003) Prevalence of impaired left ventricular systolic function and heart failure in a middle aged and elderly urban population segment of Copenhagen. Heart 89: 1422-1429. [Crossref]
- Abhayaratna WP, Smith WT, Becker NG, Marwick TH, Jeffery IM, (2006) Prevalence of heart failure and systolic ventricular dysfunction in older Australians: the Canberra Heart Study. Med J Aust 184: 151-154. [Crossref]
- Mureddu GF, Agabiti N, Rizzello V, Forastiere F, Latini R, et al. (2012) Prevalence of preclinical and clinical heart failure in the elderly. A population‐based study in Central Italy. Eur J Heart Fail 14: 718-729. [Crossref]
- Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, et al. (2009) Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circ 119: 3070-3077. [Crossref]
- Velagaleti RS, Vasan RS (2007) Heart failure in the twenty-first century: is it a coronary artery disease or hypertension problem? Cardiology clinics 25: 487-495. [Crossref]
- Gerber Y, Weston SA, Enriquez-Sarano M, Manemann SM, Chamberlain AM, et al. (2016) Atherosclerotic burden and heart failure after myocardial infarction. JAMA cardiology 1: 156-162. [Crossref]
- Gerber Y, Weston SA, Berardi C, McNallan SM, Jiang R, et al. (2013) Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol 178: 1272-1280. [Crossref]
- Vedin O, Lam CS, Koh AS, Benson L, Teng TH, et al. (2017) Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a nationwide cohort study. Circ-Heart Fail 10(6): e003875. [Crossref]
- Allen LA, Magid DJ, Gurwitz JH, Smith DH, Goldberg RJ, et al. (2013) Risk factors for adverse outcomes by left ventricular ejection fraction in a contemporary heart failure population. Circ-Heart Fail 6: 635-646. [Crossref]
- Lofman I, Szummer K, Dahlström U, Jernberg T, Lund LH (2017) Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid‐range, and reduced ejection fraction. Eur J Heart Fail 19: 1606-1614. [Crossref]
- Al-Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, (2001) Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 38: 955-962. [Crossref]
- Antlanger M, Aschauer S, Kopecky C, Hecking M, Kovarik JJ, et al. (2017) Heart failure with preserved and reduced ejection fraction in hemodialysis patients: prevalence, disease prediction and prognosis. Kidney Blood Press Res 42: 165-176. [Crossref]
- Van Riet EE, Hoes AW, Limburg A, Landman MA, van der Hoeven H, et al. (2014) Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur J Heart Fail 16: 772-777. [Crossref]
- Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). (2011). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 33: 1750-1757. [Crossref]
- Kontogeorgos S, Thunstrom E, Johansson MC, Fu M (2017) Heart failure with preserved ejection fraction has a better long-term prognosis than heart failure with reduced ejection fraction in old patients in a 5-year follow-up retrospective study. Int. J. Cardiol 232: 86-92. [Crossref]
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, et al. (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 14: 803-869. [Crossref]
- Eyuboglu M (2017) Prognostic factors in the heart failure with reduced ejection fraction. Int J Cardiol 235: 187. [Crossref]
- Xu Y, Shi Y, Zhu Z, Cui C, Li B, et al. (2013) Prognosis of patients with heart failure and reduced ejection fraction in China. Exp Ther Med 6: 1437-1442. [Crossref]
- Lodge FM, Yousef Z (2016) The pathophysiology of heart failure. Primary Care Cardiovascular Journal S12-S16. [Crossref]
- Morrow T (2017) Pathophysiology of heart failure. Master of Science in Nursing (MSN) Student Scholarship. 246. [Crossref]
- Casey G (2013) Heart failure. Kai Tiaki Nursing, New Zealand, 19: 20-24. [Crossref]
- Sutton MG, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circ 101: 2981-2988. [Crossref]
- Marin-Garcia J (2016) Cell death in the pathogenesis and progression of heart failure. Heart Failure Reviews 21: 117-121. [Crossref]
- Briasoulis A, Androulakis E, Christophides T, Tousoulis D (2016) The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Failure Reviews 21: 169-176. [Crossref]
- Sousa-Pinto B, Ferreira-Pinto MJ, Santos M, Leite-Moreira AF (2014) Central nervous system circuits modified in heart failure: pathophysiology and therapeutic implications. Heart Failure Reviews 19: 759-779. [Crossref]
- Kelder JC, Cramer MJ, van Wijngaarden J, van Tooren R, Mosterd A, et al. (2011) The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure clinical perspective. Circ 124: 2865-2873. [Crossref]
- Mant J, Doust J, Roalfe A, Barton P, Cowie MR, et al. (2003) Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. NIHR Journals Library. [Crossref]
- Wong CM, Hawkins NM, Jhund PS, MacDonald MR, Solomon SD, et al. (2013) Clinical characteristics and outcomes of young and very young adults with heart failure: the CHARM programme (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity). J Am Coll Cardiol 62: 1845-1854. [Crossref]
- Wong CM, Hawkins NM, Petrie MC, Jhund PS, Gardner RS, et al. (2014) Heart failure in younger patients: the Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). Eur Heart J 35: 2714-2721. [Crossref]
- Davie AP, Francis CM, Love MP, Caruana L, Starkey IR, et al. (1996) Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ 312: 222. [Crossref]
- Thomas JT, Kelly RF, Thomas SJ, Stamos TD, Albasha K, et al. (2002) Utility of history, physical examination, electrocardiogram, and chest radiograph for differentiating normal from decreased systolic function in patients with heart failure. Am J Med 112: 437-445. [Crossref]
- Roberts E, Ludman AJ, Dworzynski K, Al-Mohammad A, Cowie MR, et al. (2015) The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ 350: h910. [Crossref]
- Zaphiriou A, Robb S, Murray‐Thomas T, Mendez G, Fox K, et al. (2005) The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: results of the UK natriuretic peptide study. Eur J Heart Fail 7: 537-541. [Crossref]
- Fuat A, Murphy JJ, Hungin AP, Curry J, Mehrzad AA, et a;. (2006) The diagnostic accuracy and utility of a B-type natriuretic peptide test in a community population of patients with suspected heart failure. Br J Gen Pract 56: 327-333. [Ctrossref]
- Krishnaswamy P, Lubien E, Clopton P, Koon J, Kazanegra R, Wanner E, et al. (2001) Utility of B-natriuretic peptide levels in identifying patients with left ventricular systolic or diastolic dysfunction. Am J Med 111: 274-279. [Crossref]
- Madamanchi C, Alhosaini H, Sumida A, Runge MS (2014) Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol 176: 611-617. [Crossref]
- Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, et al. (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28: 2539-2550. [Crossref]
- Marwick TH, Raman SV, Carrio I, Bax JJ (2010) Recent developments in heart failure imaging. JACC: Cardiovascular Imaging 3: 429-439. [Crossref]
- Dokainish H, Nguyen JS, Bobek J, Goswami R, Lakkis NM (2011) Assessment of the American Society of Echocardiography-European Association of Echocardiography guidelines for diastolic function in patients with depressed ejection fraction: an echocardiographic and invasive haemodynamic study. Eur J Echocardiogr 12: 857-864. [Crossref]
- Kirkpatrick JN, Vannan MA, Narula J, Lang RM (2007) Echocardiography in heart failure: applications, utility, and new horizons. J Am Coll Cardiol 50: 381-396. [Crossref]
- Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, et al. (2015) Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. Eur Heart J Cardiovasc Imaging 16: 1031-1041. [Crossref]
- Garbi M, Habib G, Plein S, Neglia D, Kitsiou A, et al. (2014) Appropriateness criteria for cardiovascular imaging use in clinical practice: a position statement of the ESC/EACVI taskforce. Eur Heart J Cardiovasc Imaging 15: 477-482. [Crossref]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, et al. (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16: 233-271. [Crossref]
- Jensen-Urstad K, Bouvier F, Hojer J, Ruiz H, Hulting J, et al. (1998) Comparison of different echocardiographic methods with radionuclide imaging for measuring left ventricular ejection fraction during acute myocardial infarction treated by thrombolytic therapy. Am J Cardiol 81: 538-544. [Crossref]
- Alfakih K, Reid S, Jones T, Sivananthan M (2004) Assessment of ventricular function and mass by cardiac magnetic resonance imaging. European Radiology 14: 1813-1822. [Crossref]
- Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, et al. (2013) Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol 61: 77-84. [Crossref]
- Jenkins C, Bricknell K, Hanekom L, Marwick TH (2004) Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J Am Coll Cardiol 44: 878-886. [Crossref]
- Gutiérrez-Chico JL, Zamorano JL, de Isla LP, Orejas M, Almería C, et al. (2005) Comparison of left ventricular volumes and ejection fractions measured by three-dimensional echocardiography versus by two-dimensional echocardiography and cardiac magnetic resonance in patients with various cardiomyopathies. Am J Cardiol 95: 809-813. [Crossref]
- Jacobs LD, Salgo IS, Goonewardena S, Weinert L, Coon P, et al. (2005) Rapid online quantification of left ventricular volume from real-time three-dimensional echocardiographic data. Eur Heart J 27: 460-468. [Crossref]
- Jenkins C, Chan J, Hanekom L, Marwick TH (2006) Accuracy and feasibility of online 3-dimensional echocardiography for measurement of left ventricular parameters. J Am Soc Echocardiogr 19: 1119-1128. [Crossref]
- Jenkins C, Leano R, Chan J, Marwick TH (2007) Reconstructed versus real-time 3-dimensional echocardiography: comparison with magnetic resonance imaging. J Am Soc Echocardiogr 20: 862-868. [Crossref]
- Bicudo LS, Tsutsui JM, Shiozaki A, Rochitte CE, Arteaga E, et al. (2008) Value of real time three‐dimensional echocardiography in patients with hypertrophic cardiomyopathy: comparison with two‐dimensional echocardiography and magnetic resonance imaging. Echocardiography 25: 717-726. [Crossref]
- Chukwu EO, Barasch E, Mihalatos DG, Katz A, Lachmann J, et al. (2008) Han J, Reichek N, Gopal AS. Relative importance of errors in left ventricular quantitation by two-dimensional echocardiography: insights from three-dimensional echocardiography and cardiac magnetic resonance imaging. J Am Soc Echocardiogr 21: 990-997. [Crossref]
- Marsan NA, Westenberg JJ, Roes SD, van Bommel RJ, Delgado V, et al. (2011) Three-dimensional echocardiography for the preoperative assessment of patients with left ventricular aneurysm. Ann Thorac Surg 91: 113-121. [Crossref]
- Jenkins C, Moir S, Chan J, Rakhit D, Haluska B, et al. (2008) Left ventricular volume measurement with echocardiography: a comparison of left ventricular opacification, three-dimensional echocardiography, or both with magnetic resonance imaging. Eur Heart J 30: 98-106. [Crossref]
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, et al. (2002) Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346: 877-883. [Crossref]
- Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, et al. (2002) Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (copernicus) study. J Am Heart Assoc 106: 2194-2199. [Crossref]
- Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, et al. (2006) Quantitative assessment of left ventricular size and function: side-by-side comparison of real-time three-dimensional echocardiography and computed tomography with magnetic resonance reference. Circ 114: 654-661. [Crossref]
- Kuecherer HF, Kee LL, Modin G, Cheitlin MD, Schiller NB (1991) Echocardiography in serial evaluation of left ventricular systolic and diastolic function: importance of image acquisition, quantitation, and physiologic variability in clinical and investigational applications. J Am Soc Echocardiogr 4: 203-214. [Crossref]
- Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, et al. (2000) Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur Heart J 21: 1387-1396. [Crossref]
- Bernard Y, Meneveau N, Boucher S, Magnin D, Anguenot T, et al. (2001) Schiele F, Vuillemenot A, Bassand JP. Lack of agreement between left ventricular volumes and ejection fraction determined by two‐dimensional echocardiography and contrast cineangiography in postinfarction patients. Echocardiography 18: 113-122. [Crossref]
- Chuang ML, Hibberd MG, Salton CJ, Beaudin RA, Riley MF, et al. (2000) Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction: assessment by two-and three-dimensional echocardiography and magnetic resonance imaging. J Am Coll Cardiol 35: 477-484. [Crossref]
- Spitzer E, Ren B, Zijlstra F, Van Mieghem NM, Geleijnse ML (2017) The role of automated 3D echocardiography for left ventricular ejection fraction assessment. Cardiac Failure Review 3:97. [Crossref]
- Dorosz JL, Lezotte DC, Weitzenkamp DA, Allen LA, Salcedo EE (2012) Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: a systematic review and meta-analysis. J Am Coll Cardiol 59: 1799-1808. [Crossref]
- Qi X, Cogar B, Hsiung MC, Nanda NC, Miller AP, et al. (2007) Live/real time three‐dimensional transthoracic echocardiographic assessment of left ventricular volumes, ejection fraction, and mass compared with magnetic resonance imaging. Echocardiography 24: 166-173. [Crossref]
- American Society of Echocardiography Coding and Reimbursement Newsletter. [Crossref]
- Rosenthal MB (2011) Hard choices: alternatives for reining in Medicare and Medicaid spending. N Engl J Med 364: 1887-1890. [Crossref]
- Nicholson C (2014) Chronic heart failure: pathophysiology, diagnosis and treatment. Nursing Older People 26: 29-38. [Crossref]
- Consensus Trial Study Group (1987) Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med 316: 1429-1435. [Crossref]
- SOLVD Investigators (1991) Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325: 293-302. [Crossref]
- Kober L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, et al. (1995) A clinical trial of the angiotensin-converting–enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 333: 1670-1676. [Crossref]
- Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown Jr EJ, et al. (1992) Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement Trial. N Engl J Med 327: 669-677. [Crossref]
- Julian DG, Moss AJ, Murray GD, Poole-wilson P, Simoons M, et al. (1993) Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Lancet 342: 821-828. [Crossref]
- Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, et al. (2003) Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 349: 1893-1906. [Crossref]
- Maggioni AP, Anker SD, Dahlström U, Filippatos G, Ponikowski P, et al. (2013) Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12 440 patients of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 15: 1173-1184. [Crossref]
- McMurray JJ, Östergren J, Swedberg K, Granger CB, Held P, et al. (2003) Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. The Lancet 362: 767-771. [Crossref]
- Cohn JN, Tognoni G (2001) A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 345: 1667-1675. [Crossref]
- Hjalmarson Å, Goldstein S, Fagerberg B, Wedel H, Waagstein F, et al. (2000) Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). Jama 283: 1295-1302. [Crossref]
- Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, et al. (2001) Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 344: 1651-1658. [Crossref]
- Merit-HF Study Group (1999) Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in-congestive heart failure (MERIT-HF). The Lancet 353: 2001-2007. [Crossref]
- Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, et al. (2005) Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 26: 215-225. [Crossref]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, et al. (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709-717. [Crossref]
- Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, et al. (2011) Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 364: 11-21. [Crossref]
- Faris R, Flather M, Purcell H, Henein M, Poole-Wilson P, et al. (2002) Current evidence supporting the role of diuretics in heart failure: a meta analysis of randomised controlled trials. Int J Cardiol 82: 149-158. [Crossref]
- Faris RF, Flather M, Purcell H, Poole‐Wilson PA, Coats AJ (2006) Diuretics for heart failure. Cochrane Database Syst Rev 1. [Crossref]
- Digitalis Investigation Group (1997) The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 336: 525-533. [Crossref]
- Beck‐da‐Silva L, de Bold A, Fraser M, Williams K, Haddad H (2005) BNP‐Guided Therapy Not Better Than Expert's Clinical Assessment for β‐Blocker Titration in Patients With Heart Failure. Congestive Heart Failure 11: 248-255. [Crossref]
- Jourdain P, Jondeau G, Funck F, Gueffet P, Le Helloco A, et al. (2007) Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol 49: 1733-1739. [Crossref]
- Pfisterer M, Buser P, Rickli H, Gutmann M, Erne P, et al. (2009) BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. Jama 301: 383-392. [Crossref]
- Eurlings LW, van Pol PE, Kok WE, van Wijk S, Lodewijks-Van Der Bolt C, et al. (2010) Management of chronic heart failure guided by individual N-terminal pro–B-type natriuretic peptide targets: results of the PRIMA (Can PRo-brain-natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality?) study. J Am Coll Cardiol 56: 2090-2100. [Crossref]
- Berger R, Moertl D, Peter S, Ahmadi R, Huelsmann M, et al. (2010) N-terminal pro–B-Type natriuretic peptide–guided, intensive patient management in addition to multidisciplinary care in chronic heart failure: A 3-Arm, prospective, randomized pilot study. J Am Coll Cardiol 55: 645-653. [Crossref]
- Persson H, Erntell H, Eriksson B, Johansson G, Swedberg K, et al. (2010) Improved pharmacological therapy of chronic heart failure in primary care: a randomized Study of NT‐proBNP Guided Management of Heart Failure–SIGNAL‐HF (Swedish Intervention study–Guidelines and NT‐proBNP AnaLysis in Heart Failure). Eur J Heart Fail 12: 1300-1308. [Crossref]
- Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, et al. (2007) Association between performance measures and clinical outcomes for patients hospitalized with heart failure. Jama 297: 61-70. [Crossref]
- Michalsen A, König G, Thimme W (1998) Preventable causative factors leading to hospital admission with decompensated heart failure. Heart 80: 437-441. [Crossref]
- Sanders-van Wijk S, Maeder MT, Nietlispach F, Rickli H, et al. (2014) Long-term results of intensified, n-terminal-pro-b-type natriuretic peptide–guided versus symptom-guided treatment in elderly patients with heart failureclinical perspective: Five-year follow-up from TIME-CHF. Circ-Heart Fail 7: 131-139. [Crossref]
- Felker GM, Hasselblad V, Hernandez AF, O'connor CM (2009) Biomarker-guided therapy in chronic heart failure: a meta-analysis of randomized controlled trials. Am Heart J 158: 422-430. [Crossref]
- Porapakkham P, Porapakkham P, Zimmet H, Billah B, Krum H (2010) B-type natriuretic peptide–guided heart failure therapy: a meta-analysis. Arch Intern Med 170: 507-514. [Crossref]


