Abstract
To better characterize the clinical and pathologic features of granulomatous reaction to Pneumocystis jirovecii in non-HIV/AIDS patients, nine cases of this uncommon pathology were reviewed. Patients included 7 males and 2 females (mean age, 63 years). The most common symptom was dyspnea (4/9). Primary medical diagnoses included lymphomas (5/9) and solid malignancies (4/9). Radiology findings included nodular (4/8) and diffuse (3/8) infiltrates, and solitary nodule (1/8). Diagnostic procedures with the highest yield were open lung biopsy (5/9) and autopsy (3/9) and transbronchial biopsy (1/9); one false negative result was initially obtained with bronchoalveolar lavage. Follow-up showed death from disease (5/7) and resolution of disease (2/7). Histologically, clusters of Gomori methenamine silver-positive Pneumocystis organisms were identified in all cases. Organisms were identified within well- (5/9) and poorly- (4/9) formed necrotizing (6/9) and non-necrotizing (3/9) granulomas ranging in size from 0.1 to 2.5 cm (mean, 0.7cm); granulomas were multiple (8/9) or single (1/9). Eosinophils (6/9), giant cells (5/9), and a fibrous rim around granulomas (4/9) were seen. Foamy pink exudates were present centrally within two granulomas. Only one case demonstrated the classic intra-alveolar foamy exudates containing Pneumocystis. In non-HIV/AIDS patients, granulomatous Pneumocystis jirovecii pneumonia occurs most commonly in males with lymphoproliferative and solid malignancies. The diagnosis may be overlooked as conventional radiologic and pathologic features are absent. When suspected, open lung biopsy is most likely to yield diagnostic material. Attention to organism morphology avoids misdiagnosis as Histoplasma.
Key words
pneumocystis, pneumonia, granulomas, pulmonary, immunocompromise
Introduction
Pneumocystis jirovecii (formerly Pneumocystis carinii) pneumonia has been conventionally described as a bilateral, diffuse pulmonary disease having a histologic appearance of intra-alveolar eosinophilic foamy exudates containing cysts of P. jirovecii [1] Numerous case reports and rare series [2,3] with few cases of granulomatous P. jirovecii pneumonia have described the granulomas as an unusual histologic finding in up to 4-5% of patients with and without human immunodeficiency virus/acquired immune deficiency syndrome. Recent studies have suggested that the granulomatous response to P. jirovecii is likely due to host factors rather than microorganism genotypes [4]. Awareness of a granulomatous reaction with P. jirovecii is important since diagnostic procedures traditionally employed in the diagnosis of P. jirovecii pneumonia, such as bronchoalveolar lavage, may be of low yield [5]. Diagnostic delay must be avoided as mortality with P. jirovecii pneumonia is high [6]. Once diagnostic material is obtained, careful attention to microorganism morphology is necessary to avoid misdiagnosis. Nine cases of granulomatous P. jirovecii pneumonia were reviewed to better characterize its clinical and pathologic features in patients with lymphoproliferative and solid malignancies.
Materials and methods
Thirty-one cases diagnosed as “granulomatous Pneumocystis” infection from 1988 to 2009 were retrieved from tissue archives. Eleven non-pulmonary cases and 11 pulmonary HIV/AIDS cases were excluded. Clinical history and follow-up data were obtained from patient records. Chest radiographs were available in 8 cases. Histomorphologic and histochemical details were reviewed. Hematoxylin and eosin (H&E) stained sections were available for each case (range 1-22 slides, mean 7). Gomori methenamine silver (GMS) stains, performed on paraffin-embedded tissue, to identify microorganisms were available in all cases. The diagnostic features utilized to separate P. jiroveceii from Histoplasma capsulatum in GMS stained sections are presented in Table 1.
Table 1. Diagnostic features of P. jiroveceii and Histoplasma capsulatum.
Pneumocystis jiroveceii |
Histoplasma capsultum |
Thin-walled cysts |
Thin-walled cysts |
2 to 5 microns |
2 to 5 microns |
Collapsed forms common |
Collapsed forms common |
Typically spherical |
More oval forms |
Large capsular dots |
Smaller capsular dots |
Non-budding |
Budding |
Results
The clinical results are presented in Table 2. Seven males and two females, ranging in age from 30 to 87 years (mean, 63) comprised the study group. Presenting symptoms were most commonly dyspnea, cough, and fever; one patient presented with a right lung mass. The most common medical diagnoses were lymphoma (5/9) and solid tumors (4/9). Chest radiographs reported nodular infiltrates (4/8), diffuse infiltrates (3/8), or solitary nodule (1/8). One case had no imaging. Definitive diagnostic procedures included open lung biopsy (5/9), autopsy (3/9), and less commonly transbronchial biopsy (1/20). One nondiagnostic bronchoalveolar lavage was pergformed. Follow-up data were available for 7 patients. While resolution of disease was observed (2/7), patients typically died of disease (5/7).
Table 2. Clinical findings in granulomatous Pneumocystis pneumonia.
Demographics
Male
Female
Mean age, years
|
7/9
2/9
63 |
Symptoms
Dyspnea
Cough
Fever
Solitary nodule on CXR |
4/9
2/9
2/9
1/9 |
Primary diagnoses
Lymphoma/leukemia
Solid malignancy |
5/9
4/9 |
Radiology (8 CXRs)
Nodular infiltrates
Diffuse infiltrates
Solitary nodule |
4/8
3/8
1/8 |
Diagnostic procedure
Open biopsy
Autopsy
Transbronchial biopsy |
5/9
3/9
1/9 |
Nondiagnostic procedures
Bronchoalveolar lavage |
1/9 |
Follow-up
Dead of Disease
Resolved |
7/9
2/9 |
Pathologic findings are listed in Table 3 and demonstrated in Figure 1. Pneumocystis organisms were identified within granulomas in all cases on GMS stained sections and showed characteristic thin-walled, spherical non-budding cysts. Dark GMS-positive intracystic foci (capsular dots) were also a consistent feature. Although additional histochemical studies were employed including Ziehl-Neelsen, Brown-Hopps, and PAS, no other microorganisms were identified to account for the granulomas in any case. Granulomas containing P. jiroveceii ranged from 0.1 to 2.5 cm (mean, 0.7cm). Granulomas were well-formed (5/9), poorly formed (4/9), necrotizing (6/9), and non-necrotizing (3/9), multiple (8/9) and single (1/9). Histology of granulomas showed epithelioid histiocytes and a surrounding rim of lymphocytes (9/9). Eosinophils (6/9) and giant cells (5/6) were present within and around granulomas. Other findings included a peri-granulomatous fibrous rim (4/9) and central, intra-granuloma foamy eosinophilic exudates (2/9). Conventional intra-alveolar foamy eosinophilic exudates were seen in only one case.
Table 3. Pathologic findings in granulomatous Pneumocystis pneumonia.
Pneumocystis organisms
Gomori methenamine-silver positive, within granulomas
Thin-walled, nonbudding cysts
Capsular dots |
9/9
9/9
9/9 |
Comorbid infections to account for granulomas (by histochemistry)
Gomori methenamine-silver
Ziehl-Neelsen
Brown-Hopps
PAS
Gridley’s Fungus
Brown-Brenn
Fite |
0/9
0/9
0/8
0/1
0/1
0/1
0/1 |
Granulomas
Mean size (range), cm
Well-formed
Poorly-formed
Necrotizing
Non-necrotizing
Multiple
Single |
0.7 (0.1 to 2.5)
5/9
4/9
6/9
3/9
8/9
1/9 |
Granuloma-associated histology
Epithelioid histiocytes and lymphocytes
Eosinophils
Giant cells
Fibrous rim
Central, intra-granuloma foamy eosinophilic exudate
Associated classic intra-alveolar foamy eosinophilic exudates |
9/9
6/9
5/9
4/9
2/9
1/9 |
Figure 1. Histopathologic features of granulomatous PCP
The morphology of granulomas in PCP varies from solitary well-formed necrotizing lesions (A), which may contain numerous organisms (B, GMS stain), to confluent nodules (C), well-formed non-necrotizing (D) and poorly-formed necrotizing lesions (E). Some granulomas display peripheral multinucleated giant cells with eosinophils (F), a fibrous rim (G), or central eosinophilic necrosis (H). Uncommon findings in PCP associated granulomas include cavitation (I), foamy eosinophilic exudates within granulomas (J) and within adjacent lung parenchyma (K). Thin-wall, nonbudding cysts (5-7 µm) with dark staining intracystic capsular dots are characteristic of Pneumocystis jirovecii (L, GMS stain).
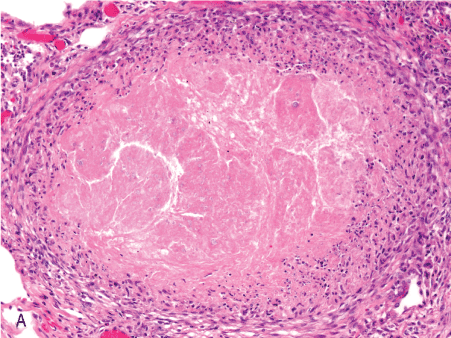
Figure 1A. 10x well-formed nec gran.
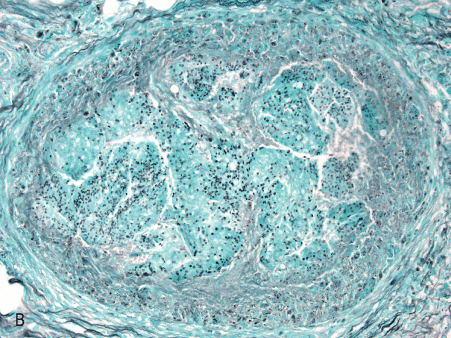
Figure 1B. 10x GMS well-formed nec gran.
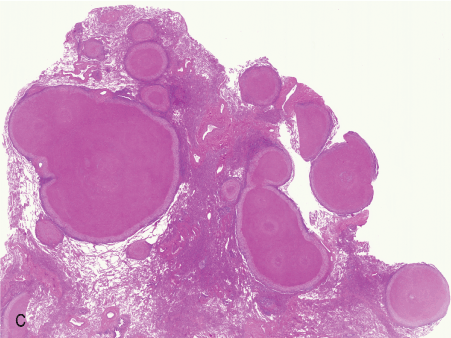
Figure 1C. 1x confluent grans.
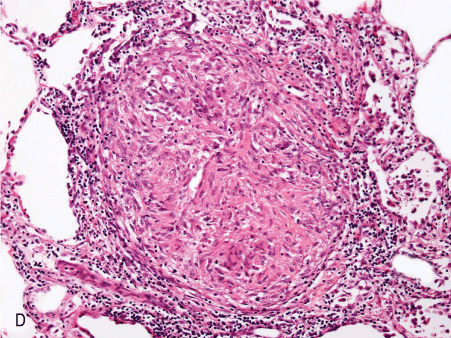
Figure 1D. 10x non nec well-formed gran.
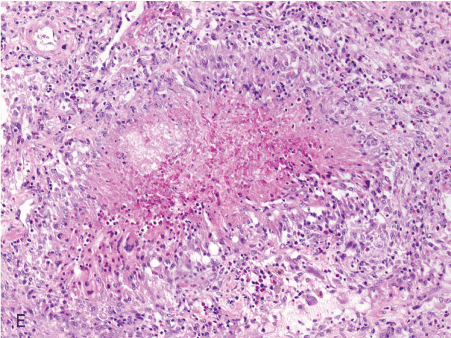
Figure 1E. 10x poorly formed nec gran.
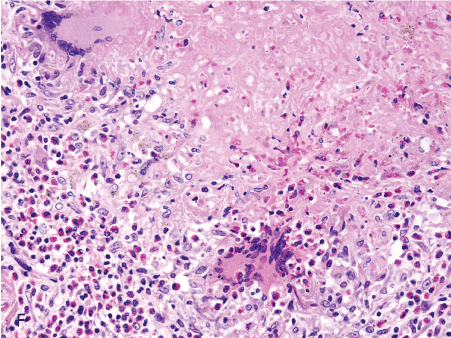
Figure 1F. 20x giant cells eosinophils in nec gran.
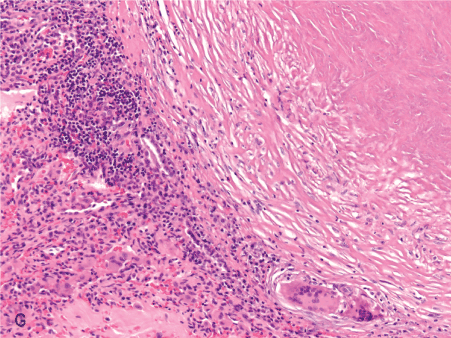
Figure 1G. 10x fibrous rim.
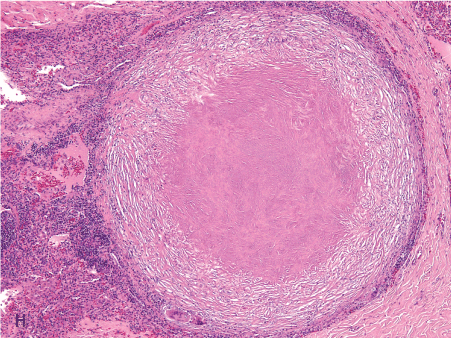
Figure 1H. 4x eo necrosis.
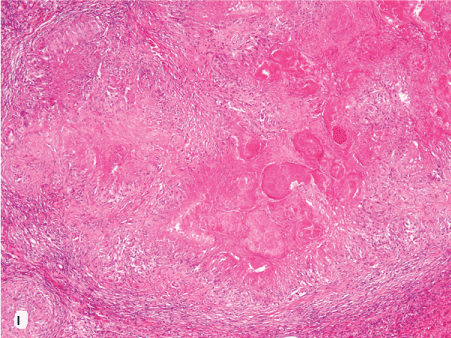
Figure 1I. 4x intragran foamy eo exudate.
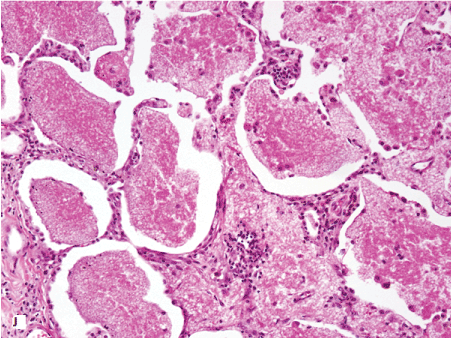
Figure 1J. 20x intraalveolar foamy eo exudate.
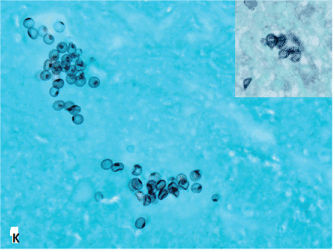
Figure 1K. 100x capsular dots GMS inset.
Discussion
A granulomatous response to P. jirovecii pneumonia has been previously described as an unusual histologic finding predominantly in patients with human immunodeficiency virus/acquired immune deficiency syndrome [7,8] The diagnosis of P. jirovecii pneumonia may be overlooked when granulomas are present since classic radiologic and pathologic findings in granulomatous P. jirovecii pneumonia are absent. In this setting, traditional procedures, such as bronchoalveolar lavage, are likely to be nondiagnostic. As the mortality of P. jirovecii pneumonia ranges from 40-54%,[6] expedient diagnosis and treatment are necessary to prevent deaths from this treatable illness. We reviewed 9 cases of granulomatous P. jirovecii pneumonia in lymphoproliferative and solid malignancies to better characterize the clinical and pathologic features of this entity in this subset of patients.
The literature has described P. jirovecii pneumonia as a bilateral pulmonary disease having a histologic and radiologic appearance of diffuse alveolar and interstitial infiltrates, often with a perihilar distribution [6,9]. Dyspnea and cough are the most common presenting symptoms [1]. Atypical pathologic manifestations of Pneumocystis pneumonia described in patients with human immunodeficiency virus/acquired immune deficiency syndrome [3] and patients with neoplastic diseases [2] have included interstitial pulmonary fibrosis, absence of alveolar exudates, granulomatous inflammation, hyaline membranes, giant cell reaction, desquamative interstitial pneumonia-like histology, and interstitial lymphoid infiltrates. Investigations into the relationship between genotypic variation of P. jirovecii and these atypical pathologic manifestations, particularly the granulomatous reaction, have been undertaken. These studies have concluded that identical organisms are involved despite variable histologic tissue response, [4,10] and that host factors are likely more contributory to the atypical pathologic manifestations of Pneumocystis infection. Such host factors may include CD4/CD8 cell counts, exposure time to Pneumocystis surface glycoproteins, absence of IgA Pneumocystis antibodies, and prior Pneumocystis exposure [11-13]. Diagnosis of conventional Pneumocystis pneumonia has traditionally been and continues to rely on bronchoalveolar lavage [14]. However, in the case of granulomatous Pneumocystis pneumonia, bronchoalveolar lavage has been demonstrated to be of low yield [5]. Bronchial brushings, needle aspiration and transbronchial biopsy have not shown more favorable results [7,15-17]. A prior study has shown open lung biopsy is safe in immunocompromised patients and renders superior diagnostic information [18].
Similar to previous reports, our study demonstrated that granulomatous reaction to P. jirovecii pneumonia occurs most commonly in male patients and with lymphoproliferative and solid malignancies. Such associations do not differ from usual P. jirovecii pneumonia. In addition to their primary medical diagnoses, five patients were known to have received immunosuppressive treatments (i.e., chemotherapy, radiation, or corticosteroids). The preponderance of males (7/9) in our study is consistent with previous reports. While dyspnea and cough are common symptoms, patients may present with a solitary lung nodule. Chest radiograph findings of solitary pulmonary nodule, while rare with P. jirovecii pneumonia, must nevertheless include granulomatous P. jirovecii pneumonia in the differential diagnosis in the appropriate clinical setting. The present cases did not have complete data available on host factors that may contribute to the granulomatous response to P. jirovecii such as CD4/CD8 cell counts and immunoglobulin antibody profiles. None of the current patients had prior infection or exposure to Pneumocystis recorded. We found that less invasive procedure, i.e., bronchoalveolar lavage, conventionally utilized to obtain diagnostic material in P. jirovecii pneumonia cases was nondiagnostic. Open lung biopsy should be considered early in cases of possible granulomatous P. jirovecii pneumonia to expedite diagnosis. While specific treatment data were not completely available for our study, two of the present cases were noted in the medical record to have resolved. This outcome is comparable to that reported with P. jirovecii pneumonia in general [6]. None of the present cases of granulomatous P. jirovecii pneumonia documented resolution with TMP/SMX treatment.
Histologically, the pulmonary granulomas seen with P. jirovecii usually have the typical appearance of infectious-type granulomas. They are most commonly multiple, well-formed, and necrotizing with thin-walled, spherical, non-budding cysts of P. jirovecii present within the granulomas. While central foamy eosinophilic exudates are seen in some pulmonary P. jirovecii granulomas, conventional findings of intra-alveolar foamy eosinophilic exudates were seen in only one case.
Because there is overlap in morphologic features, P. jirovecii and Histoplasma capsulatum are often difficult to separate in GMS stained tissue sections (Table 1). Both organisms appear as thin-walled structures that are similar in size range. Either can appear collapsed or distorted in tissue sections. Pneumocystis is distinguished from fungal yeasts by its lack of budding forms. In contrast, Histoplasma multiplies by budding. Budding forms are usually not numerous but when present are the most definitive feature in separating the two organisms. Care must be taken, however, to avoid identifying abutting or slightly overlapping forms as budding. When budding is not identified, the two most important features in avoiding misdiagnosis of P. jirovecii as Histoplasma are shape and size of the organisms and size of the intracystic bodies (so-called ‘capsular dot’). Pneumocystis is typically spherical in shape while Histoplasma most frequently is comprised of oval forms. The size range of the two organisms is similar, however, Histoplasma on average is slightly smaller. Capsular dots represent focal thickening of the cyst wall of Pneumocystis [19] and the capsular dots of Pneumocystis are larger than those of the Histoplasma. Additionally, multiple dots may be seen in Pneumocystis. Rarely, Cryptococcus yeast forms may pose a differential diagnostic challenge when these organisms present at the smaller end of their usual size range (i.e., 4-6 um, range 2-20 um). They are oval to elliptoid with single, narrow based budding. They can appear to have a halo that represents a mucinous capsule, readily identified with mucin stains. Correct identification of P. jirovecii will rely on a careful assessment of all the morphologic features mentioned. Immunohistochemistry, direct immunofluorescence and real-time PCR can also assist in detecting P. jirovecii [20,21].
Future studies exploring the role of host immune factors in the granulomatous response to P. jirovecii may help elucidate the pathogenesis of this unusual tissue reaction to P. jirovecii.
References
- Peters SG, Prakash UB (1987) Pneumocystis carinii pneumonia. Review of 53 cases.Am J Med82: 73-78. [Crossref]
- Luna MA1, Cleary KR (1989) Spectrum of pathologic manifestations of Pneumocystis carinii pneumonia in patients with neoplastic diseases.Semin Diagn Pathol6: 262-272. [Crossref]
- Travis WD, Pittaluga S, Lipschik GY, Ognibene FP, Suffredini AF, et al. (1990) Atypical pathologic manifestations of Pneumocystis carinii pneumonia in the acquired immune deficiency syndrome. Review of 123 lung biopsies from 76 patients with emphasis on cysts, vascular invasion, vasculitis, and granulomas.Am J Surg Pathol14: 615-625. [Crossref]
- Totet A, Duwat H, Daste G, Berry A, Escamilla R, et al. (2006) Pneumocystis jirovecii genotypes and granulomatous pneumocystosis. Med Mal Infect 36: 229-231. [Crossref]
- Wakefield AE, Miller RF, Guiver LA, Hopkin JM (1994) Granulomatous Pneumocystis carinii pneumonia: DNA amplification studies on bronchoscopic alveolar lavage samples. J Clin Pathol 47: 664-666. [Crossref]
- Miller RF, Allen E, Copas A, Singer M, Edwards SG (2006) Improved survival for HIV infected patients with severe Pneumocystis jirovecii pneumonia is independent of highly active antiretroviral therapy.Thorax61: 716-721. [Crossref]
- Cupples JB, Blackie SP, Road JD (1989) Granulomatous Pneumocystis carinii pneumonia mimicking tuberculosis.Arch Pathol Lab Med113: 1281-1284. [Crossref]
- Lewin-Smith M, Clack WP (1998) Granulomatous Pulmonary Infection with Pneumocystis carinii. Pathology Case Reviews 3: 29-34.
- DeLorenzo LJ, Huang CT, Maguire GP, Stone DJ (1987) Roentgenographic patterns of Pneumocystis carinii pneumonia in 104 patients with AIDS. Chest 91: 323-327. [Crossref]
- Totet A, Duwat H, Magois E, Jounieaux V, Roux P, et al. (2004) Similar genotypes of Pneumocystis jirovecii in different forms of Pneumocystis infection.Microbiology150: 1173-1178. [Crossref]
- Blumenfeld W, McCook O, Griffiss JM (1992) Detection of antibodies to Pneumocystis carinii in bronchoalveolar lavage fluid by immunoreactivity to Pneumocystis carinii within alveoli, granulomas, and disseminated sites. Mod Pathol 5: 107-113. [Crossref]
- Theus SA, Andrews RP, Steele P, Walzer PD (1995) Adoptive transfer of lymphocytes sensitized to the major surface glycoprotein of Pneumocystis carinii confers protection in the rat. J Clin Invest 95: 2587-2593. [Crossref]
- Vahid B, Bibbo M, Marik PE (2007) Role of CD8 lymphocytes and neutrophilic alveolitis in Pneumocystis jiroveci pneumonia. Scand J Infect Dis 39: 612-614. [Crossref]
2021 Copyright OAT. All rights reserv
- Vélez L, Correa LT, Maya MA, Mejía P, Ortega J, et al. (2007) Diagnostic accuracy of bronchoalveolar lavage samples in immunosuppressed patients with suspected pneumonia: analysis of a protocol.Respir Med101: 2160-2167. [Crossref]
- Bondoc AY, White DA (2002) Granulomatous Pneumocystis carinii pneumonia in patients with malignancy. Thorax 57: 435-437.
- Gal AA, Plummer AL, Langston AA, Mansour KA (2002) Granulomatous Pneumocystis carinii pneumonia complicating hematopoietic cell transplantation. Pathol Res Pract 198: 553-558. [Crossref]
- Lauffer L, Kini JA, Costello P, Godleski J (2004) Granulomatous Pneumocystis carinii pneumonia in a non-AIDS patient: an atypical presentation. J Thorac Imaging 19: 196-199. [Crossref]
- Ellis ME, Spence D, Bouchama A, Antonius J, Bazarbashi M, et al. (1995) Open lung biopsy provides a higher and more specific diagnostic yield compared to broncho-alveolar lavage in immunocompromised patients. Fungal Study Group. Scand J Infect Dis 27: 157-162. [Crossref]
- Watts JC, Chandler FW (1985) Pneumocystis carinii pneumonitis. The nature and diagnostic significance of the methenamine silver-positive "intracystic bodies". Am J Surg Pathol 9:744-51.
- Fillaux J, Malvy S, Alvarez M, Fabre R, Cassaing S, et al. (2008) Accuracy of a routine real-time PCR assay for the diagnosis of Pneumocystis jirovecii pneumonia.J Microbiol Methods75: 258-261. [Crossref]
- Forbes BA, Sahm DF, Weissfeld AS. Bailey and Scott's Diagnostic Microbiology, 12th edition, 2007, Mosby Elsevier, St Louis pp695-696.











