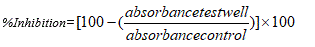Background: The quest for continuous search for the use of plants for the cure of diabetes mellitus necessitated the research work.
Objective: The search for total and complete remedy for the treating of diabetes mellitus has necessitated the beaming of search light on bridelia micrantha for its potential anti-diabetic inhibitors.
Materials and method: The bark of the tree plant was obtained, air dried and turned to powdered. Extraction was done using methanol and chloroform solvents and the in-vitro studies of the inhibitory potentials of the crude extracts were done using Gao et.al method with slight modification. The IC50 values were calculated using Graph pad prism 5.0software.
Results: Methanolic crude extract was found to be the most potent inhibitor of α-glucosidase with 1.06 ± 0.1 μg/mL IC50 value and the chloroform crude extract had 2.84 ± 0.1 μg/mL IC50 which was better than standard inhibitor acarbose (234.6 ±2.01μM)). The IC50 (7.35 ± 1.0μg/mL) of chloroform extract showed less potent inhibitor against glucoamylase than that of methanolic extract with IC50 (1.12 ± 0.10 μg/mL). The β-glucosidase screening of the extracts showed that they do not potent and selective inhibitors.The high inhibitory effects of the extracts against glucoamylase and α-glucosidase were confirmed by the presence of bioactive compounds present. The OSIRIS drug properties of some identified compounds was screened using online OSIRIS property explorer server and were found to possess various drug properties.
Conclusion: The in vitro assays of these extracts indicated that they are therapeutically interesting and could serve as important tools for treatment of diabetes.
anti-diabetes, ic50, bridelia micrantha, α-glucosidase, β-glucosidases, gluco amylase, osiris drug properties, GC-MS
Diabetes mellitus disease has been a major ailment around the world and Nigeria not being exempted from the dreaded disease. The searching for the complete eradication of the disease through the use of plants has consistently been a major task for the pharmaceutical chemists. Diabetes mellitus is said to be a group of metabolic disorders manifested by innate or acquired failure to transport glucose from blood stream to cells [1]. There are many anti-diabetes inhibitors and among which are α-glucosidase, β-glucosidase. α -glucosidase inhibitors are oral drugs made for DM type 2 and it works by disallowing the digestion of carbohydrates and thereby reducing the impact of carbohydrate on blood sugar. Generally, plants are considered to be a source for the most active, potent hypoglycaemic properties [2,3]. Natural drugs from plants are considered to be nontoxic with lesser side effects than synthetic drugs. It has been reported that medicinal plants possessing anti-diabetic activities could be a useful tool for the discovery of safer hypoglycaemic agents [4]. These plants are said to be the major source for discovering new compounds with therapeutic value for drug development against most common and very prevalent disease, diabetes mellitus. The plants which have therapeutic application possess bioactive composites viz., alkaloids, glycosides, tannins, flavonoids, saponins, phenolics and vitamins [5]. B. micrantha is a medium sized semi-deciduous to deciduous tree that grows up to 20 m tall and belongs to the family Euphorbiaceae [6]. In Nigeria, different parts of the plant are used traditionally in the treatment of some ill-health by different cultural groups [7]. Pharmacological properties such as antidiabetic, antioxidant [6,8], anti-inflammatory [7], hepatoprotective [9] and abortifacient [10] activities of the plant have been reported. Bark of B. micrantha plant has been previously evaluated for its phytochemicals; Saponins Alkaloids Tannins Phytosterols Glycosides Flavonoids [11].
Research laboratory
The research work was carried out at the centre for the Advanced Drug Research (CADR), Department of Pharmacy, COMSATS Institute of Information and Technology, Abbottabad, Pakistan in the month of July, 2017.
Materials and instruments
All the chemicals, solvents used are of analytical grade, α-glucosidase (from Saccharomyces cerevisiae), substrate p-nitrophenylα-D-glucopyranoside (pNPG), β-glucosidase (from sweet almonds) and 96 well plates were purchased from Sigma Aldrich. ELIZA micro plate reader.
Plant source
B. micrantha belong to the family of Euphorbiaceae. The barks of the plant were obtained from a small farm in Akure South Local Government Area of Ondo State in Nigeria on the 5th of April, 2017 and identified at the Department of crop and soil science, Federal University of Technology Akure, Nigeria.
Plant preparation
The barks were collected and air dried for one month and later grinded into powdered sample using grinder. The commercial grinder is made in China, brand MPN, having the following specifications; power (2.2kw), dimension (34×38×75cm), roller length (26cm), roller diameter (14cm), voltage (220v/50Hz).
Crude extract preparation
Two hundred grams of powdered B. micrantha were soaked in 1000 ml of Chloroform and methanol for five days and filtered through whatman filter paper. The extract was concentrated using a rotary evaporator at 35°C and the dried extract was stored at room temperature for further use. Ten milligram (10 mg) of dried crude extracts were dissolved in 1 ml of 100% Dimethyl sulfoxide (DMSO) and labelled as stock (10 mg/ml), working solution was made as 1 mg/ml.
α-glucosidase inhibition study
Assay for α-glucosidase inhibition was performed by slight modification of a previously published method [12]. Briefly, solutions of α-glucosidase (from Saccharomyces cerevisiae) and its substrate p-nitrophenyl α-D-glucopyranoside (pNPG) were prepared in phosphate buffer (70 mM, pH 6.8). Buffer was used for the preparation of inhibitor solutions. The inhibition assays were conducted by adding inhibitor solution (10 μL) to 70 μL buffer and 10 μL of enzyme solution (2.5unit/mL) in 70 mM phosphate buffer (pH 6.8) followed by pre-incubation at 37 °C for 5 min. After pre-incubation, 10 μL of 10 mM substrate (pNPG) prepared in phosphate buffer was added to the mixture to initiate enzymatic reaction. The reaction mixture was incubated at 37 °C for 30 minutes. Acarbose was used as a positive control. The α-glucosidase activity was determined by measuring the p-nitrophenol released from pNPG at 405 nm using an Eliza micro plate reader. The experiment was performed in triplicates.
β- glucosidase inhibition study
The evaluation of inhibitory activity against β-glucosidase was performed with slight modification of the previously published method [13]. Briefly, β-glucosidase (from sweet almonds) enzyme and p-nitrophenyl β-D-glucopyranoside (pNPG) as substrate were prepared in 0.07 M phosphate buffer (pH 6.8). The inhibition assays were conducted by adding inhibitor solution (10 μL) to 70 μL buffer and 10 μL of enzyme solution (2.0 unit/mL) in 70 mM phosphate buffer (pH 6.8) followed by pre-incubation at 37 °C for 5 min. After pre-read, 10 μL of substrate was added to the mixture and then incubated at 37 °C for 30 min and final reading was obtained. Negative control contained 10 μL of 10% DMSO instead of inhibitor. The experiment was performed in triplicates and the % inhibition was calculated.
Maltase glucoamylase inhibition study
Assay for glucoamylase inhibition was carried out by slight modification of a previously published method [12]. Shortly, solutions of glucoamylase (from maltase) enzyme and its substrate p-nitrophenyl α-D-glucopyranoside (pNPG) were prepared in phosphate buffer (70 mM, pH 6.8). Buffer was used for the preparation of inhibitor solutions. The inhibition assays were conducted by adding inhibitor solution (10 μL) to 70 μL buffer and 10 μL of enzyme solution (2.5unit/mL) in 70 mM phosphate buffer (pH 6.8) followed by pre-incubation at 37 °C for 5 min. After pre-incubation, 10 μL of 10 mM substrate (pNPG) prepared in phosphate buffer was added to the mixture to initiate enzymatic reaction. The reaction mixture was incubated at 37 °C for 30 min. Acarbose was used as a positive control. The glucoamylase activity was determined by measuring the p-nitrophenol released from pNPG at 405 nm using an Eliza micro plate reader. Each experiment was performed in triplicates. The % inhibition was calculated.
Statistical analysis: The total percentage inhibitions were calculated by method of [12]:
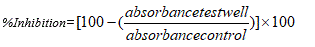
IC50 (i.e., the concentration of sample inhibiting 50%) values of potent inhibitors were determined by testing the serial dilutions of inhibitors and were calculated by using the program PRISM 5.0 (GraphPad, San Diego, California, USA).
GC/MS analysis
GC-MS analysis of methanol and chloroform extracts of B.Micrantha barks was performed using TurboMass GC System, fitted with an Elite-5 capillary column (30 m, 0.25 mm inner diameter, 0.25 μm film thickness; maximum temperature 350 °C, and coupled to a Perkin Elmer Clarus 600C MS. Helium was used as gas carrier at a constant flow rate of 1.0 mL/min. The injection, transfer line and ion source temperatures were 280 °C. The ionizing energy was 70 eV. The oven temperature was programmed from 70 °C (hold for 2 min) to 280 °C (hold for 10 min) at a rate of 5 °C/min. The crude extract was solubilised with chloroform and filtered with syringe filter (Corning, 0.45 μm). Volumes of 1 μL of the crude extracts were injected with a split ratio 1:20. The data were obtained by collecting the mass spectra within the scan range 50-550 m/z. The identification of chemical compounds in the extracts was based on GC retention time; the mass spectra matched those of standards available at NIST library.
Tables 1-4, Figure 2 and 3.
Table 1. Inhibition potency of crude extracts against α-glucosidase, maltase glucoamylase and β-glucosidase
Extracts |
α-glucosidase
IC50 + SEM (μg/mL) |
β-glucosidase
% inhibition+ SEM |
Maltase glucoamylase
IC50+SEM (μg/mL) |
CHCl3 extract |
2.84 ± 0.08 |
24.53+3.64 |
7.35 ± 1.02 |
MeOH extract |
1.06 ± 0.11 |
24.16+1.54 |
1.12+0.10 |
Acarbosea |
234.6 ±2.01(μM) |
Not tested |
234.6 ±2.01(μM) |
Castonospermineb |
Not tested |
59.98%[14] |
Not tested |
a α-glucosidase standard
b β-glucosidase standard
+ SEM: standard Error mean
Table 2. Identified compounds in the chromatogram of methanolic extract
Compound name |
Molecular formula |
CAS |
Molecular Retention time
weight (g.mol-1) (minutes) |
Valeric acid |
C21H42O2 |
125164-54-7 |
326 11.822 |
L-Ascorbic acid,6-octadecanoate |
C24H42O7 |
10605-09-1 |
442 31.442 |
Oxalic acid ,butyl-6-ethyloct-3-yl-ester |
C16H30O4 |
900309-34-3 |
286 13.608 |
Menthol |
C10H20O |
1490-04-6 |
156 42.89 |
Phytol |
C20H40O |
150-86-7 |
296 28.639 |
Stearic acid,2-phenyl-M-dioxan-5-yl ester trans |
C28H46O4 |
10564-35-9 |
446 10.842 |
Phenol3.5-Bis(1,1-dimethyl ethyl) |
C14H22O |
1138-52-9 |
206 20.241 |
Myristic acid vinyl ester |
C16H30O2 |
5809-91-6 |
254 49.638 |
Vitamin A Aldehyde |
C20H28O |
116-31-4 |
284 16.784 |
3-Methyl-2-(2-oxopropyl)Furan |
C8H10O2 |
87773-62-4 |
138 20.841 |
1H-Imidazole,1-(1ioxooctadecyl) |
C21H88ON2 |
17450-32-7 |
334 20.526 |
D-mannitol, 1-decylsulfonyl |
C16H34O7S |
900154-76-1 |
370 50.274 |
Table 3. Identified compounds in the chromatogram of chloroform extract
Compound name |
Molecular formula |
CAS |
Molecular Retention time
weight (g.mol-1) (minutes) |
3-methyl-2-(2-oxopropyl) Furan |
C8H10O2 |
87773-62-4 |
138 11.707 |
Z,Z-6,28-Heptatriactontadiene-2-one |
C37H70O |
133530-21-9 |
538 51.313 |
1,10-Hexadecanediol |
C16H34O2 |
39516-54-6 |
258 19.895 |
(Z)-14-tricosenyl formate |
C24H46O2 |
77899-10-6 |
366 21.176 |
3-T-Butyl-oct-6-yn-1-ol |
C12H24O |
900185-34-1 |
184 24.527 |
Cis-1-chloro-9-octadecene |
C18H35Cl |
16507-61-2 |
286 20.576 |
4-N-Hexylthiane,s,s-dioxide |
C11H22O2S |
70928-52-8 |
218 27.183 |
OSIRIS drug properties and toxicity profile
Table 4. Drug properties of some of identified compounds in the crude extracts determined by OSIRIS property explorer
Compound |
Drug likeness |
Mutagenic |
Tumorigenic |
cLogS |
CLogP |
Polar surface area(Å2) |
%Absoption |
H-bond Acceptor |
H-bond Donor |
Irritability |
Valeric acid |
-7.0646 |
None |
None |
-1.269 |
1.0641 |
37.3 |
96.13 |
2 |
1 |
None |
Menthol |
-25.216 |
High |
None |
-2.501 |
2.4112 |
20.23 |
102.02 |
2 |
1 |
None |
Phytol |
-3.7661 |
None |
High |
-4.633 |
7.4212 |
20.23 |
102.02 |
1 |
1 |
High |
1H-imidazole (identified compound] having imidazole ring) |
0.44659 |
None |
None |
-0.431 |
-0.1802 |
28.68 |
99.11 |
2 |
1 |
None |
Ascorbic acid |
0.023806 |
None |
None |
-0.349 |
-2.8448 |
90.15 |
77.9 |
6 |
4 |
None |
(molecular aspect) |

Figure 1. Bark of Bridelia micranth.

Figure 2. Chromatogram of Methanolic extract of B. micranth.
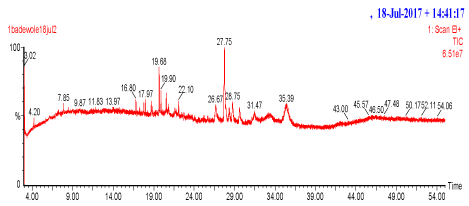
Figure 3. Chromatogram of chloroform extract of B. micrantha.
It is worthy to note that from the result of table 1, the IC50 of methanolic extract (1.06+0.1μg/mL) showed high inhibitory potential against α-glucosidase than that of chloroform extract IC50 (2.84+0.1μg/mL). However, the two results were better than the acarbose standard (234.6 ±2.01μM) against α-glucosidase. The IC50 both for the extracts of B.micrantha against α-glucosidase were better than the ethanolic extracts of Andrographis paniculata and andrographolide as reported by Rammohan [15] when compared. The extract of A. Paniculata showed α-glucosidase inhibitory effect in a concentration-dependent manner (IC50 of 17.2 ± 0.15 mg/mL) and andrographolide demonstrated a similar (IC50 of 11.0 ± 0.28 mg/mL) against α-glucosidase. Furthermore, the α-glucosidase of the B.micrantha extracts were better than the methanolic extracts of Artocarpus altilis (IC50 129.85+10.29μg/mL), A.heterophyllus (IC50 76.90+9.55μg/mL), Cinnamomus zeylanicum (IC50 140.01+0.08 μg/mL) and piper betel (IC50 96.56+12.93μg/mL) at concentrations ranged from 20 to 100 μg/mL as reported by Sindhu [16]. In addition, the methanolic extract of B.micrantha (IC50 1.06+0.1μg/mL) showed good potent and selective inhibitor than A.calamus (IC50 1.26mg/mL) and N.sativa (IC50 1.53mg/mL) as reported by Balaji [17] against α-glucosidase. The IC50 (1.12+0.10 μg/mL) of methanolic extract of B.micrantha was better than the chloroform extract of IC50 (7.35+1.02 μg/mL) when tested against maltase glucoamylase and the results were better than the standard acarbose (IC50 234.6 ±2.01μM). However, the IC50 both for the extracts of B.micrantha against maltase glucoamylase showed good inhibitory potentials than the methanolic extracts of Artocarpus altilis (IC50 118.88+11.14μg/mL), A.heterophyllus (IC50 70.58+9.66 μg/mL), Cinnamomus zeylanicum (IC50 130.55+10.5 μg/mL) and piper betel (IC50 84.63+13.09 μg/mL) at concentrations ranged from 20 to 100 μg/mL as reported by Sindhu [16]. The β-glucosidase screening of the B.micrantha extracts showed that they do not potent and selective inhibition, the methanolic extract had 24.16+1.54% and chloroform extract had 24.53+3.64% inhibitory potential against β-glucosidase and these values were less than the Castonospermine standard (59.98%) as reported by Verma [14].
Moreover, the good potent and inhibitory potentials of the extracts of B.micrantha against α-glucosidase and maltase glucoamylase are good indication that the plant possesses therapeutic properties. The identification of bioactive compounds as revealed by gas chromatography Mass spectrophotometer has shown that the efficacy of the plant being used for the treatment of diabetes mellitus may not be unconnected to the presence of the these bioactive compounds both in the chloroform and methanolic extracts as many heterocyclic compounds have been found to possess various pharmacological activities against different ailments.
In addition, in the characterization, the results of GC-MS profile can be employed as a tool for the identification of novel compounds [18] as revealed in tables 2 to 3. It is interesting to note that some of the identified compounds in the crude extracts were screened computationally using online OSIRIS property explorer server [19] and were found to possess various drug properties as shown in Table 4.It has been reported that molecular properties which include bioavailability, hydrophobicity and membrane permeability are linked with some molecular descriptors as cLog P (partition coefficient),cLogS (solubility) number of H-bond acceptors and H-bond donors and molecular weight. It is documented that Lipinski's rule of five [20] is widely used to predict molecular drug-likeness. According to the rule, a drug like molecule has log P≤5, molecular weight <500 g/mol, hydrogen bond acceptors ≤10, hydrogen bond donors ≤5 and molar refractivity between 40-130. Molecules violating more than one of these rules are not expected to be viable drug candidates. The solubility parameter, log S, is another important parameter for determining drug likeness. The absorption of a compound is considerably influenced by its solubility. Generally, high log S values correspond to good absorption. Molecular polar surface area (PSA) is a very useful parameter for the prediction of drug transport properties (PSA must be ≤140 Å2). It is used to estimate the percentage of absorption using the expression %ABS=109-0.345PSA [21]. The online OSIRIS property explorer server has revealed the relevance and various drug properties of some of the identified compounds in the crude extracts using GC-MS and this could serve as a tool for the pharmaceutical chemists to further research on the plant as a potential anti-diabetic agent.
This study discovered the potent anti-diabetic inhibitory potentials of the methanolic and chloroform crude extracts of B. micrantha against α-glucosidase and maltase glucoamylase which can be highly beneficial for the treatment of diabetes mellitus type 2. Also, the online OSIRIS server explorer has revealed the drug properties of some of the identified compounds which will be beneficial to the scientists for further investigation. This study could be explored by researchers and a new anti-diabetic agent may be arrived at.
Dr. Adewole E. sincerely appreciates ‘The world academy of science’ (TWAS) for the Postdoctoral fellowship opportunity granted at the centre for Advanced Drug Research, COMSATS Institute of Information Technology, Abbottabad, Pakistan in 2017.
- Palanisamy UD, Ling LT, Manaharan T, Appleton D (2011) Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chem Toxicol 127: 21-27.
- Patel DK, Kumar R, Laloo D, Hemalatha S (2012) Natural medicines from plant source used for therapy of diabetes mellitus: an overview of its pharmacological aspects. Asian Pac J Trop Biomed 2: 239-250.
- Patel DK, Prasad SK, Kumar R, Hemalatha S (2012) An overview on antidiabetic medicinal plants having insulin mimetics property. Asian Pac J Trop Biomed 2: 320-330. [Crossref]
- Sunila C, Agastian P, Kumarappan C, Ignacimuthu S (2012) In vitro antioxidant, antidiabetic and antilipidemic activities of Symplocos cochinchinensis (Lour.) S. Moore bark. Food Chem Toxicol 50: 1547-1553.
- Ghani A (2003) Medicinal Plants of Bangladesh. second editon 55: 402-500
- Omeh YN, Onoja SO, Ezeja MI, Okwor PO (2014) Subacute antidiabetic and in vivo antioxidant effects of methanolic extract of Bridelia micrantha (Hochst Baill) leaf on alloxan-induced hyperglycaemic rats. J Complement Integr Med 11: 99-105.
- Nwaehujor CO, Igile GO, Ode JO, Udegbunam RI (2014) Anti-Inflammatory activities of methanol leaf extract of Bridelia micrantha (Hochst) Baill. (Euphorbiaceae) in wistar rats. J Appl Pharm Sci 5: 68-73.
- Adika OA, Madubunyi II, Asuzu IU (2012) Anti-diabetic and antioxidant effects of the methanol extract of Bridelia micrantha(Hochst) Baill. (Euphorbiaceae) leaves on alloxan-induced diabetic albino mice. Comp Clin Pathol 21: 945-951.
- Nwaehujor CO, Udeh NE (2011) Screening of ethyl acetate extract of Bridelia micrantha for hepatoprotective and anti-oxidant activities on wistar rats. Asian Pac J Trop Med 4: 796-800.
- Steenkamp V (2003) Traditional herbal remedies used by South African women for gynaecological complaints. J Ethnopharmacol 86: 97-108. [Crossref]
- Mburu C, Kareru1 PG, Kipyegon C, Madivoli ES, Maina EG, et al. (2016) Phytochemical Screening of Crude Extracts of Bridelia micrantha. European Journal of Medicinal Plants 16: 1-7.
- Ma HY, Gao HY, Sun L, Huang J, Xu XM, et al. (2011) Constituents with alpha-glucosidase and advanced glycation end-product formation inhibitory activities from Salvia miltiorrhiza Bge. J Nat Med 65: 37-42.
- Pérez M, Muñoz FJ, Muñoz E, Fernández M, Sinisterra JV, et al. (2008) Synthesis of novel glycoconjugates and evaluation as inhibitors against ß-glucosidase from almond. J Mol Catal B Enzym 52: 153-157.
- Verma N, Behera BC, Sharma BO (2012) Glucosidase inhibitory and radical scavenging properties of lichen metabolites salazinic acid, sekikaic acid and usnic acid. Hacett J Biol Chem 40: 7-21.
- Rammohan S, Zaini Asmawi M, Amirin S (2008) In vitro a-glucosidase and a-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochimica Polonica 55: 391-398
- Sindhu SN, Vaibhavi K, Anshu M (2013) In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. European Journal of Experimental Biology 3: 128-132.
- Balaji RM, Chitra J, Meenakshi K, Sundaram (2015) Studies on Antidiabetic Activity of Indian Medicinal Plants Using –Amylase and -Glucosidase Inhibitory Activity - A Pathway to Antidiabetic Drugs. World Journal of Medical Sciences 12: 207-212.
- Shettar AK, Sateesh MK, Kaliwal BB, Vedamurthy AB (2017) In vitro antidiabetic activities and GC-MS phytochemical analysis of Ximenia americana extracts. South African Journal of Botany 111: 202-211.
- Sander T (2001) OSIRIS property explorer. Allschwil: Actelion Pharmaceuticals Ltd. Available at http://www.organicchemistry. org/prog/peo/index.html.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46: 3-26. [Crossref]
- Wang RX, Fu Y, Lai LH (1997). ‘A new atom-additive method for calculating partition co-efficients. J Chem Inf Comput Sci 37: 615-621.