Abstract
Background
Lung volume reduction surgery (LVRS) has been shown to improve dyspnoea, pulmonary function, exercise tolerance and quality of life in properly selected patients with pulmonary emphysema. However, the ultimate impact of LVRS on gas exchange is still a matter of debate. The current work tries to provide an insight into this matter.
Methods
Study group of 39 patients undergone to LVRS and reached a follow-up time of 2 years. Pre and postoperative measurements of arterial blood gases (ABG) at rest and the diffusing capacity of the lung (DLCO), 6-min walking distance test and dyspnoea score were recorded at 3, 6, 12, 18 and 24 months after LVRS.
Results
After LVRS, an improvement of preoperative partial pressure of carbon dioxide (PaCO2) (Mean ± SD: 38.5 ± 0.9 mm Hg) of 8% occurred in 3 months and was retained up to 12 months compared to preoperative value. The decrease of PaCO2 correlated weakly with the increase of the forced expiratory volume in one second (FEV1) and vital capacity (VC). Mean preoperative partial pressure of oxygen (PaO2) (65.2 ± 1.5 mm Hg) in 6 months after LVRS was temporarily improved by 8%. The individual gas exchange parameter alveolar-arterial oxygen gradient (AaDO2), and therefore, PaO2 were not predictable by assessment of lung mechanics, other biometric parameters, or emphysema morphology. Preoperatively and 6 months after LVRS the same fraction of patients (10 %) fulfilled the ABG criterion PaO2< 55 mm Hg for long-term oxygen treatment (preop. 10/101 patients; 6 months postop. 8/82 patients.
Conclusion
We observed a slight improvement in PaO2 for up to 6 months after LVRS, which is secondary to an increase in alveolar ventilation as reflected by a drop in PaCO2. Gas exchange as assessed by AaDO2 and DLCO remained unchanged and a weak correlation between fractional change of FEV1 and VC and change in PaCO2 have been observed. Overall, LVRS temporarily improved alveolar ventilation and resting gas exchange, but on an individual basis, improved or worsened gas exchange and alveolar ventilation at rest.
Keywords
Thoracoscopic lung volume reduction surgery, emphysema, gas exchange, chronic obstructive pulmonary disease, pulmonary function, dyspnea.
Introduction
Emphysema is a progressive atypical and permanent dilatation of the airspaces distal to terminal bronchioles by mainly inflammatory processes including proteinases and apoptosis, declining the alveolar and the capillary surface area, i.e. the surface for gas exchange and decreasing lung elasticity [1]. A reduction in lung elastic recoil occurs that predisposes lung hyperinflation and is accompanied by decreased inspiratory capacity. An increase in functional residual capacity occurs which my lead to contractile dysfunction of the inspiratory muscles [1-5]. Abnormal configuration of the diaphragm generated by emphysema may further lead to dyspnoea [2,3,6].
LVRS is an efficient and approved surgical treatment for patients with emphysema and hyperinflation [7]. It represent a bridge and sometimes even substitution to lung transplantation [8] in highly selected patients [8]. LVRS improves lung compliance by better matching the size of the lungs to the size of the thorax [9] and results i) Improvement in lung elastic recoil at analogous thoracic inspiratory volume [10,11], expiratory flow [12] and the mechanical efficiency of healthier parts of the lung; ii) Improvement of FEV1, vital capacity (VC), total lung capacity (TLC) and residual volume (RV) [13]; iii) Reduction in static hyperinflation and dynamic hyperinflation and air trapping causing the improvement of exercise capacity [14-16]; iv) Improving respiratory muscles’ function by returning inspiratory muscles including the diaphragm to their optimal length-tension ratio [4,11,16]; v) small increase in DLCO, but on average no crucial changes in gas exchange [9]; vi) improvement in quality of life [13] and in part in survival [17].
Choice of appropriate candidates, usually by a multidisciplinary approach, can greatly affect the success of LVRS [8]. Accordingly, considering emphysema morphology, target location (s), pulmonary function parameters, cardiac comorbidities, etc. are of high importance [8]. Most centres use comparable LVRS inclusion criteria [8] but in general, patients with heterogeneous/homogeneous emphysema morphology, substantial impaired diffusion capacity, moderate pulmonary arterial hypertension, a history of previous LVRS and alpha 1 -antitrypsin (A1AT) deficiency can be considered as candidates for LVRS [17]. The large multicentre National Emphysema Treatment Trial (NETT) evidenced the improvement of lung function, exercise capacity, dyspnoea and eventually survival rate of patients (n=1218) who undergone LVRS surgery compared to those that received medical therapy predominantly in the subset of subjects with upper lobe dominant emphysema and low baseline exercise capacity [18]. A high-risk subgroup was recognised, which had a baseline % predicted (FEV1) of ≤20%, harbouring either a homogeneous distribution of pulmonary emphysema or % predicted (DLCO) <20% [18,19].
Exclusion criteria mentioned by Caviezel et al. (2018) recommends daily steroid dose of > 20 mg, CT morphology of significant bronchiectasis, lung function parameters measure of FEV1 < 20% and DLCO < 20% in the homogeneous emphysema, 6-minute walk distance (6 MWD) > 600 m and gas exchange of paCO2 > 50 mm Hg and paO2 < 45 mm Hg in homogeneous emphysema [8]. In patients with A1AT deficiency, more heterogeneous emphysema and the less frequent the infection exacerbations seem to be associated with successful LVRS [20,21]
In patients with severe COPD different levels of hypoxemia or hypercapnia have been observed [16]. Some studies have suggested exclusion of patients with moderate to severe hypercapnia (PaCO2 > 50 to 55 mm Hg) from LVRS [18,22] due to critical level of parenchymal lung destruction [22]. However, the changes in post-LVRS PaCO2 exhibits marked intersubjective variability, LVRS is supposed to enable the lung and chest wall to function more efficiently as a pump, and therefore, augment alveolar ventilation and alleviate baseline resting PaCO2 [16]. Accordingly, patients with higher baseline levels of PaCO2 demonstrate the greatest reduction in PaCO2 post-LVRS, [16] One study suggests that the heterogeneity of emphysema in CT scan is more critical than blood gas analysis for assessment of prognosis [23].
One area of concern is the effect of LVRS on gas exchange. We performed a retrospective study, which has been conducted between years 1994 to 1998, we aim to shed the light on the postoperative outcome of LVRS on lungs diffusing capacity and resting gas exchange. Hence, in a group of patients who were eligible to participate in such a study, the fundamental parameters related to blood gas and gas exchange as well as lung function parameters, exercise test and dyspnoea score have been measured at defined intervals in a single-centre closed cohort population of 39 patients for two years after bilateral thoracoscopic LVRS. The closed cohort study allowed best to study physiological changes over time, studied in those 39 patients over two years.
Methods
Patients
In this retrospective study of a single centre study, patient data from every eligible patient for LVRS have been collected from the patients with severe emphysema who undergone bilateral LVRS by video-assisted thoracoscopy (VAT) by one single surgeon (WW) between August 1994 and December 1998. Ethical approval and written patient consent were given. We defined two major groups. From the whole cohort of 101 patients, all 39 patients were the subject of study as they could reach the five postoperative episodes of follow-up in 24 months in order to pathophysiologically best analyze a close cohort. Therefore, we called them study group. 101 consecutive patients (38 women) with severe emphysema underwent bilateral LVRS by video-assisted thoracoscopy (VAT) at our institution between August 1994 and December 1998. Their mean age at operation was 63 years (SE: ± 1 y; range: 38 - 78 years). 13 of them had ZZ homozygotous a1-antiprotease deficiency.
Preoperatively, all patients were severely symptomatic with a mean modified Medical Research Council (MRC) dyspnea score of 3.6 ± 0.1 (mean ± SE). They had severe airflow obstruction with a mean forced expiratory volume in one second (FEV1) of 0.78 ± 0.02 L which was 28 ± 1 % predicted, a mean total lung capacity of 8.27 ± 0.14 L (137 ± 2 % predicted), a mean residual volume of 5.36 ± 0.10 L (241 ± 5 % predicted) and a mean RV/TLC ratio of 0.65 ± 0.01. Mean PaCO2 was normal with 39 ± 1 mm Hg, whereas mean PaO2 was 66 ± 2 mm Hg. Carbon monoxide (CO) diffusing capacity was decreased to 43 ± 2 % of predicted. Nine deaths occurred in the 2 years follow-up after LVRS, one of them being perioperatively, 8 postoperatively, and with no significant difference between preoperatively hypercapnic patients compared to the rest of the cohort. Loss of follow-up - excluding death - occurred during the 2 years postoperatively in four patients, one of them by lung transplantation. (Table 1)
|
Whole cohort |
Study group |
Number of subjects |
101 |
39 |
Mean age |
63 ± 1 |
65 ± 1 |
Gender |
|
|
Female |
38 |
13 |
Male |
63 |
26 |
Patients with alpha 1-antiprotease deficiency |
13 |
3 |
Preoperative residual volume (% predicted) |
254 ± 9%; p=0.017 |
228 ± 8%; p=0.017 |
Pulmonary hyperinflation |
|
|
TLC |
8.27 ± 0.14 L = 137 ± 2% pred. |
|
RV/TLC |
0.660; p=0.048 |
0.624; p=0.048 |
Pulmonary function |
|
|
FEV1 |
0.77 ± 0.02 L/s = 28 ± 1% pred. |
|
VC |
2.91 ± 0.08; = 81 ± 2% pred. |
|
Dyspnoea scale (mMRC score) |
3.6 ± 0.1 |
3.5 ± 0.1 |
Table 1. Demographic characteristics of patients in both groups at baseline
Clinical and functional evaluation
Evaluation has been performed with recording medical history, radiographic, computed tomography (CT) and scintigraphy examinations preoperatively and post operatively ( five evaluation episodes in 3, 6, 12, 18 and 24 month after operation). In addition, clinical evaluation such as pulmonary function tests, arterial blood gas analysis at rest, determination of the 6 MWD, and the assessment of dyspnoea were carried out at each time point. Appraisal condition was upon patients’ stable status, otherwise, they examinations were postponed to maximally to one month.
Measure of dyspnoea scale: Dyspnoea was rated in accordance with the definition of the American thoracic society's Modified Medical Research Council (MRC) Scale [24]. Hereupon, the degree of dyspnoea is described by grading with an integer from zero to four. Zero indicates breathlessness as a result of strenuous exercise, while four represents the disability of the patient to depart from the house or breathlessness while dressing.
Measure of Pulmonary function: Pulmonary function testing was performed after inhalation of two puffs of salbutamol, adhering to standard criteria [25] with the Sensor Medics Autobox plethysmograph (Yorba Linda, CA, USA).
6-minute walk test: For assessment of 6 MWD, the patients walked uncoached and without oxygen supplementation along the hospital hallway.
Arterial blood gas analysis at rest: Arterial blood gas was determined at rest, while breathing room air in an upright sitting position at our institution using an AVL 993 haemoximeter (AVL Medical Instruments Inc., Schaffhausen, Switzerland) after puncture of about 1 ml of blood from the patients’ radial artery with a powder pre-heparinized syringe. The sample was analysed within 2 minutes after the puncture.
Measure of alveolar-arterial oxygen gradient (AaDO2): AaDO2 was calculated using the following formula [26]:
AaDO2 (mm Hg) = PAO2 - PaO2 = (FIO2 (Pb - PH2O) - PaCO2/R - PaO2 |
PAO2: alveolar oxygen partial pressurePaO2: arterial oxygen pressure
FIO2: fraction of inspired O2. It is assumed to be 0.21
Pb: barometric (atmospheric) pressure = 760 mm Hg at sea level
PH2O: vapor pressure of water, which is 47 mm Hg at body temperature and Pb=760 mm Hg
PaCO2: the CO2 tension of alveolar gas
R: respiratory coefficient, for people consuming a standard diet = 0.8
AaDO2 was only calculated, as direct measurement might influence resting blood gases, and therefore lead to more exact, but potentially less clinically relevant values.
Surgical technique
LVRS was performed bilaterally by video-assisted thoracoscopy. The most destroyed zones of lung parenchyma, were specified by CT scans and perfusion scintigrams as the target areas for resection and removed using buttressed or non-buttressed endoscopic staplers (Endo-GIA 30 and 60, Auto Suture, United States Surgical Corporation™, Norwalk, CT, USA, or ECL-45, Ethicon Endo-Surgery, Cincinnati, OH, USA) [27]. In cases which with homogeneous emphysema morphology no special target areas could be detected. Thus, the resection was performed mostly in the upper lobes. Approximate volume of 20 to 30% of lung volume on each side was removed, as estimated during operation by the surgeon. Bilateral thoracoscopic LVRS was followed by mean hospital stay of 16 ± 1 days and mean drainage time of 9.7 ± 0.7 days).
Target zones for LVRS were selected using CT scans.
Data analysis
Descriptive statistics, two-tailed tests, and linear regressions were used. Paired or unpaired t-test or analysis of variance followed by Tukey post hoc test, where appropriate, were performed to detect differences in values within the same or between groups. Survival differences between groups were analysed using a stratified Cox regression model and using Kaplan-Meier survival curves. Data were analysed using the SYSTAT for Windows® software package, release 8.03 (SPSS Inc., Chicago IL, USA). Results were expressed as mean values and standard error. A p-value ≤ 0.05 was considered significant.
Literature survey
PubMed Subject Headings (MeSH) database was used to find other studies. The terms implemented in the advanced MeSH search engine were pneumonectomy and pulmonary gas exchange in the form ("pneumonectomy" [mesh]) and ("pulmonary gas exchange" [mesh]). This resulted in 391 articles (July 2022), which were further screened. Studies with animal species as well as studies with insufficient/irrelevant data and those with a follow-up of fewer than 12 months were excluded. In addition, studies authored in some languages such as Russian, Japanese, etc. were excluded due to our limited knowledge of those languages. However, this method seems not to be sufficient to have all studies. For example, the study by Pompeo, et al. (2012) [28] did not include 391 results. Therefore, supplementary searches in PubMed and Google Scholar have been performed to provide better coverage in finding most relevant studies.
Results
Preoperative and postoperative characteristics in the study group
Preoperative and postoperative measurements including gas exchange, lung function, exercise test and dyspnoea scale in study group of 39 patients upon 24 months follow up have been presented in the table 2.
|
|
Preoperative measurements |
Postoperative measurements |
|
|
|
After 3 Months |
After 6 Months |
After 12 Months |
After 18 Months |
After 24 Months |
Blood gas and gas exchange parameters |
PaCO2 (mm Hg) |
38.5 ± 0.9 |
35.6 ± 0.7* |
36.4 ± 0.7* |
35.8 ± 0.8* |
36.9 ± 1.1 |
36.9 ± 0.9 |
PaO2 (mm Hg) |
65.2 ± 1.5 |
70.4 ± 1.7* |
69.5 ± 1.7* |
67.7 ± 1.6 |
64.7 ± 2.0 |
65.3 ± 1.7# |
AaDO2 (mm Hg) |
28.7 ± 1.7 |
27.6 ± 2.0 |
28.0 ± 1.7 |
30.3 ± 1.9 |
31.0 ± 2.6 |
31.1 ± 1.8# |
DLCO (ml/min×mm Hg) |
3.83 ± 0.22 |
4.06 ± 0.19 |
4.13 ± 0.19 |
3.89 ± 0.20 |
3.68 ± 0.24 |
3.57 ± 0.20# |
DLCO (% pred.) |
46 ± 3 |
48 ± 2 |
49 ± 2 |
46 ± 2 |
44 ± 3 |
43 ± 2# |
Lung function parameters |
FEV1 (L/s) |
0.79 ± 0.04 |
1.26 ± 0.10* |
1.21 ± 0.09* |
1.14 ± 0.09* |
1.10 ± 0.09* |
1.02 ± 0.08*# |
FEV1 (% pred.) |
28 ± 1 |
45 ± 2* |
43 ± 2* |
40 ± 2* |
40 ± 2* |
37 ± 2*# |
IVC (L) |
2.99 ± 0.14 |
3.69 ± 0.19* |
3.77 ± 0.18* |
3.71 ± 0.19* |
3.48 ± 0.20* |
3.34 ± 0.18*# |
IVC (% pred.) |
82 ± 3 |
100 ± 2* |
102 ± 2* |
100 ± 3* |
96 ± 3* |
92 ± 3*# |
RV (L) |
5.13 ± 0.16 |
3.88 ± 0.15* |
3.90 ± 0.17* |
4.04 ± 0.17* |
4.09 ± 0.18* |
4.29 ± 0.20*# |
RV (% pred.) |
277 ± 8 |
171 ± 7* |
172 ± 8* |
177 ± 7* |
179 ± 8* |
186 ± 9*# |
RV/TLC |
0.63 ± 0.01 |
0.51 ± 0.02* |
0.51 ± 0.02* |
0.52 ± 0.02* |
0.54 ± 0.02* |
0.55 ± 0.02*# |
Exercise test |
6 MWD (m) |
266 ± 14 |
364 ± 12* |
370 ± 14* |
404 ± 19* |
344 ± 18* |
338 ± 19* |
Dyspnoea scale |
mMRC score |
3.5 ± 0.1 |
1.5 ± 0.2* |
1.5 ± 0.2* |
1.6 ± 0.1* |
1.9 ± 0.2* |
2.0 ± 0.2*# |
| |
|
|
|
|
|
|
|
|
*compared to preoperatively: p£ 0.05; #compared to 3 months postoperatively: p£ 0.05
6 MWD: 6-minute walk distance; AaDO2: alveolar-arterial oxygen gradient; DLCO: diffusing capacity for carbon monoxide; FEV1: first second of forced expiration; IVC: inspiratory vital capacity; PaCO2: partial pressure of carbon dioxide; PaO2: alveolar oxygen partial pressure; pred.: predicted; RV: Residual volume; TLC: Total lung capacity.
Table 2. Gas exchange, lung functional, exercise test and dyspnea parameters study group (n=39, Mean Values ± standard error
Table 3a. Summary of results of clinical trials issuing Lung Volume Reduction Surgery with 12 or 24 months follow up
(In excel sheet)
Parameters of alveolar ventilation and gas exchange at rest: PaCo2: Alveolar ventilation in terms of mean PaCO2 slightly decreased between preoperative and 3 months postoperative stages, reflected by a slight drop of mean PaCO2 of 8% or 2.9 mm Hg from 38.5 ± 0.9 mm Hg to 35.6 ± 0.7 mm Hg (table 2, figure 1 and 2), corresponding to an individual PaCO2 decrease of 4.2 ± 1.2%. Compared to preoperative values, this decrease remained significant up to 12 months after the operation, which was no longer the case 24 months after LVRS (data is not shown). The 3 months postoperative PaCO2 was the lowest postoperative value observed.
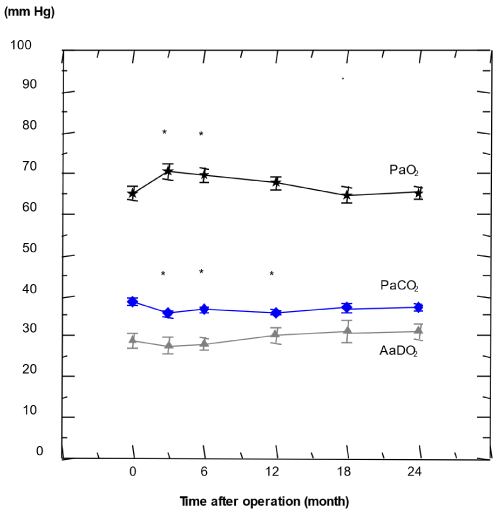
Figure 1. Resting blood gas parameters PaCO2, PaO2, and the calculated AaDO2 in the study group (n= 39) from preoperative values (set as 0 months) up to 24 months after LVRS; Results are given as mean and standard error (p < 0.05 vs. preop.)
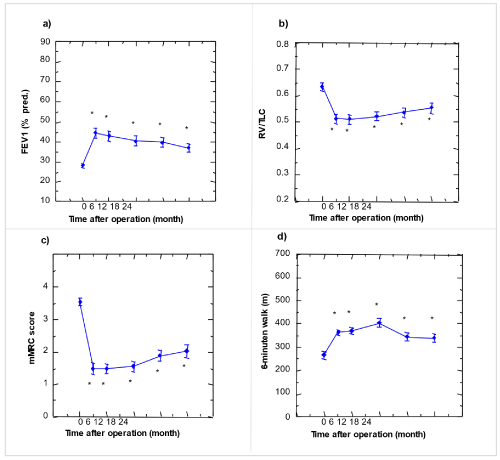
Figure 2. Lung function parameters, exercise test measure and dyspnoea scale, preoperatively up to 24 months after LVRS in the study group (n=39)
a) FEV1; b) RV/TLC quotient; c) 6-minutes walking distance; d) mMRC score. Results are given as mean and standard error. (* p < 0.05 compared to preoperative 0: preoperative)
Figure 3. LVRS induced changes in lung function and alveolar ventilation in study group (n=39)
PaO2: A significant increase of the mean PaO2 of 8% or 5.2 mm Hg from 65.2 ± 1.5 to 70.4 ± 1.7 mm Hg was observed 3 months after LVRS if compared to preoperative values (table 2, figure 1 and 2), whereas the individual PaO2 increased by 7.2 ± 1.5%. The increase was less pronounced, but still significant 6 months after LVRS, whereas 12 months after LVRS it was no longer different from preoperative value [29-35].
AaDO2: During the whole of the study period the main parameter of pulmonary gas exchange, mean AaDO2, was almost unchanged at rest (table 2, figure 1 and 2). At 6 months after LVRS the fraction of hypoxemic patients with a PaO2 of 55 mm Hg or lower remained unchanged at 10% of patients if compared to the preoperative situation. Furthermore, on an individual basis, the 3 months postoperative AaDO2 was erratic when preoperative resting blood gases were known. The correlation of both pre- and postoperative PaCO2 with their corresponding PaO2 (preop.: r=-0.37; p<0.0001; 3 months postop.: r= -0.28; p=0.01) was weak which suggested a rather high variation in gas exchange, i.e. of AaDO2 before as well as after LVRS. This suggests the rather erratic individual evolution of gas exchange by LVRS (figure 1).
DLCO: There was no significant increase of DLCO after LVRS. However, there was a significant decrease of DLCO 24 months after operation compared to DLCO at three, six, or twelve months postoperatively (table 2).
KCO (DLCO/VA): There was a steady and highly significant decline of 16% of the KCO from preoperatively of 0.92 ± 0.05 ml/min*mm Hg*L to 24 months postoperatively of 0.79 ± 0.04 ml/min*mm Hg*L, but no change by LVRS, i.e., between preoperative and 3 months postoperative KCO.
Overall, between 3 and 24 months postoperatively, mean PaO2, AaDO2, and DLCO deteriorated slightly, but significantly. Such a slight and significant deterioration was also observed in the lung functional parameters including FEV1, VC, RV, and RV/TLC, and in MRC dyspnea score, but not in 6-minute walking distance (table 2, figure 1 and 2).
Static and dynamic lung volumes: FEV1: Related data in the table 2 and figure 2 show a significant increase of FEV1 from the preoperative value of 0.79 ± 0.04 (L/s) to 1.26 ± 0.10 three months after surgery. At 6,12,18 and 26 months after LVRS, still FEV1 shows a greater value that before operation. However, in 3 months after LVRS FEV1 reached its maximum value.
IVC (L): IVC reaches its maximum value at 3 (3.69 ± 0.19) and 6 months (3.77 ± 0.18) after LVRS, while before operation it was less and after 6 months also it has downward trend (table 2).
RV (L): Preoperative value of RV shows 5.13 ± 0.16 (L), while three months post operation it is significantly reduced to 3.88 ± 0.15 (L). Although 6,12,18, and 24 months postoperative measures shows increasing trend, it still could not reach its preoperative value (table 2).
RV/TLC: Related values shown in table 2 and figure 2, demonstrate a significant decline of RV/TLC from a preoperative value of 0.63 ± 0.01 to 0.51 ± 0.02 three months after LVRS. In the rest of measuring times, it harbored increasing but nevertheless it did not reach its preoperative value.
Exercise test: Preoperational 6-min walking distance shows 266 ± 14(m), while postoperative values are between 364 ± 12 in 3 months after LVRS to maximum of 404 ± 19 (m) twelve months after LVRS. Generally, all postoperative values are significantly more than preoperational measure (table 2 and figure 2).
Dyspnoea score: There was a substantial improvement in modified MRC dyspnoea score from 3.5 ± 0.1 preoperatively to 1.5 ± 0.2 three months after LVRS. This result was deteriorated up to 24 months (2.0 ± 0.2; p<0.05 vs. preop.; table 2 and figure 2), however it is still better than preoperative baseline [36-39].
Blood gas versus lung function relationships
Correlations of preoperative and 3 months postoperative blood gas parameters in study group (n=39): Preoperative PaCO2 and PaO2 correlated weakly (r= -0.37; p<0.0001). Also, preoperative PaCO2 correlates with AaDO2 (r= -0.35; p=0.001). Three months postoperatively, PaCO2 weakly correlated with PaO2 (r=-0.28; p=0.01) as well as PaCO2 with AaDO2 (r=-0.29; p=0.007) (figure 2).
A higher negative correlation was found between the difference in preoperative to 3 months postoperative PaCO2 and preoperative PaCO2 (r=-0.59; p<0.0001).
Correlations of preoperative and postoperative blood gas parameters with lung functional, dyspnoea and walking test parameters: Whereas weak correlations of PaCO2 were found with lung volumes, resting arterial PaO2 did neither correlate with lung functional parameters, nor with MRC dyspnoea score, 6 MWD, or DLCO. The same was true with the gas exchange parameter AaDO2. Also, multiple regression did not reveal relations between 3 months postoperative PaO2 or DPaO2 and the lung functional parameters including DLCO.
At 3 months after LVRS, VC showed the best correlation with PaCO2 (r=-0.43; p<0.0001), followed by FEV1 (r=-0.37; p=0.001) and RV/TLC (r=0.34; p=0.001).
Are there predictors of blood gas parameters after LVRS (n=39)?
There was a significant decline of mean PaCO2 between preoperative and 3 months postoperative resting ABG. Therefore, the question was addressed whether changes in PaCO2 were related to lung functional parameters. There were significant correlations found between ΔPaCO2 and the fractional change of FEV1 compared to preoperative value (Δ%FEV1pre-3mo; r=-0.45; p=0.0001) as well as between PaCO2 and the fractional change of VC compared to preoperative VC (Δ%VCpre-3mo; r=-0.37; p=0.002), but not with the change in RV/TLC quotient between preoperative
and 3 months postoperative value (ΔRV/TLC) (figure 3).
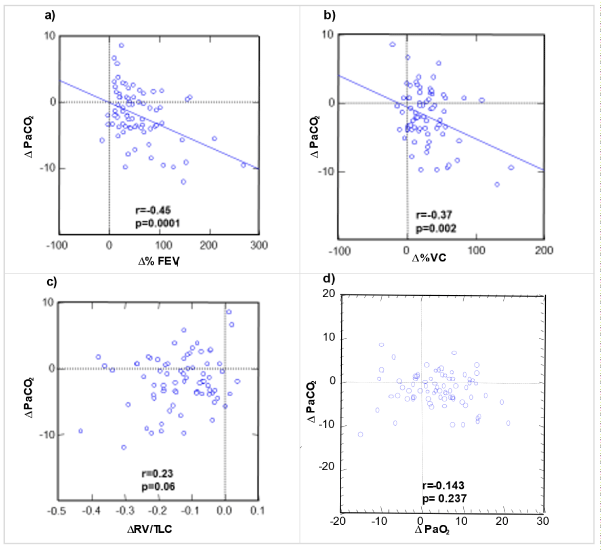
Relationships between preoperative and 3 months postoperative change in PaCO2 (alveolar ventilation) and the fractional changes of FEV1 (a) and VC (b), RV/TLC ratio (c), and PaCO2 (d). The solid lines represent regression lines where significance level was achieved and underline the negative correlations of FEV1 change and VC change with the change in resting PaCO2, and, thus, the importance of lung volumes for the alveolar ventilation at rest.
Importantly, there was no correlation between ΔPaCO2 and ΔPaO2 (r=0.14; p=0.24; figure 4d3d). Thus, in an individual patient with given preoperative PaCO2 pre and PaO2 pre and a given PaCO2 3 months the corresponding individual’s PaO2 3 months is not predictable. Accordingly, as at a given PaCO2 3 months the PaO2 3 months is not predictable, the resulting AaDO2 is also not predictable. No correlation between preoperative and 3 months postoperative changes in resting PaCO2 (alveolar ventilation) and the corresponding change in PaO2 at rest means that in case of an individual preoperative blood gas analysis with given PaCO2 pre and PaO2 pre and a given postoperative PaCO2 3 months, the corresponding individual’s PaO2 3 months is not predictable. Consequently, the resulting AaDO2 of an individual patient is also not predictable. In the severely debilitated emphysema patient before LVRS, both RV/TLC (r=0.34; p=0.001) and the related FEV1 (r=-0.32; p=0.002) correlated with PaCO2. No correlation was found between ΔPaCO2 and ΔPaO2 (figure 3).
LVRS- induced changes in PaCO2 between preoperative and 3 months postoperative controls, on the other hand, did not correlate with the changes in RV/TLC attained by LVRS, but with corresponding changes in FEV1 or VC. Both regression lines passed near zero of both ΔPaCO2 and the %change of the lung function parameter and illustrate the weak relationship between corresponding LVRS-mediated differences in PaCO2 and gains in FEV1 or VC that apparently determined alveolar ventilation in our study population. Thus, in an individual patient with given preoperative PaCO2 pre and PaO2 pre and a given PaCO2 3 months the corresponding individual’s PaO2 3 months is not predictable. Accordingly, as at a given PaCO2 3 months the PaO2 3 months is not predictable, the resulting AaDO2 is also not predictable. This means that blood gas changes induced by LVRS do not predict resting AaDO2 as the central parameter of resting pulmonary gas exchange.
Evolution of 12 preoperatively hypercapnic patients (PaCO2 > 45 mm Hg)
12 patients (4 women) had preoperatively PaCO2 > 45 mm Hg (mean, 47.0 ± 0.04 mm Hg; range, 45.3 - 48.7 mm Hg). Biometric data like age, sex, body mass index, or number of pack-years smoking history did not differ from the rest of the study population; however, only preoperative, but not postoperative FEV1 (preop.: 23.3 ± 1.7 vs 28.9 ± 0.7 % pred.; p=0.012), VC (preop.: 71.3 ± 5.9% vs. 82.6 ± 1.7%; p=0.039) and inspiratory capacity (24.6 ± 1.7 vs. 30.5 ± 0.8 IC/pred. TLC; p=0.018) were lower in hypercapnic patients. Hypercapnic patients gained significantly more increase in FEV1 as well as in VC than the rest of the study population (p=0.02 and p=0.006, respectively), and decreased PaCO2 between preoperatively and 3 months postoperatively by - 6.9 ± 1.3 mm Hg in contrast to the remainder of patients where a decrease of - 1.3 ± 0.4 mm Hg was observed (p<0.0001 for both groups). Neither preoperatively, nor postoperatively, RV/TLC, DLCO, or 6-minute walking distance differed significantly. Preoperatively, the hypercapnic patients' PaCO2 was significantly higher (p<0.0001) and AaDO2 (23.6 ± 1.4 vs. 28.1 ± 1.1 mm Hg; p=0.038) significantly lower than that of the rest of the population. However, PaO2 was significantly lower (60.1 ± 2.5 vs. 66.3 ± 0.9 mm Hg; p=0.013) than in the remainder of the study population. Figure 4 shows the evolution over time of the PaCO2 values of the preoperatively hypercapnic patients and the rest of the population and demonstrated a new increase of PaCO2 in the evolution with a difference compared to the rest of the study population which was above significance level 18 and 24 months after LVRS.
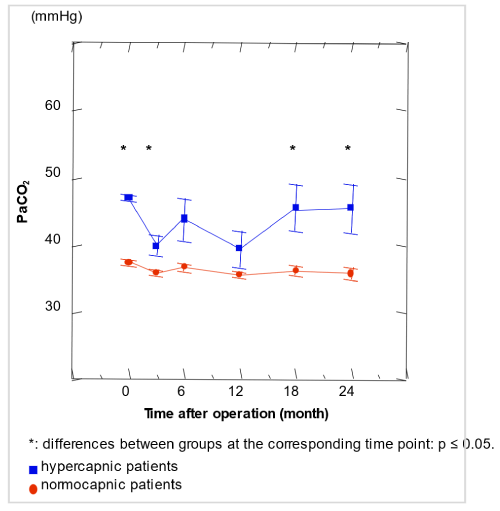
Figure 4. Evolution of preoperatively hypercapnic patients resting PaCO2 (n=12) compared with the rest of the LVRS study group (n=27 normacapnic); N=39 and Results are given as mean and standard error
Patients with a preoperative PaO2 £ 55 mm Hg
Preoperatively 10 of 101 (10%) patients fulfilled the criterion of PaO2 ≤ 55 mm Hg which is main criterion for long-term oxygen supplementation. At 6 months and at 24 postoperatively, 8 patients of 82 (10%) respectively 7 of 39 patients (18%) fulfilled this criterion. 8 of the 10 patients (80%) with a preoperative PaO2 ≤ 55 mm Hg fulfilled within the 24 months following LVRS again this criterion at least once. On the other hand, 17 patients of the whole study cohort who did not have preoperative PaO2 ≤ 55 mm Hg developed hypoxemia with this criterion within the 24 months after LVRS.
Discussion
Some surgical lung volume reduction studies showed no gas exchange improvements [40], some improvements in PaCO2 [16] or in PaO2 [41], and a number of them in both [42]. The current study can be categorized into the latter group, as it exhibits significant but only temporary improvements of both PaCO2 and PaO2 at rest. As we showed, both the gas exchange parameters AaDO2 and PaO2 were uncorrelated to parameters of lung mechanics, which might be an indication of more complex changes by LVRS. These data suggest that after LVRS gas exchange is basically unpredictable and not favorably changed. Therefore, LVRS may have improved or worsened the individual’s gas exchange in an unpredictable way.
The arising question is whether the temporary improvement of PaO2 at 3 and 6 months post-LVRS is explained by the temporary improvement of alveolar ventilation. Both mean PaCO2 and mean PaO2 improved between preoperatively and 3 months post-LVRS, whereas AaDO2 was unchanged. This finding suggests that the mean gas exchange was unchanged between those two assessments. If we assume AaDO2 to be “constant”, i.e., the same for an individual preoperatively as well as 3 months postoperatively, then the equation ΔPaO2 = ΔPaCO2/respiratory quotient (RQ) derived from the simplified alveolar gas equation assumes a linear relationship between the two parameters (RQ has not been measured in our setting and is taken as 0.8).
The comprehensive analyses of literature conducted by van Dijk and co-workers corroborate our prediction [9]. They accentuate that even if there might be a slight improvement in postoperative DLCO, relatively variable effects on an individual level can be achieved, ranging from a negative to a large beneficial effect [9]. However, due to the various methods which have been employed to receive data, it seems not possible to conclude whether this increase remains statistically significant [9].
This assumption is considered to be reasonable, based on observations with patients who suffered from somewhat less severe COPD [43]. In COPD patients AaDO2 chiefly depends on ventilation-perfusion inequality [44]. As there seemed to represent no relation between any of our measured parameters and changes of preoperative and 3 months postoperative AaDO2 in our population, LVRS seemed to influence ventilation-perfusion inequalities and accordingly AaDO2 in a rather erratic way. As bronchodilators decrease PaO2 in COPD patients in the range of 3 - 4 mm Hg probably by vessel dilation that chiefly results in ventilation-perfusion inequality [45], it seems plausible that LVRS, by increasing elastic recoil and thus improving obstructive lung function generally in a considerably more significant order of magnitude than any bronchodilator, may also similarly exert critical effects on vessels geometry and thus possibly also vessel tone.
Correlations of PaCO2 changes with the lung volumes VC and FEV1 suggest a primary role of lung mechanics for alveolar ventilation (figure 3). The findings suggest that hyperinflation in terms of RV/TLC influenced alveolar ventilation in preoperative severely obstructive patients in about the same magnitude as FEV1. Accordingly, good correlations between changes in FEV1 respectively VC and VA derived from the DLCO manoeuvre have been found, whereas changes of TLC did not show a relationship with improved VA [42]. Therefore, the increase in VA respectively the changes of PaCO2 seems to be associated with an improvement in spirometry parameters following LVRS but not with changes in hyperinflation [42].
Surprisingly, no correlation was found between ΔPaCO2 and ΔPaO2. This means that in an individual preoperative blood gas analysis with given PaCO2 pre and PaO2 pre and a given postoperative PaCO2 3 months the corresponding individual’s PaO2 3 months seemed erratic. Due to this dissociation of any individual 3 months postoperative PaCO2 from its corresponding PaO2 the resulting patients AaDO2 was also not predictable despite the assumed preoperative AaDO2 given by preoperative PaCO2, PaO2 and RQ. This further suggests that in an individual LVRS patient the 3 months postoperative resting AaDO2 could not be deduced from the patient's preoperative value. The alternative explanation that RQ may chiefly account for the striking variation between ΔPaO2 and ΔPaCO2 seems not very likely, but cannot be firmly excluded, as it has not been measured.
Surveying the influence of LVRS on PaCO2 indicates that mainly patients with high preoperative PaCO2 (e.g. hypercapnic patients) had 3 months postoperatively lowered PaCO2 values. This is corroborated by the negative correlation (r=-0.59; p<0.0005) between preoperative PaCO2 and the difference between pre- and three months postoperative PaCO2, which was not found in every patient and makes the finding of several LVRS groups who described no change in mean PaCO2 [16,43] well conceivable. These data suggest that the temporary improvement of PaO2 by LVRS in our population is not explained by changes in alveolar ventilation but presumably mainly by the unpredictable alterations in ventilation-perfusion heterogeneity. The same was concluded by other studies based on pre- and postoperative blood gas analysis of 46 patients undergoing LVRS [43].
The evolution of preoperative hypercapnic patients shows that when comparing 12 preoperatively hypercapnic patients with a PaCO2 > 45 mm Hg to the rest of the study population (n=27), preoperative FEV1 (% pred.) and VC (% pred.) were both significantly lower than in the rest of the population, therefore showing their more severe obstructive lung function and/or air trapping. No further biometric differences between both groups were found. Three months postoperatively, there were no more lung functional differences found. Hypercapnic patients had exclusively preoperatively more severe obstructive lung function and profited by LVRS with a more pronounced decline of PaCO2 (figure 4), as previously have been described by some studies [16] but not all LVRS centres [46] and showed more lung functional improvement than the rest of the study population up to two years after LVRS. Therefore, we agree with Shade and O’Brien who proposed not to exclude patients from LVRS solely on the presence of resting hypercapnia [16,46]. However, contrary to the more important lung functional parameters, the effect on PaCO2 was no longer seen at 18 or 24 months after LVRS in our study population. Although patients with severe hypercapnia have been excluded from most LVRS studies, patients with modest hypercapnia had a PaCO2 improvement to the normal or near-normal range [47], as also shown in the studied population here.
The number of patients with important hypoxemia did not change by LVRS in our study group. Long-term oxygen therapy (LTOT) necessity may importantly interfere with or be associated with important consequences on daily life [48], in particular with a positive impact on cognitive performance [49]. As the indication is primarily based on resting PaO2, the impact of a low resting PaO2 (e.g., PaO2 ≤ 55 mm Hg) is high. Pathophysiologically one would expect that by both an unchanged AaDO2 after LVRS as observed and a postoperative reduction of PaCO2 due to improved lung mechanical properties less patients might be severely hypoxemic, e.g., be below the threshold of indication for LTOT. Our opposed finding that the proportion of patients with a resting PaO2 ≤ 55 mm Hg before operation and 6 months after LVRS remained the same underscores that in our population LVRS did not act primarily beneficially on gas exchange.
This finding further emphasizes that the obvious benefits of LVRS, i.e., alleviation of dyspnoea at rest and on exertion and improvement of exercise capacity, were chiefly based on lung and respiratory mechanical properties [50,51], and primarily not on blood gases. The pattern of emphysema determined by CT scan inspection is associated with a change in breathing pattern and consequently gas exchange upon maximum exercise after LVRS [52]. Another study has shown that the proportions of patients reporting use of supplemental oxygen at rest and with exercise fall significantly 6 months after LVRS (53 to 15% for use at rest and 95 of 46% for use on exercise) [53]. To assess the proportion of patients requiring oxygen supplementation before and 6 months after LVRS with an objective parameter, they used PaO2 ≤ 59 mm Hg instead of PaO2 ≤ 55 mm Hg. Therefore, their results are not directly comparable with ours and the proportion fulfilling the indication for LTOT before and after LVRS in their population remains unclear.
The presented data show a profit to the patients that are at two years follow-up at still clearly improved FEV1 (that is at 24 months still 230 ml or 29% better than preoperatively, considered above the range of the minimal clinical importance), residual volume RV (that was at 24 months still 840 ml or 19.5% less than preoperatively) and 6-minute walk (that was at 24 months still by 72 m or 27% improved, considered above the range of the minimal clinical importance). We have also to keep in mind that all studied intervention groups are highly specifically selected patient groups [54-56]. Pathophysiologically one may speculate that surgical intervention versus endoscopic intervention may have similar effects on vessels and airways and may not be of a huge difference in terms of ventilation-perfusion pattern change or concerning the reduction of the alveolar gas exchange surface. Whether a real difference to endoscopic procedures exists remains therefore open, as to our knowledge no study has so far been published with similar follow-up with endoscopic procedures and gas exchange parameters. The tables 3a and 3b give evidence on all other published studies found including bronchoscopic lung volume reduction patient studies.
|
|
Preoperative measurements |
Postoperative measurements |
|
|
|
After 3 Months |
After 6 Months |
After 12 Months |
After 18 Months |
After 24 Months |
Blood gas and gas exchange parameters |
PaCO2 (mm Hg) |
38.5 ± 0.9 |
35.6 ± 0.7* |
36.4 ± 0.7* |
35.8 ± 0.8* |
36.9 ± 1.1 |
36.9 ± 0.9 |
PaO2 (mm Hg) |
65.2 ± 1.5 |
70.4 ± 1.7* |
69.5 ± 1.7* |
67.7 ± 1.6 |
64.7 ± 2.0 |
65.3 ± 1.7# |
AaDO2 (mm Hg) |
28.7 ± 1.7 |
27.6 ± 2.0 |
28.0 ± 1.7 |
30.3 ± 1.9 |
31.0 ± 2.6 |
31.1 ± 1.8# |
DLCO (ml/min×mm Hg) |
3.83 ± 0.22 |
4.06 ± 0.19 |
4.13 ± 0.19 |
3.89 ± 0.20 |
3.68 ± 0.24 |
3.57 ± 0.20# |
DLCO (% pred.) |
46 ± 3 |
48 ± 2 |
49 ± 2 |
46 ± 2 |
44 ± 3 |
43 ± 2# |
Lung function parameters |
FEV1 (L/s) |
0.79 ± 0.04 |
1.26 ± 0.10* |
1.21 ± 0.09* |
1.14 ± 0.09* |
1.10 ± 0.09* |
1.02 ± 0.08*# |
FEV1 (% pred.) |
28 ± 1 |
45 ± 2* |
43 ± 2* |
40 ± 2* |
40 ± 2* |
37 ± 2*# |
IVC (L) |
2.99 ± 0.14 |
3.69 ± 0.19* |
3.77 ± 0.18* |
3.71 ± 0.19* |
3.48 ± 0.20* |
3.34 ± 0.18*# |
IVC (% pred.) |
82 ± 3 |
100 ± 2* |
102 ± 2* |
100 ± 3* |
96 ± 3* |
92 ± 3*# |
RV (L) |
5.13 ± 0.16 |
3.88 ± 0.15* |
3.90 ± 0.17* |
4.04 ± 0.17* |
4.09 ± 0.18* |
4.29 ± 0.20*# |
RV (% pred.) |
277 ± 8 |
171 ± 7* |
172 ± 8* |
177 ± 7* |
179 ± 8* |
186 ± 9*# |
RV/TLC |
0.63 ± 0.01 |
0.51 ± 0.02* |
0.51 ± 0.02* |
0.52 ± 0.02* |
0.54 ± 0.02* |
0.55 ± 0.02*# |
Exercise test |
6 MWD (m) |
266 ± 14 |
364 ± 12* |
370 ± 14* |
404 ± 19* |
344 ± 18* |
338 ± 19* |
Dyspnoea scale |
mMRC score |
3.5 ± 0.1 |
1.5 ± 0.2* |
1.5 ± 0.2* |
1.6 ± 0.1* |
1.9 ± 0.2* |
2.0 ± 0.2*# |
| |
|
|
|
|
|
|
|
|
*compared to preoperatively: p£ 0.05; #compared to 3 months postoperatively: p£ 0.05
6 MWD: 6-minute walk distance; AaDO2: alveolar-arterial oxygen gradient; DLCO: diffusing capacity for carbon monoxide; FEV1: first second of forced expiration; IVC: inspiratory vital capacity; PaCO2: partial pressure of carbon dioxide; PaO2: alveolar oxygen partial pressure; pred.: predicted; RV: Residual volume; TLC: Total lung capacity.
Table 2. Gas exchange, lung functional, exercise test and dyspnea parameters study group (n=39, Mean Values ± standard error
Table 3a. Summary of results of clinical trials issuing Lung Volume Reduction Surgery with 12 or 24 months follow up
(In excel sheet)
There are a number of limitations to this research. One important constraint is that the data were obtained and analyzed about two and a half decades ago. However, besides the still underestimated role of rehabilitation and maintenance or increase of daily physical activity, only few pharmacological and non-pharmacological treatment options have changed, and also the surgical techniques have only moderately changed. Another limitation is the retrospective nature of this study. Patients were assessed in a clinically circumscribed and for clinicians usually well perceivable, but not clearly definable “stable condition". For ethical reasons repeated arterial blood gas measurements at each time point could not be performed, leaving open how much variation might have occurred due to factors including pain in a blood gas study. AaDO2 was only calculated, as direct measurement may influence resting blood gases, and therefore lead to more exact, but less clinically relevant values. Bronchodilators and their timing may have influenced the results. The fact that virtually all patients regularly used bronchodilators may have lessened their influence of on the blood gas results.
Conclusions
In conclusion, we observed in the retrospective single LVRS center study with a complete 2 years follow up of 39 patients a slight improvement in resting PaO2 for up to 6 months after LVRS. This temporary improvement coincided with a significant drop in PaCO2 up to 12 months after LVRS. Mean gas exchange as assessed by AaDO2 remained unchanged during the study period if compared to the preoperative value. The weak correlations between LVRS-mediated changes of FEV1 and VC with those temporary changes of PaCO2 corroborated a role of the lung volumes for the PaCO2 improvement 3 months after LVRS. On the other hand, for any individual patient, LVRS could unpredictably improve or worsen gas exchange, and the fraction of patients meeting oxygen supplementation criteria at rest remained unaltered after LVRS. The results clearly underscore that at two or more years sustained beneficial effects of LVRS are, contrary to blood gas parameters, based on lung mechanics, with important outcomes such as 6-minute walk or FEV1 improvement being still above the minimal clinical importance [16]. Both gas exchange improvement and worsening might ensue after LVRS. This specific uncertainty on gas exchange alterations should be taken into account when patients are given advice before LVRS, also concerning the need of further oxygen supplementation. Whether similar gas exchange results are obtained in bronchoscopic lung volume procedures may be conceivable, but to our knowledge has not been shown so far. Therefore, the results of the presented study and further published LVRS studies hint to the superior improvement of lung functional parameter after LVRS compared to bronchoscopic lung volume reduction. Whereas it may be conceivable pathophysiologically that similar gas exchange influences may occur by bronchoscopic lung volume reduction, to our knowledge this has not been published so far.
Author contribution statements
W.W. was the surgeon performing operative procedures and he also implicated the study designs. J.H., P.L. and U.S. performed data analysis and wrote the whole manuscript. Y.H. corrected the manuscript, rewrote parts of the manuscript and carried out the submission procedure.
Statement of ethics
The authors have no ethical conflicts to disclose.
Patients consent
Written informed consent was obtained from the patient to be enrolled in a prospective study on the outcome after LVRS for publication of this study, which was approved by the hospital’s ethical committee.
Funding
Supported by Grant No. 3200-043358;95.1 from the Swiss National Science Fund and by a grant from the Zürich Lung League. In addition, the work of YH and JH was supported by the Lungen-und Atmungsstifung Bern.
Acknowledgement
The authors thank Mrs. Sabine Wyss Kohl and Ms. Rahel Naef, study nurses, and the pulmonary laboratory staff of the Pulmonary Division for technical assistance.
Conflict of interest
None.
References
- https://goldcopd.org/2022-gold-reports-2/
- Rochester DF (1984) The respiratory muscles in COPD State of the art. Chest 1984, 85: 47s-50s. [Crossref]
- Cassart M, Hamacher J, Verbandt Y, Wildermuth S, Ritscher D, et al. (2001) Effects of lung volume reduction surgery for emphysema on diaphragm dimensions and configuration. Am J respir crit care med 163: 1171-1175. [Crossref]
- Criner RN, Yu D, Jacobs MR, Criner GJ (2018) Effect of lung volume reduction surgery on respiratory muscle strength in advanced emphysema. Chronic Obstr Pulm Dis (Miami, Fla.) 6: 40-50. [Crossref]
- https://www.ncbi.nlm.nih.gov/books/NBK482217/
- Agrawal S, Gupta N, Gonuguntla HK (2021) Evidence-based review of bronchoscopic lung volume reduction. Adv Respir Med 89: 43-48. [Crossref]
- Weder W, Ceulemans LJ, Opitz I, Schneiter D, Caviezel C (2021) Lung volume reduction surgery in patients with homogeneous emphysema. Thorac Surg Clin 31: 203-209. [Crossref]
- https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0043-103363
- van Dijk M, Klooster K, Ten Hacken NHT, Sciurba F, Kerstjens HAM. Et al. (2020) The effects of lung volume reduction treatment on diffusing capacity and gas exchange. Eur Respir Rev 29: 190171. [Crossref]
- Gelb AF, McKenna RJ Jr, Brenner M, Epstein JD, Zamel N (2001) Lung function 5 yr after lung volume reduction surgery for emphysema. Am J Respir Crit Care Med 163: 1562-1566. [Crossref]
- Russi EW, Stammberger U, Weder W (1997) Lung volume reduction surgery for emphysema. Eur Respir J 10: 208-218.
- Sharafkhaneh A, Goodnight-White S, Officer TM, Rodarte JR, Boriek AM (2005) Altered thoracic gas compression contributes to improvement in spirometry with lung volume reduction surgery. Thorax 60: 288-292. [Crossref]
- Hamacher J, Büchi S, Georgescu CL, Stammberger U, Thurnheer R, et al. (2002) Improved quality of life after lung volume reduction surgery. Eur Respir J 19: 54-60. [Crossref]
- Lammi MR, Marchetti N, Criner GJ (2014) Reduced dynamic hyperinflation after lvrs is associated with improved exercise tolerance. Respir Med 108: 1491-1497. [Crossref]
- Fessler HE, Scharf SM, Ingenito EP, McKenna RJ Jr, Sharafkhaneh A (2008) Physiologic basis for improved pulmonary function after lung volume reduction. Proc Am Thoracic Soc 5, 416-420. [Crossref]
- Shade D Jr, Cordova F, Lando Y, Travaline JM, Furukawa S, et al. (1999) Relationship between resting hypercapnia and physiologic parameters before and after lung volume reduction surgery in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159: 1405-1411. [Crossref]
- Caviezel C, Schneiter D, Opitz I, Weder W (2018) Lung volume reduction surgery beyond the nett selection criteria. J Thoracic Dis 10: S2748-S2753. [Crossref]
- Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, et al. (2003) A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. Engl J Med 2003 348: 2059-2073. [Crossref]
- Fishman A, Fessler H, Martinez F, McKenna RJ Jr, Naunheim K, et al. (2001) Patients at high risk of death after lung-volume-reduction surgery. Engl J Med 345: 1075-1083. [Crossref]
- Cassina PC, Teschler H, Konietzko N, Theegarten D, Stamatis G (1998) Two-year results after lung volume reduction surgery in alpha1-antitrypsin deficiency versus smoker's emphysema. Eur Respir J 12: 1028-1032. [Crossref]
- Tutic M, Bloch KE, Lardinois D, Brack T, Russi EW, et al. (2004) Long-term results after lung volume reduction surgery in patients with alpha1-antitrypsin deficiency. J Thorac Cardiovasc Surg 128: 408-413. [Crossref]
- Gaissert HA, Trulock EP, Cooper JD, Sundaresan RS, Patterson GA (1996) Comparison of early functional results after volume reduction or lung transplantation for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 111: 296-306. [Crossref]
- You B, Zhao Y, Hou S, Hu B, Li H (2018) Lung volume reduction surgery in hypercapnic patients: A single-center experience from china. J Thoracic Dis 10: S2698-S2703. [Crossref]
- https://pubmed.ncbi.nlm.nih.gov/7149469/
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, et al. (1993) Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, european community for steel and coal. Official statement of the european respiratory society. Eur Respir J 16: 5-40. [Crossref]
- Curran-Everett D (2006) A classic learning opportunity from fenn, rahn, and otis (1946): The alveolar gas equation. Adv Physiol Educ 30: 58-62. [Crossref]
- Stammberger U, Klepetko W, Stamatis G, Hamacher J, Schmid RA, et al. (2000) Buttressing the staple line in lung volume reduction surgery: A randomized three-center study. Annals Thoracic Surg 70: 1820-1825. [Crossref]
- Pompeo E, Rogliani P, Tacconi F, Dauri M, Saltini C, et al. (2012) Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg 143: 47-54. [Crossref]
- McKenna RJ Jr, Brenner M, Fischel RJ, Gelb AF (1996) Should lung volume reduction for emphysema be unilateral or bilateral? J Thorac Cardiovasc Surg 112: 1331-1338. [Crossref]
- Geddes D, Davies M, Koyama H, Hansell D, Pastorino U, et al. (2000) Effect of lung-volume-reduction surgery in patients with severe emphysema. Engl J Med 343: 239-245. [Crossref]
- Meyers BF, Yusen RD, Guthrie TJ, Patterson GA, Lefrak SS, et al. (2004) Results of lung volume reduction surgery in patients meeting a national emphysema treatment trial high-risk criterion. J Thorac Cardiovasc Surg 127: 829-835. [Crossref]
- Laghi F, Jubran A, Topeli A, Fahey PJ, Garrity ER Jr, et al. (2004) Effect of lung volume reduction surgery on diaphragmatic neuromechanical coupling at 2 years. Chest 125: 2188-2195. [Crossref]
- Pompeo E, Mineo TC (2007) Two-year improvement in multidimensional body mass index, airflow obstruction, dyspnea, and exercise capacity index after non-resectional lung volume reduction surgery in awake patients. Annals Thoracic Surg 84: 1862-1869. [Crossref]
- Tacconi F, Pompeo E, Forcella D, Marino M, Varvaras D, et al. (2008) Lung volume reduction reoperations. Annals Thoracic Surg 85: 1171-1177. [Crossref]
- Caviezel C, Schaffter N, Schneiter D, Franzen D, Inci I, et al. (2017) Outcome after lung volume reduction surgery in patients with severely impaired diffusion capacity. Annals Thoracic Surg 105:379-385. [Crossref]
- Criner GJ, Sue R, Wright S, Dransfield M, Rivas-Perez H, et al. (2018) A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (liberate). Am J Respir Crit Care Med 198: 1151-1164. [Crossref]
- Shah PL, Slebos DJ, Cardoso PF, Cetti E, Voelker K, et al. (2011) Bronchoscopic lung-volume reduction with exhale airway stents for emphysema (ease trial): Randomised, sham-controlled, multicentre trial. Lancet (London, England) 378: 997-1005. [Crossref]
- Venuta F, Anile M, Diso D, Carillo C, De Giacomo T, et al. (2012) Long-term follow-up after bronchoscopic lung volume reduction in patients with emphysema. Eur Respir J 39: 1084. [Crossref]
- Yang L, Hsu K, Williamson JP, Peters MJ, Ho-Shon K, et al. (2019) Changes in ventilation and perfusion following lower lobe endoscopic lung volume reduction (elvr) with endobronchial valves in severe copd. Clin Respir J 13: 453-459. [Crossref]
- Leyenson V, Furukawa S, Kuzma AM, Cordova F, Travaline J, et al. (2000) Correlation of changes in quality of life after lung volume reduction surgery with changes in lung function, exercise, and gas exchange. Chest 118, 728-735. [Crossref]
- Snyder ML, Goss CH, Neradilek B, Polissar NL, Mosenifar Z, et al. (2008) Changes in arterial oxygenation and self-reported oxygen use after lung volume reduction surgery. Am J Respir Crit Care Med 178: 339-345. [Crossref]
- Homan S, Porter S, Peacock M, Saccoia N, Southcott AM, et al. (2001) Increased effective lung volume following lung volume reduction surgery in emphysema. Chest 120: 1157-1162. [Crossref]
- Albert RK, Benditt JO, Hildebrandt J, Wood DE, Hlastala MP (1998) Lung volume reduction surgery has variable effects on blood gases in patients with emphysema. Am J Respir Crit Care Med 158: 71-76. [Crossref]
- Wagner PD, Dantzker DR, Dueck R, Clausen JL, West JB (1977) Ventilation-perfusion inequality in chronic obstructive pulmonary disease. J Clin Invest 59: 203-216. [Crossref]
- Khoukaz G, Gross NJ (1999) Effects of salmeterol on arterial blood gases in patients with stable chronic obstructive pulmonary disease. Comparison with albuterol and ipratropium. Am J Respir Crit Care Med 160: 1028-1030. [Crossref]
- O'Brien GM, Furukawa S, Kuzma AM, Cordova F, Criner GJ (1999) Improvements in lung function, exercise, and quality of life in hypercapnic copd patients after lung volume reduction surgery. Chest 115: 75-84. [Crossref]
- Yusen RD, Trulock EP, Pohl MS, Biggar DG (1996) Results of lung volume reduction surgery in patients with emphysema. The washington university emphysema surgery group. Semin Thoracic Cardiovasc Surg 8: 99-109. [Crossref]
- Frey JG, Rochat T, Pichard C, Dousse N, Janssens JP, et al. (1998) Chronic obstructive pulmonary disease patients undergoing home oxygen therapy: A study of clinical parameters, nutritional status and ambulatory capacity. Revue des maladies respiratoires 15: 69-78. [Crossref]
- Kozora E, Emery CF, Ellison MC, Wamboldt FS, Diaz PT, et al. (2005) Improved neurobehavioral functioning in emphysema patients following lung volume reduction surgery compared with medical therapy. Chest 128: 2653-2663. [Crossref]
- Stammberger U, Bloch KE, Thurnheer R, Bingisser R, Weder W, et al. (1998) Exercise performance and gas exchange after bilateral video-assisted thoracoscopic lung volume reduction for severe emphysema. Eur Respir J 12: 785-792. [Crossref]
- Hamacher J, Bloch KE, Stammberger U, Schmid RA, Laube I, et al. (1999) Two years' outcome of lung volume reduction surgery in different morphologic emphysema types. Annals Thoracic Surg 68: 1792-1798. [Crossref]
- Criner GJ, Belt P, Sternberg AL, Mosenifar Z, Make BJ, et al. (2009) Effects of lung volume reduction surgery on gas exchange and breathing pattern during maximum exercise. Chest 135: 1268-1279. [Crossref]
- Yusen RD, Lefrak SS, Gierada DS, Davis GE, Meyers BF, et al. (2003) A prospective evaluation of lung volume reduction surgery in 200 consecutive patients. Chest 123: 1026-1037. [Crossref]
- Klooster K, ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, et al. (2015) Endobronchial valves for emphysema without interlobar collateral ventilation. Engl J Med 373: 2325-2335. [Crossref]
- Herth FJ, Noppen M, Valipour A, Leroy S, Vergnon JM (2012) Efficacy predictors of lung volume reduction with zephyr valves in a european cohort. Eur Respir J 39: 1334-1342. [Crossref]
- Posthuma R, Vanfleteren L (2020) The stelvio trial, a game changer for bronchoscopic lung volume reduction in patients with severe emphysema. Breathe 16: 200004. [Crossref]




