Objectives: To assess inter-ventricular synchrony with fusion beats pacing in the context of a patient with Right Bundle Branch Block (RBBB).
Methods: Case report of a patient with mild LV systolic dysfunction and RBBB with intermittent complete heart block requiring permanent pacemaker implantation. The RV pacing lead was placed in the lower septum thus achieving fusion beats pacing with narrow QRS complex duration. Inter-ventricular and atrio-ventricular synchrony studies were used to assess and optimise fusion beats pacing.
Results: Inter-ventricular synchrony was achieved with fusion beats pacing with identical left ventricular and right ventricular pre-ejection times and no evidence of inter-ventricular mechanical delay. Atrio-ventricular synchrony was also achieved with optimised sensed and paced AV delays. There was corresponding improvement in both LV systolic function and heart failure symptoms.
Conclusion: This case report demonstrated the potential benefit of fusion beats pacing. Even though further studies are still warranted, fusion beats pacing might prove to be an alternative to CRT implantation in patients with RBBB
right ventricular pacing, fusion beats, inter-ventricular synchrony
In this case report, we describe an alternative method of achieving inter-ventricular synchrony in an 83 years old female who presented on October 21, 2018 with shortness of breath and syncope. She has a background of ischaemic heart disease and Parkinson’s disease. A coronary angiogram in 2011 showed a mild plaque disease only. On examination, she was haemodynamically stable with a heart rate of 65 bpm and BP135/84 mmHg. The rest of her examination was unremarkable. The ECG showed sinus rhythm with Right Bundle Brunch Block (RBBB) and QRS duration of 157 ms (Figure 1) and the chest X-ray showed clear lung fields. The echocardiogram showed mild LV systolic dysfunction with dyssynchronous septal motion and no significant valve disease.
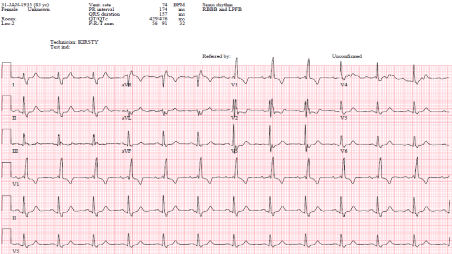
Figure 1. Intrinsic Rhythm (RBBB)
The patient was monitored overnight on telemetry which revealed multiple episodes of non-conducted P-waves with pauses up to 15 seconds indicating an intermittent complete heart block. A permanent pacemaker (Vitatron Q70 DR MRI DDD(R)) was therefore implanted via the axillary approach. The right ventricular lead was actively fixed to lower septum (R wave amplitude of 5.8 mv, impedance of 860 ohms and threshold of 1v at 0.5 ms), and the atrial lead was actively fixed into the right atrial appendage (P wave amplitude of 4.4 ms, impedance of 572 ohms and threshold of 1.25 v at 0.4 ms). The pacemaker was programmed DDD with base rate of 60 bpm, sensed AV delay of 160 ms and paced AV delay of 180 ms and upper sensor/tracking rate of 130 bpm. However, it was noted that the paced rhythm had a narrow QRS complex with duration of 110-115 ms. Telemetry and 12 leads ECG confirmed narrow QRS complexes in keeping with fusion beats (Figures 2 and 3).
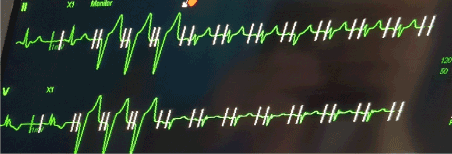
Figure 2. Fusion Beats pacing: First 2 beats are intrinsic rhythm followed by 3 beats of predominantly RV pacing. Subsequent rhythm is fusion beats.
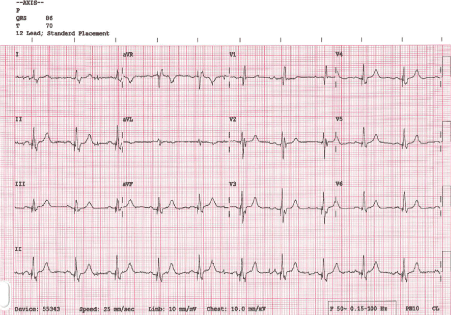
Figure 3. 12 leads ECG showing Fusion Beats QRS morphology
Right ventricular pacing has been traditionally achieved through the placement of active or passive leads in the Right Ventricular (RV) apex due to the less challenging aspect of the procedure and the perceived benefit of stability and reduced risk of displacement [1]. This approach results in dyssynchronous ventricular contraction and subsequent iatrogenic Left Bundle Brunch Block (LBBB) (Figure 4) which is associated with worse outcomes in the long term [2]. The outcomes are worse in patients with impaired LV systolic function and high pacing burden due to the inter-ventricular dyssynchrony that results in reduced cardiac output and adverse remodelling [3].
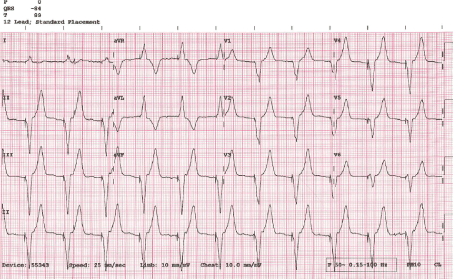
Figure 4. Predominantly RV pacing
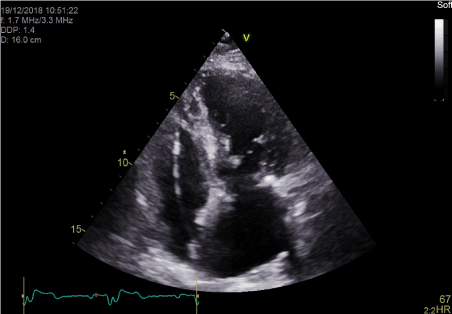
Figure 5. Apical Four chamber echocardiographic view showing position of RV active fixation lead in lower septum.
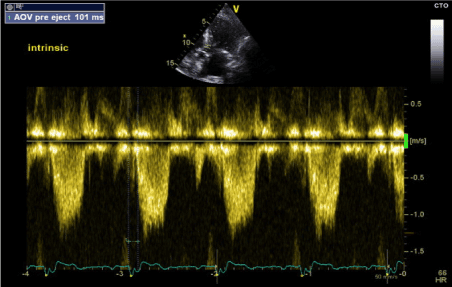
Figure 6. LV-PEP measurement in intrinsic Rhythm (RBBB) (PW Doppler in LVOT)
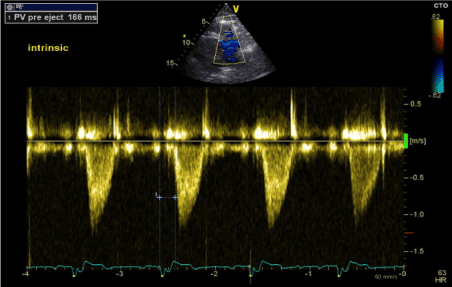
Figure 7. RV-PEP measurement in intrinsic Rhythm (RBBB) (PW Doppler in RVOT)
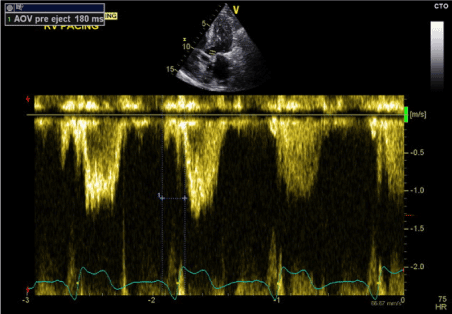
Figure 8. LV-PEP in predominantly RV pacing (LBBB) (PW Doppler in LVOT)
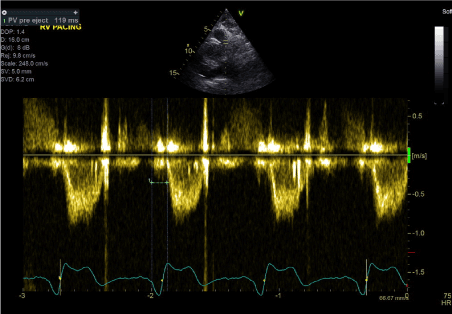
Figure 9. RV-PEP in predominantly RV pacing (LBBB) (PW Doppler in RVOT)
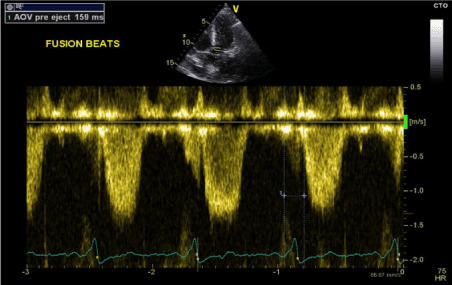
Figure 10. LV-PEP measurement in fusion beats rhythm (PW Doppler in LVOT)
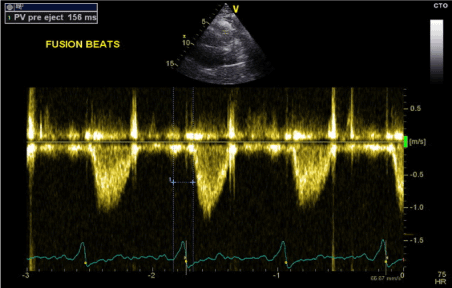
Figure 11. RV-PEP measurement in fusion beats rhythm (PW Doppler in RVOT)
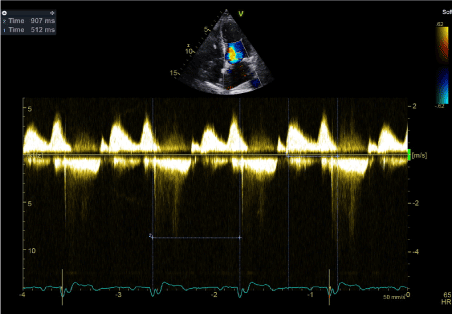
Figure 12. Transmitral Doppler Wave confirming absence of AV dyssynchrony

Figure 4. Predominantly RV pacing
Multiple approaches have been recommended to avoid the detrimental effect of RV apical pacing, including reduced RV pacing burden and adopting alternative pacing strategies that results in narrow QRS complex and more physiological ventricular contraction [3]. Cardiac Resynchronisation Therapy (CRT), His Bundle pacing and septal pacing are the main alternative strategies used. Both CRT and His Bundle pacing are challenging and require expertise and fulfilment of guideline-based criteria [4,5]. On the other hand, septal pacing is less technically challenging and can result in more physiologically pacing being closer to the conduction system and can result in narrow QRS complex, which is a surrogate marker of inter-ventricular synchrony [3,6].
Moreover, the use of CRT in patients with RBBB and LV systolic dysfunction has been controversial, with multiple studies showing less benefit from CRTs especially if the QRS duration is less than 150 ms [7,8]. As a result, the European Heart Rhythm Association (EHRA) indicated that a CRT can only be used in RBBB if the QRS duration was > 150 ms (Class IIa) with a lower recommendation class for QRS duration 120-150 ms (class IIb) [9]. In this case where the patient had RBBB, we were able to achieve narrow QRS complexes on RV lower septal pacing (Figure 5), and therefore achieve inter-ventricular synchrony. This was mainly due to the resulting fusion beats.

Figure 5. Apical Four chamber echocardiographic view showing position of RV active fixation lead in lower septum.
Fusion beats are the result of multiple electrical impulses originating from different parts of the heart and acting on same region of the heart at the same time [10,11]. In this case, the patient’s native electrical conduction coincided with the pacing impulse leading to ventricular activation [11]. As the patient had underlying RBBB, activation at lower septum lead to retrograde activation of the His-BBB pathway and fusion with anterograde activation from the native impulses. The results are narrow QRS complexes and absence of either RBBB (native conduction) or LBBB (RV pacing), which indicate synchronous LV and RV contractions [12]. Therefore, cardiac output can be improved by the prevention of the detrimental effect of both chronic RV pacing and loss of synchronous contraction with native RBBB pattern [12,13].
One way to maintain this fusion beat pacing is by optimising paced and sensed AV delay in order to achieve narrow QRS complex fusion beats. We achieved this using the same CRT optimisation method by adjusting paced and sensed AV delay and monitoring QRS duration on 12 leads ECG. We also used echocardiographic confirmation to assess for presence or absence of inter-ventricular dyssynchrony.
The dyssynchony study was used to assess inter-ventricular dyssyncrony (delay between RV and LV activation) using assessment of LV and RV pre-ejection times and inter-ventricular mechanical delay [14,15]. This relays on obtaining a pulse wave flow velocity at LVOT level (apical five chamber view) and RVOT level (parasternal short axis view) and measuring the time difference between the onset of electrical activation (onset of QRS complex) and onset of aortic flow (LV Pre-Ejection Period: LV-PEP) and pulmonary flow (RV Pre-Ejection Period: RV-PEP). Inter-Ventricular Mechanical Delay (IVMD) is calculated as the difference in time between LV-PEP and RV-PEP. IVMD of > 40 ms is considered a sensitive marker of inter-ventricular dyssynchrony [15]. We therefore applied these echocardiographic criteria during all three stages of rhythm assessment: intrinsic rhythm, predominantly RV pacing and fusion beats pacing.
During intrinsic rhythm assessment, we programmed the pacemaker to VVI back up pacing at 30 bpm resulting in unmasking of patients underlying intrinsic rhythm with RBBB morphology (Figure 1). On transthoracic echocardiography, there was paradoxical septal motion with LV contraction proceeding RV contraction and overall mild LV systolic dysfunction. LV-PEP was 101 ms and RV-PEP was 166 ms (Figures 6 and 7). The IVMD was calculated at 65 ms confirming inter-ventricular dyssynchrony.

Figure 6. LV-PEP measurement in intrinsic Rhythm (RBBB) (PW Doppler in LVOT)

Figure 7. RV-PEP measurement in intrinsic Rhythm (RBBB) (PW Doppler in RVOT)
During the second stage (predominantly RV pacing), the pacemaker was programmed to DDD mode with shortened AV delay to achieve RV paced rhythm. The result was AS-VP pacing pattern with LBBB morphology and QRS duration of 155 ms (Figure 4). Similarly, transthoracic echocardiography showed marked paradoxical septal motion and overall mild LV systolic dysfunction. LV-PEP measured 180 ms and RV-PEP was 119 ms (Figures 8 and 9). The resulting IVMD was 61ms in keeping with inter-ventricular dyssynchrony (RV contraction proceeding LV contraction).

Figure 8. LV-PEP in predominantly RV pacing (LBBB) (PW Doppler in LVOT)

Figure 9. RV-PEP in predominantly RV pacing (LBBB) (PW Doppler in RVOT)
The final stage was to optimise the pacemaker settings to achieve fusion beats while pacing in DDD mode. We achieved this by programming the paced AV delay to 180 ms and sensed AV delay to 160 ms. The result was fusion beats pacing with QRS duration of 110-115 ms (Figures 2 and 3). Transthoracic echocardiography showed improvement of LV systolic function with normal septal motion. The LV-PEP and RV-PEP measured at this stage were almost identical with LVPEP measuring 159 ms and RVPEP measuring 156 ms (Figures 10 and 11). The resulting IVMD was 3 ms confirming synchronous LV and RV contractions.

Figure 10. LV-PEP measurement in fusion beats rhythm (PW Doppler in LVOT)

Figure 11. RV-PEP measurement in fusion beats rhythm (PW Doppler in RVOT)
We therefore optimised the pacing settings and we were able to achieve fusion beat pacing with ECG and echocardiographic confirmations of absence of inter-ventricular dyssynchrony with paced AV delay of 180-200 ms and sensed AV delay of 150-170 ms. We subsequently assessed the AV delay settings for the presence or absence of Atrio-Ventricular (AV) dyssynchrony. This is defined as a suboptimal delay between atrial and ventricular contractions which impacts negatively on LV filling. It is mainly due to short ventricular filling time and superimposed atrial contraction on the passive filling stage [15].
Assessment of AV dyssynchrony is performed using the mitral inflow pattern (ventricular filling time). AV dyssynchrony is considered to be present if mitral inflow interval is less than 40% of the RR interval, which implies that the ventricular filling time is < 40% of the cardiac cycle [14,15]. In this case, mitral inflow pattern was measured using trans-mitral Doppler wave. Ventricular filling time was calculated as the time interval from the onset of E wave to the end of the A wave. The ventricular filling time was 512 ms while the cardiac cycle length (RR interval) was 907 ms. Therefore, the ventricular filling time was 56% in keeping with optimal AV delay settings and confirming the absence of AV dyssynchrony (Figure 12).

Figure 12. Transmitral Doppler Wave confirming absence of AV dyssynchrony
The pacemaker settings were optimised accordingly with fusion beats pacing and adequate AV delay intervals (paced AV delay of 180 ms and sensed AV delay of 160 ms). We were therefore able to maximise AV contraction and LV filling time, reduce inter-ventricular mechanical delay and increase cardiac output. This was confirmed by the patient reporting significant improvement in her symptoms of dyspnoea and fatigue when assessed one-month post pacemaker implantation and programming.
In this case, we discussed a patient with sinus rhythm and RBBB who presented with symptomatic intermittent complete heart block. Following DDD pacemaker implant with RV lead placed in the lower septum, we were able to achieve fusion beats pacing by optimising the AV delay. This resulted in narrow QRS complex morphology. Subsequent dyssynchrony study confirmed the absence of inter-ventricular dyssynchrony and overall improvement of LV systolic function with fusion beats pacing. This was maintained with optimised AV delay programming and there was symptomatic benefit at follow up. This case demonstrated the potential benefit of fusion beats pacing in patient with RBBB by achieving synchronous biventricular contraction and avoiding the deleterious effect of both intrinsic RBBB and iatrogenic LBBB. Even though further studies are still warranted, fusion beats pacing might prove to be an alternative to CRT implantation in patients with RBBB.
- Raatikainen MJP, Arnar DO, Merkely B, Nielsen JC, Hindricks G, et al. (2017) A Decade of Information on the Use of Cardiac Implantable Electronic Devices and Interventional Electrophysiological Procedures in the European Society of Cardiology Countries: 2017 Report from the European Heart Rhythm Association. Europace 19: ii1–ii90. [Crossref]
- Manolis AS (2006) The deleterious consequences of right ventricular apical pacing: time to seek alternate site pacing. Pacing Clin Electrophysiol 29: 298–315. [Crossref]
- Kaye GC, Linker NJ, Marwick TH, Pollock L, Graham L, et al. (2015) Effect of right ventricular pacing lead site on left ventricular function in patients with high-grade atrioventricular block: results of the Protect-Pace study. Eur Heart J 36: 856-862. [Crossref]
- Grines CL, Bashore TM, Boudoulas H, Olson S, Shafer P, et al. (1989) Functional abnormalities in isolated left bundle branch block. The effect of interventricular asynchrony. Circulation 79: 845-853. [Crossref]
- Barba-Pichardo R, Moriña-Vázquez P, Fernández-Gómez JM, Venegas-Gamero J, Herrera-Carranza M (2010) Permanent His-bundle pacing: seeking physiological ventricular pacing. Europace 12: 527–33. [Crossref]
- Vijayaraman P, Bordachar P, Ellenbogen KA (2017) The Continued Search for Physiological Pacing: Where Are We Now? J Am Coll Cardiol 69: 3099-3114. [Crossref]
- Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, et al. (2011) Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 123: 1061-1072. [Crossref]
- Cleland JGF, Daubert J-C, Erdmann E, Freemantle N, Gras D, et al. (2500) The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352: 1539–49. [Crossref]
- Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, et al. (2013) 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 34: 2281–2329. [Crossref]
- Gianfranchi L, Bettiol K, Sassone B, Verlato R, Corbucci G, et al. (2008) Fusion beat in patients with heart failure treated with left ventricular pacing: may ECG morphology relate to mechanical synchrony? A pilot study. Cardiovasc Ultrasound 6:1. [Crossref]
- Ellenbogen KA, Kay GN, Lau CP, Wilkoff BL, editors. Clinical cardiac pacing defibrillation and resynchronization therapy. 3rd ed. Philadelphia: Elsevier; 2007: 1005–62.
- Hutten H, Tchapeller B, Fritz L (2006) Some Interesting Properties of Cardiac Fusion Beats. World Congress on Medical Physics and Biomedical Engineering 3436-3440.
- Niazi I, Baker J, Corbisiero R, Love C, Martin, et al. (2017) Safety and efficacy of Multipoint Pacing in cardiac resynchronization therapy: the MultiPoint Pacing Trial. JACC Clin Electrophysiol 3: 1510–1518. [Crossref]
- Bader H, Garrigue S, Lafitte S, Reuter S, Jaïs P, et al. (2004) Intra-left ventricular electromechanical asynchrony:A new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol 43: 248–56. [Crossref]
- Serri K, Lafitte S, Amyot R, Sauvé C, Roudaut R (2007) Echocardiographic evaluation of cardiac dyssynchrony. Can J Cardiol 23: 303-310. [Crossref]












