Abstract
During or after chemotherapy, a peripheral neuropathy might rise. Neuropathic pain in such cases is often difficult to treat, and pain is sometimes even increasing in intensity after the cessation of chemotherapy. In 2016 we started a topical phenytoin project to treat pain due to chemotherapy induced peripheral neuropathy (CIPN) and meanwhile we monitored the efficacy and safety of a special phenytoin 5% or 10% cream in great detail in 6 patients suffering from CIPN. We will present in some detail one exemplary case from this cohort, responding to application of the cream. In all 6 cases a fast onset of perceptible pain relief was reported by patients. This is of great importance, as a fast onset will support compliance and help patients to rapidly perceive perspective in their chronic burden.
Introduction
Pain due chemotherapy induced peripheral neuropathy (CIPN) is often refractory to treatment, and there are not yet analgesics with clear clinical relevant analgesic effect. CIPN develops particularly related to the use of platinum, alkaloids, taxanes, thalidomide, lenalidomide, and bortezomib therapies [1]. The CIPN prevalence in the first month, after 3 months, and after 6 months or more is around 70%, 60%, and 30% respectively [2]. Almost 100% of the patients treated at high dose levels, will develop CIPN [3]. Platinum compounds can produce “coasting”: worsening of CIPN after cessation of therapy [4].
Duloxetine, recently tested in a phase III study, only marginally decreased pain compared to placebo [5]. To date, in our clinic for neuropathic pain, we have registered in detail a cohort of 73 patients suffering from neuropathic pain, using topical cream containing phenytoin 5% and 10%. Most patients reported clinical relevant pain reduction and did not report any systematic adverse events. From these patients, 6 patients suffering from CIPN were monitored in greater detail, and results will be reported here. We will also present one exemplary patient, in order to be able to discuss in some detail the time to perceptible pain relief after administering the cream, sometimes also defined as ‘onset of action’.
Case presentation
A 48-year old male suffering from CIPN due to the treatment of de novo acute myeloid leukemia, according to the ‘NOVE regimen’, consisted of mitoxantrone 10 mg/m2 day 1 to 5, and of etoposide 100 mg/m2 day 1 to 5, in 2014. Although currently treated with the maximal dose of pregabalin 600 mg daily, the patient scored his pain still with 8.5 on the 11-point numerical rating scale (NRS). Both feet were painful, characterized as tingling and pins and needles, pain scored 8.5 on the 10-point NRS. We prescribed phenytoin sodium 5% cream, and after applying one fingertip unit (containing 0.6 grams of phenytoin cream), patient reported a perceptible pain relief within 15 minutes, and scored the pain subsequently as 0 on the NRS. However, the duration of pain relief was only 3.5 hours. He had to apply the cream 3 times daily. We also prescribed amitriptyline 10% cream, though this cream reduced pain for only 1 to 1.5 hours. The addition of phenytoin 10% to the amitriptyline cream however, prolonged the effect considerably, with an even faster onset of perceptible pain relief: 3 minutes. At the moment, he only uses phenytoin 10% cream, which now completely abolishes pain, and he reports a duration of sufficient analgesia of 12 hours, with a fast onset of pain relief within minutes.
Further results
In table 1 we summarized the clinical effects of applying phenytoin 10% cream to painful localized areas. Three patients were male, and three were female, average age 55.8 years (SD: 8.3). Mean daily application times was 1.7 (SD: 1.4). The amount of phenytoin cream per application was 0.9 grams (SD: 0.8) In 4 out of 6 patients pain reduction was greater than 50%, which is clearly a clinical relevant response. In 2 cases the reduction was around 30%, still considered to be clinically meaningful. In all cases the onset to perceptible pain relief was within 30 minutes, and in one patient a fast onset of perceptible pain relief of 2 minutes was reported by the patient. The duration of sufficient analgesia varied between 2.5 hours and 70 hours and patients applied cream once daily up to thrice daily. Because of skewed distribution instead of means we also report the median and interquartile range (IQR) for duration of effect, 9.0 hours (3.6;35.5) and onset of perceptible pain relief, 11.0 minutes (6.5;31.3).
Table 1. Results of the application of phenytoin 10% cream in pain in CIPN in 6 patients.
Patient # |
Baseline NRS |
Post application NRS |
Onset of relief in minutes |
Percentage pain reduction |
Duration of effect in hours |
1 |
8.5 |
0 |
20 |
100 |
5 |
2 |
7 |
0 |
2 |
100 |
12 |
3 |
9.5 |
2 |
8 |
79 |
70 |
2021 Copyright OAT. All rights reserv
4 |
8 |
6 |
25 |
25 |
24 |
5 |
7 |
4 |
30 |
43 |
2.5 |
6 |
8 |
2 |
10 |
75 |
4 |
In none of the patients adverse events were reported. From our overall data pool, we collected blood from 16 cases to measured phenytoin plasma levels, after few hours of application to ensure sampling around T-max., in all these cases plasma levels were below the limit of detection.
Discussion
Chemotherapy induces toxic adverse effects to the peripheral nervous system and considerably reduces sleep quality and quality of life. Approximately 30% to 40% of patients treated with chemotherapy will develop CIPN, the higher the dose, the greater the chances to develop CIPN [6]. Adverse events frequently lead to a reduction of chemotherapy dosage or even to discontinuation of therapy. Clinical effects of analgesics in this population are small [7].
Neuro-inflammation might be one of the triggering mechanisms leading to CIPN [8]. As phenytoin possesses some anti-inflammatory activity, next to its sodium blocker properties, we felt it might be worth while trying phenytoin cream in the treatment of pain in CIPN. Phenytoin is the first modern anticonvulsant, introduced in the clinic in 1937 [9]. Phenytoin has been repurposed for a number of indications, and neuropathic pain is the most recent indication [10]. Phenytoin is thought to possess a number of mechanisms of action when applied on the skin, and its sodium channel blocking properties clearly contributes to its analgesic effect. It is a lipophilic compound which easily can penetrate the outer layers of the skin to the target tissues in the epidermis. As said, one additional mechanism of action might be linked to the immunomodulatory role of phenytoin, which might help downregulation peripheral inflammatory mechanisms in the small nerve fibers in the epidermis (Figure 1). On this epidermal tissue level, cross talk between sodium-containing overactive keratinocytes, immune-competent cells and nerve endings lead to pain and peripheral sensitization [11]. As we could rule out analgesia acting via central mechanisms, because levels of phenytoin in the blood were below the threshold of detection, a local mechanism of action seems logical, especially since the action to symptomatic relief is so fast. Systemic absorption via the transdermal route would lead to effective steady state plasma levels, only after many hours to days.
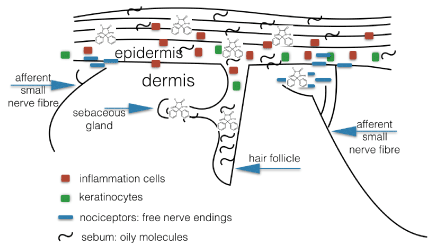
Figure 1. Mechanism of action of phenytoin in the epidermis in patients suffering from CIPN Phenytoin is a lipophilic compound and has great affinity to the lipid containing compartments in the skin: the sebum, the follicles and glands, and these compartments serve as slow release reservoirs. Phenytoin can inhibit overactive nerve endings, as well as immune competent cells and keratinocytes, as these all carry sodium channels. By inhibiting hyperactivity of these cells, phenytoin inhibits pain and downregulates peripheral sensitization phenomena.
Topical treatment of pain in CIPN using analgesic creams is rare [12,13]. One study evaluated a topical cream on the base of 2% ketamine plus 4% amitriptyline cream, but due to under-dosing this did not lead to effective analgesia [14]. In a study evaluating the combination of ketamine 1.5%, amitriptyline 3% and baclofen 0.75% gel, only in a trend toward improvement was seen [15]. Apart from the low dose selected there were other methodological flaws in these trials, as we discussed elsewhere [16]. The concentration of compound in analgesic creams needs to be as high as possible, without inducing local irritating effects, and we compounded since many years amitriptyline in 10% cream, ketamine in 10% cream and phenytoin 10% creams [17,18].
In general, patients suffering from localized peripheral neuropathic pain like to rub-in cream there where it hurts. In our current data pool, consisting of more than 70 patients, we have monitored a relative fast onset of perceptible pain relief after applying 10% phenytoin cream, mostly within 30 minutes. In these 6 CIPN patients we also monitored such a fast onset of perceptible pain relief, and some patients even reported such onset within some minutes after application.
Here, we specifically focused on the time to perceptible pain relief. A fast onset of action helps patients to have confidence in the therapy, and the faster the onset of action/relief is noticed, the more likely it will be that patients are compliant with therapy.
Patient-related definitions for onset of action are to be preferred above mechanistic onset of action studies. We always ask patients how long it takes from application of cream on the localized pain area to perceptible pain relief. Most orally taken (co-)analgesics for neuropathic pain are characterized with a slow onset of action, days up to weeks before pain relief sets in. When pain reduction starts slowly, classical habituation phenomena might blur the perceptible onset of action of pain relief, and can lead to low compliance. A fast onset of perceptible pain relief therefore, is quite relevant and unfortunately this fact has been underestimated in literature to date. Topical analgesia based on phenytoin cream seems to be a new modality of treatment of pain in CIPN, especially due to its fast onset of perceptible pain relief. Clearly, more data need to be gathered, including data from randomized placebo-controlled trials.
Conflict of Interest
Authors are patent holders of two patents related to the topical formulations of phenytoin in the treatment of pain: 1) Topical phenytoin for the use in the treatment of peripheral neuropathic pain and 2) Topical pharmaceutical composition containing phenytoin and a (co-) analgesic for the treatment of chronic pain.
References
- Smith EM, Bridges CM, Kanzawa G, Knoerl R, Kelly JP 4th, et al. (2014) Cancer treatment-related neuropathic pain syndromes--epidemiology and treatment: an update. Curr Pain Headache Rep 18: 459. [Crossref]
- Seretny M (2014) Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 155: 2461-2470.
- Park HJ (2014) Chemotherapy induced peripheral neuropathic pain. Korean J Anesthesiol 67: 4-7. [Crossref]
- Siegal T, Haim N (1990) Cisplatin-induced peripheral neuropathy. Frequent off-therapy deterioration, demyelinating syndromes, and muscle cramps. Cancer 66: 1117-1123.
- Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, et al. (2013) Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. Jama 309: 1359-1367.
- Staff NP, Grisold A, Grisold W, Windebank AJ (2017) Chemotherapy-induced peripheral neuropathy: A current review. Ann Neurol 81: 772-781.
- Kim PY, Johnson CE (2017) Chemotherapy-induced peripheral neuropathy: a review of recent findings. Curr Opin Anaesthesiol 30: 570-576.
- Lees JG, Makker PG, Tonkin RS, Abdulla M, Park SB, et al. (2017) Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur J Cancer 73: 22-29.
- Keppel Hesselink JM, Kopsky DJ (2017) Phenytoin: 80 years young, from epilepsy to breast cancer, a remarkable molecule with multiple modes of action. J Neurol 264: 1617-1621. [Crossref]
- Kopsky DJ, Keppel Hesselink JM (2017) Topical phenytoin for the treatment of neuropathic pain. J Pain Res 10: 469-473. [Crossref]
- Keppel Hesselink JM, Kopsky DJ, Bhaskar AK (2017) Skin matters! The role of keratinocytes in nociception: a rational argument for the development of topical analgesics. J Pain Res 10: 1-8.
- Cortellini A, Verna L, Cannita K, Napoleoni L, Parisi A, et al. (2017) Topical Menthol for Treatment of Chemotherapy-induced Peripheral Neuropathy. Indian J Palliat Care 23: 350-352.
- Fallon MT, Storey DJ, Krishan A, Weir CJ, Mitchell R, et al. (2015) Cancer treatment-related neuropathic pain: proof of concept study with menthol--a TRPM8 agonist. Support Care Cancer 23: 2769-2777.
- Gewandter JS, Mohile SG, Heckler CE, Ryan JL, Kirshner JJ, et al. (2014) A phase III randomized, placebo-controlled study of topical amitriptyline and ketamine for chemotherapy-induced peripheral neuropathy (CIPN): a University of Rochester CCOP study of 462 cancer survivors. Support Care Cancer 22: 1807-1814.
- Barton DL1, Wos EJ, Qin R, Mattar BI, Green NB, et al. (2011) A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support Care Cancer 19: 833-841.
- Keppel Hesselink JM, Kopsky DJ, Stahl SM (2017) Bottlenecks in the development of topical analgesics: molecule, formulation, dose finding and phase III design. J Pain Res 10: 635-641.
- Kopsky DJ, Keppel Hesselink JM (2012) High doses of topical amitriptyline in neuropathic pain: two cases and literature review. Pain Pract 12: 148-153.
- Kopsky DJ, Keppel Hesselink JM, Bhaskar A, Hariton G, Romanenko V, et al. (2015) Analgesic effects of topical ketamine. Minerva Anestesiol 81: 440-449. [Crossref]

