Abstract
Background: Once environmental stressors enter an ecosystem, all levels can be affected and in a number of different ways, e.g., fecundity. A specialized bioassay, an ELISA detecting inhibin B, that helps measure primate fertility status appears to do the same in non-primates. In mammals, inhibin B is a protein hormone expressed by ovarian granulosa cells and is vital in the regulation of ovarian function, including folliculogenesis. In males, inhibin B is expressed by Sertoli cells and plays a role in testicular function, including spermatogenesis.
Methods: This study measured and compared inhibin B levels between different echinoderms, sea urchins (Lytechinus variegatus) and sand dollars (Dendraster excentrus), and contrasted levels between female and male gametes.
Results: In the sea urchin, inhibin B content was significantly higher in females (oocytes) than males (spermatozoa) with overall means of 6.361 ng/106 ova and 0.007 ng/106 spermatozoa, respectively. In the sand dollar, inhibin B content was also significantly (P<0.001) higher in females (oocytes) than males (spermatozoa) with overall means of 3.366 ng/106 oocytes and 0.057 ng/106 spermatozoa, respectively. Of particular note, inhibin B levels were notably higher in both gametes of the sand dollar than the sea urchin.
Conclusions: Inhibin B levels can be detected in echinoderms using a commercially available ELISA kit. Inhibin B levels in the echinoderm are dependent on species as well as animal sex. Inhibin B levels appear to be a good candidate biomarker for reproductive monitoring in echinoderms. Inhibin B could dramatically affect what we know regarding effects of stressors, and the urgent actions for effective and cost-effective ocean water conservation. Further studies will elucidate the role of inhibin B on echinoderm reproduction.
Keywords
echinoderm, fecundity, gamete, inhibin b, oocytes, sand dollar, sea urchin, spermatozoa, TGF-beta
Introduction
Marine invertebrates, such as the sea urchin, are a time honored model for investigational studies in developmental biology [1]. The effects of stressors, for example nutritional deficiencies, anthropogenic chemical contaminants, and biotoxins in marine animals have been very difficult to assess using traditional methods. The inability to demonstrate the magnitude and extent of effects of environmental stressors such as climate change, red tides and contaminants on marine animals represents a significant impediment to their conservation. The identification and subsequent detection of biomarkers allows scientists to correlate effects, such as impaired reproduction, with possible causes.
An enzyme-linked immunosorbent assay (ELISA) specific for inhibin B serves to detect this biomarker of fecundity potential in individual mammals in both sexes [2,3]. The inhibin B ELISA assay was developed to promote better understanding of reproductive potential and fitness of humans [4,5], and appears to function effectively for other mammals as well [6,7]. Inhibin B is a peptide hormone which belongs to the transforming growth factor-β (TGF-β) superfamily [8]. In the mammalian female, inhibin B is expressed by granulosa cells of the ovaries and has been shown to be vital in the regulation of ovarian function. As the ovarian follicle cohort size decreases, which is expected as the female ages, a decrease in inhibin B levels is observed. In the mammalian male, inhibin B is expressed by the Sertoli cells and plays a role in Leydig cell steroidogenesis (i.e., testosterone production) and regulating follicle stimulating hormone (FSH) production by the anterior pituitary. Inhibin B has been shown to be an excellent marker of male reproductive health, i.e. testicular function.
An assay for specific markers of fertility can be used to facilitate investigations into gonadal dysfunction [9-11] and even help assess reproductive potential or status [12,13]. Environmental and human-caused stressors can affect wildlife populations in several ways, some more critical than others. One of the more serious effects can involve fecundity and reproductive success. Therefore, the study objective was to investigate inhibin B levels between different echinoderms, sea urchins [14] and sand dollars [15], and to also compare levels between female and male gametes We also demonstrate the utility of the inhibin B ELISA assay to detect fecundity potential in echinoderms
Materials and Methods
Individual sea urchins (L. variegatus) and sand dollars (D. excentrus) were injected with 0.5 mL KCl (0.5M) to induce gamete shedding. Shed gametes were collected in sterile sea water and gamete concentrations were recorded via a Makler Counting Chamber (Sefi-Medical Instruments, Haifa, Israel). Following centrifugation (400xg, 10 minutes), and discarding the supernatant, the gametes were resuspended in 1 mL cold lysis buffer (10% Triton-X-100 in sterile water) and stored at -70oC until assayed for inhibin B.
The inhibin B enzyme-linked immunosorbent assay (ELISA) was conducted per the manufacturers’ (Beckman Coulter, Inc., Chaska, Minnesota, USA) instructions and processed on an automated plate processor (Dynex DSX Automated Plate Processor, Dynex technologies, Chantilly, Virginia, USA). Inhibin B standards, inhibin B controls, and duplicate gamete samples were incubated in microtitration wells which had been coated with the inhibin B capture antibody. After incubation and washing, the wells were exposed to a biotin-labelled antibody for detection. After a second incubation and washing step, the wells were incubated with streptavidin-horseradish peroxidase (HRP). After a third incubation and washing step, the wells were incubated with the substrate tetramethylbenzadine (TMB). An acidic stopping solution was then added and the degree of enzymatic turnover of the substrate was determined by dual wavelength absorbance measurement at 450 and 620 nm. The absorbance measured is directly proportional to the concentration of inhibin B present. The set of inhibin-B standards was used to plot standard curves of absorbance vs. inhibin B concentration. Based on the standard curves, the concentration of inhibin-B in the gamete samples was calculated (pg/106 cells) for each specimen collected.
Statistical calculations were performed with SigmaPlot for Windows, version 13.0.0.83 (Systat Software, Inc, San Jose, California, USA). Student’s t-test analysis was used to investigate the relationships between the measures of the study.
Results
Inhibin B levels between D. excentrus oocytes and L. variegatus oocytes were while not significantly different may still be of biological significance (P = 0.09, Figure 1), which may be a factor of small sample size (n=18 total). Inhibin B levels were significantly (P < 0.01, Figure 2) higher in D. excentrus spermatozoa than L. variegatus spermatozoa.
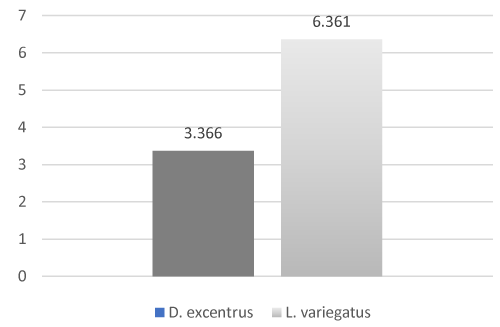
Figure 1: Inhibin B levels between D. excentrus oocytes and L. variegatus oocytes (P = 0.09)
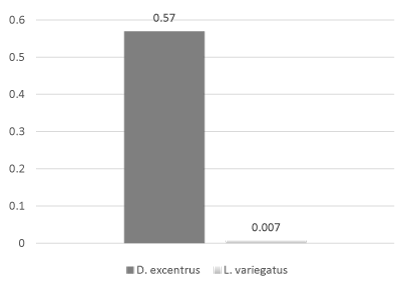
Figure 2: Inhibin B levels were significantly (P < 0.01) higher in D. excentrus spermatozoa than L. variegatus spermatozoa
Inhibin B levels in D. excentrus ranged from a low of 0.001 pg/106 spermatozoa to a high of 0.014 pg/106 spermatozoa (mean = 0.0568 + 0.014 pg/106 spermatozoa) in males. In female D. excentrus, inhibin B levels ranged from a low of 0.357 pg/106 oocytes to a high of 9.166 pg/106 oocytes (mean = 3.364 + 1.121 pg/106 oocytes). Inhibin B levels were significantly (P < 0.01, Figure 3) higher in D. excentrus spermatozoa compared to D. excentrus oocytes.
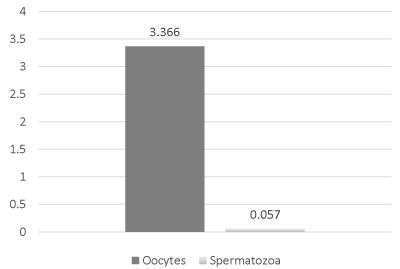
Figure 3: Inhibin B levels were significantly (P < 0.01) higher in D. excentrus spermatozoa compared to D. excentrus oocytes.
Inhibin B levels in L. variegatus spermatozoa ranged from a low of 0.005 pg/106 spermatozoa to a high of 0.236 pg/106 spermatozoa (mean = 0.007 + 0.001 pg/106 spermatozoa). In L. variegatus oocytes, inhibin B levels ranged from a low of 0.242 pg/106 oocytes to a high of 15.791pg/106 oocytes (mean = 6.361 + 1.797 pg/106 oocytes). Inhibin B levels were significantly (P < 0.001, Figure 4) higher in L. variegatus spermatozoa compared to L. variegatus oocytes.
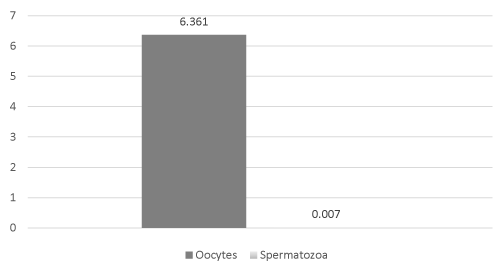
Figure 4: Inhibin B levels were significantly (P < 0.001) higher in L. variegatus spermatozoa compared to L. variegatus oocytes
Discussion
The transforming growth factor-beta (TGF-beta) superfamily is a multifunctional group of cytokines. Members of this superfamily are secreted proteins involved in many cellular functions including apoptosis, cell differentiation, cell growth and cell proliferation. Members of the TGF-beta family of genes reported to be present in echinoderms include, activin B [16], bone morphogenetic protein (BMP-4), myostatin [17], and nodal [18], which all have roles in supporting development.
We have demonstrated, for the first time, that inhibin B is present in the gametes of an echinoderm, but the question remains, “what is the biological significance of inhibins in the echinoderm?” In mammals, the primary role of inhibins is to help regulate the negative feedback system in the hypothalamus-pituitary-gonad axis. Since echinoderms lack this regulatory system, this role of inhibins is highly unlikely. Since, inhibin B content in oocytes was found to be vastly higher than in spermatozoa, one functional possibility is that inhibin B serves as a chemoattractant to facilitate spermatozoa-oocyte interactions. However, TGF-beta family members are known to play fundamental roles in regulation of basic biological process, e.g., growth, development, and homeostasis [19], inhibins most likely facilitate gamete function by a yet unreported mechanism. Note, another member of the TGF-beta superfamily (anti-mullerian hormone, AMH) found to be of great importance in mammalian gonadal function was undetectable in echinoderm gametes (unpublished observations). This observation is not surprising for AMH, while it is essential during embryonic development for the correct formation of male and female structures in mammals, AMH is most likely not required in echinoderms in this capacity.
We evaluated the use of a protein biomarker developed to assess fertility potential of male and female humans and determined that it can also be used to asses fertility potential in echinoderms. Our tests of echinoderm gametes demonstrate that the ELISA specific for inhibin B works for these represented animals of the class Echinoidea. Appropriate validation will allow us to assess fecundity potential as a function of environmental parameters including, but not limited to geographic location, physiochemical nature, and, or exposures to toxins or contaminants. Environmental factors such as stress directly affect reproduction and survival [20]. One such environmental factor is the quality of our seawater. The physiochemical nature (e.g., pH and salinity) of the seawater into which gametes are released plays a key role in fertilization and early embryonic development and survival.
Rising carbon dioxide (CO2) levels in our atmosphere are a key driver of environmental change. As CO2 levels rise, seawater absorbs it which results in a lowering of mean pH, referred to as ocean acidification. Once we have demonstrated the relationship between inhibin B content in gametes and fecundity, we can observe the impact of ocean acidification on echinoderm fecundity potential.
Detection of inhibin B or similar TGF-beta superfamily members could dramatically affect both what we know regarding effects of stressors, and the urgency of mitigation actions for effective and cost-effective conservation. Additionally, inhibin B detection has huge implications for evaluating environmental impact, mostly in assessing biodiversity hotspots [21]. Echinoderms are excellent monitors of the ocean habitat and may help in assessments of global ocean hotspots [1]. We believe that determining the fecundity/fertility potential via the use of a simple ELISA specific for a protein biomarker, such as inhibin B, in any animal (invertebrate or vertebrate) will aid in furthering our understanding of the regulatory events during fertilization and early development.
Additionally, these biomarkers have the potential for us to learn more about the reproductive capacity of invasive species which could result in programs to help eliminate their populations.
References
- Lyons DC, Kaltenbach SL, McClay DR (2012) Morphogenesis in sea urchin embryos: linking cellular events to gene regulatory network states. Wiley Interdiscip. Rev. Dev. Biol 1: 231-52.
- Kumanov P, Nandipati K, Tomova A, Agarwal A (2006) Inhibin B is a better marker of spermatogenesis than other hormones in the evaluation of male factor infertility. Fertil Steril 86: 332-338. [Crossref]
- Kwee J, Schats R, McDonnell J, Themmen A, de Jong F, et al. (2008) Evaluation of anti-Müllerian hormone as a test for the prediction of ovarian reserve. Fertil Steril 90: 737-743. [Crossref]
- Welt C, Sidis Y, Keutmann H, Schneyer A (2002) Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med (Maywood) 227: 724-752. [Crossref]
- Knight PG, Glister C (2006) TGF-beta superfamily members and ovarian follicle development. Reproduction 132: 191-206. [Crossref]
- Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, et al. (2006) Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 147: 3228-3234. [Crossref]
- Schlatt S, Pohl CR, Ehmcke J, Ramaswamy S (2008) Age-related changes in diurnal rhythms and levels of gonadotropins, testosterone, and inhibin B in male rhesus monkeys (Macaca mulatta). Biol Reprod 79: 93-99. [Crossref]
- Hu PP, Datto MB, Wang XF (1998) Molecular mechanisms of transforming growth factor-? signaling. Endocrine Reviews 19:349–63.
2021 Copyright OAT. All rights reserv
- Stewart J, Turner KJ (2005) Inhibin B as a potential biomarker of testicular toxicity. Cancer Biomark 1: 75-91. [Crossref]
- Takizawa S, Horii I (2002) Endocrinological assessment of toxic effects on the male reproductive system in rats treated with 5-fluorouracil for 2 or 4 weeks. J Toxicol Sci 27: 49-56. [Crossref]
- Uzumcu M, Kuhn PE, Marano JE, Armenti AE, Passantino L (2006) Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-Mullerian hormone production in the rat ovary. J Endocrinol 191: 549-558. [Crossref]
- Monsees TK, Franz M, Gebhardt S, Winterstein U, Schill WB, et al. (2000) Sertoli cells as a target for reproductive hazards. Andrologia 32: 239-246. [Crossref]
- Mahmoud A, Kiss P, Vanhoorne M, De Bacquer D, Comhaire F (2005) Is inhibin B involved in the toxic effect of lead on male reproduction? Int J Androl 28: 150-155. [Crossref]
- Lamarck JBM (1816) Histoire naturelle des animaux sans vertèbres. Tome troisième. Paris: Deterville/Verdière. 612 pp.
- Eschscholtz J F (1831) Zoologischer Atlas - Beschreibungen neuer Thierarten, während des Flottcapitains von Kotzebue zweiter Reise um die Welt, auf der Russisch-Kaiserlichen Kriegsschlupp Predpriaetië in den Jahren. 1823-1826.
- Sethi AJ Angerer RC, Angerer LM (2009) Gene regulatory network interactions in sea urchin endomesoderm induction. PLoS Biol 7: e1000029.
- Li S, Zhou Z2, Dong Y1, Sun H1, Gao S1, et al. (2016) Molecular characterization, expression analysis of the myostatin gene and its association with growth traits in sea cucumber (Apostichopus japonicus). Comp Biochem Physiol B Biochem Mol Biol 201: 12-20. [Crossref]
- Duboc V, Lepage T (2008) A conserved role for the nodal signaling pathway in the establishment of dorso-ventral and left-right axes in deuterostomes. J Exp Zool B Mol Dev Evol 310: 41-53. [Crossref]
- Herpin A, Lelong C, Favrel P (2004) Transforming growth factor-beta-related proteins: an ancestral and widespread superfamily of cytokines in metazoans. Dev Comp Immunol 28: 461-485. [Crossref]
- Wingfield JC, Sapolsky RM (2003) Reproduction and resistance to stress: when and how. J Neuroendocrinol 15: 711-724. [Crossref]
- Dupont S, Ortega-Martínez O, Thorndyke M (2010) Impact of near-future ocean acidification on echinoderms. Ecotoxicology 19: 449-462.




