The knee articular cartilage must withstand large compressive loads, providing a soft and lubricated surface in the contact between bone epiphysis. This structure smoothies knee movements and promotes adequate load distribution. Assessing the mechanical properties of the articular cartilage and understanding its healthy behavior can be a way of preventing pathological situations like osteoarthritis. Animal models can be useful tools for the refining and previous deliberation of the best techniques for the study of human articular cartilage. The effectiveness of the information got from these studies depends on the resemblances and differences between animal and human articular cartilage biology, chemistry and mechanical properties. Bovine models has been used for the comparison of its articular cartilage mechanical properties, with the ones of humans. There are not many studies related to the mechanical properties of swine cartilage. The difficulty in testing in human samples and the similarities between these and the swine tissues justify this work. Hereupon, this work intends to characterize this biological structure by undergoing mechanical compression tests. Both unconfined and confined ramp-stress relaxation compression tests of cylindrical cartilage discs, extracted with a defined protocol from the swine femur, were utilized to determine Young’s modulus, E, and aggregate modulus, HA, of the cartilage tissue. The obtained results indicate values of E =0.3886 MPa and HA =0.4777 MPa.
articular cartilage, cartilage biomechanics, confined compression, unconfined compression, knee joint, ramp-stress relaxation
The knee is the major and most complex synovial joint of the human skeleton and it is composed of many structures providing mechanical stabilization and support. This joint transmits loads from the body upper part to the ground and it is fundamental for human locomotion. Since the knee is frequently mechanically demanded, it is also susceptible to several types of injuries. The study of the knee joint is particularly relevant in traumatology due to the high prevalence and severity of injuries. The best comprehension of the knee behavior is obtained through the knowledge of its anatomy and physiology, as well as through the understanding of the mechanical behavior of each structure independently. Therefore, this work is concerned with the mechanical characterization of the knee articular cartilage (AC).
In a typical synovial joint, the ends of opposing bones are covered with a thin layer of AC [1], which supports high physiological stress while, simultaneously, reducing friction between articular surfaces [2]. AC is composed of a relatively small number of cells, known as chondrocytes, surrounded by a multicomponent extracellular matrix and interstitial fluid, [1,3]. This tissue is considered structurally inhomogeneous, mechanically non-linear and anisotropic [4,5]. It is important to understand the cartilage response to stress when it is mechanically stimulated, because this can be a way to prevent pathological situations like osteoarthritis, since it seems to result from a mechanically induced process [1].
The mechanical behavior of AC has been characterized using different theoretical models, like: elastic [4,6,7], biphasic and poroelastic [8-11]. More recently, and in a biomechanical simulation point of view, poroviscoelastic and fibril reinforced poroelastic [12-14], or transversely isotropic biphasic models [15-18], have also been introduced to simulate more realistically the behavior of such complex structures of AC [7]. Due to its internal structure, the mechanical response of cartilage is strongly influenced by the fluid within the extracellular matrix. Because of that, the tissue is generally called as biphasic mixture, which can be characterized as a viscoelastic material that undergoes a time‑dependent behavior [3,19]. However, these approaches are very complex. In other perspective and to simplify, several experimental investigations consider the cartilage tissue as a homogeneous and isotropic material. Indeed, under conditions of rapid dynamic loads, the cartilage behaves as a single phase of elastic solid, since there is no time for the fluid to move relative to the solid matrix [3].
To fully understand the behavior of AC it is required to determine its mechanical properties. There are three commonly accepted methods for the determination of mechanical properties of articular cartilage, i. e. unconfined compression, confined compression and indentation testing [1,3,7,20].
Animal models can become a useful tool for the refining and previous deliberation of the best experimental techniques to be used for future studies in human material tissue. The usefulness of the information obtained from these studies depends on the common aspects between the properties of human and animal cartilage [21]. Bovine models have been studied [2,4,7], and the differences with the human tissue, are not very significant [5], which can show that animal cartilage may be a good material for the validation of mechanical testing methods that allow the characterization of the tissue. There are not many studies related to the mechanical properties of swine cartilage, except for Ronken et al. [22] who compares the dynamic stiffness of human and swine AC, concluding that pig’s cartilage may serve as a standard for mechanical evaluations.
The present study intends to be a contribution for the mechanical characterization of the swine knee cartilage, by undergoing unconfined and confined compression tests. In this way information is sought that allows to define the swine AC as a better approximation to the human tissue. For the mechanical testing compression stress‑relaxation tests were performed, since they are the most common protocols to evaluate the mechanical properties of cartilage [2,4,5,7]. In the present work, the equilibrium response of swine AC was studied and the commonly used assumption that cartilage behaves like an elastic and isotropic material was adopted [4,6,7].
The authors believe that this study is a step in the direction of the characterization of swine AC in order to future study osteoarthritis and its repair and reconstruction techniques.
Sample extraction
Two fresh and normal-looking knee joint cartilage was derived from the swine’s bodies. Cylindrical cartilage discs from the condyles and superior area of femur were punched with a diameter of 6 and 10 mm, in a number of 40 samples, approximately. These samples were frozen in saline fluid until 12 hours before the day of testing.
Mechanical Testing
The stress-relaxation tests were performed in a universal machine of mechanical tests INSTRON® ElectroPlus E1000, with a 1 kN load cell. A fully automated series of stress‑relaxation steps (step 10 µm, velocity 1 µm/s) was repeated up to a 20 per cent of strain [2,4,5,7]. After a phase-test, the criterion for the beginning of a new step was set to 90 seconds of relaxation, assuming that it would be a considerable time for the relaxation of the tissue.
The mechanical behavior of the cartilage was registered on the computer, using the specific software of the test equipment. For each test, the complete time-position-load data was recorded. From these, the respective values of stress-strain were determined during the test.
In unconfined and confined compression, the Young’s modulus (E) and the aggregate modulus (HA), respectively, were determined from the linear range of the stress-strain curve, assuming a homogeneous and isotropic material. The Poisson’s ratio (v) can be calculated applying Equation (1), [3,4,7]:
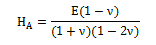 (1)
(1)
Result Analysis
All data were analyzed in Microsoft Office Excel®, which allowed the adjustment of curves that show the tissue behavior according to the imposed load. From these curves it was possible to determine the mechanical properties of interest. Data are presented as mean ± sd (standard deviation), with a total number (n) of nine experiments.
In stress-relaxation a lower test speed was considered in order to allow the tissue a considerable relaxation phase. Figure 1 shows a typical time-force response for cartilage tissue in a stress-relaxation test. In the equilibrium phase, stress and strain can be calculated, which allows to create an approximate curve of the stress‑strain response of the tissue. An example of this can be found in Figure 2.
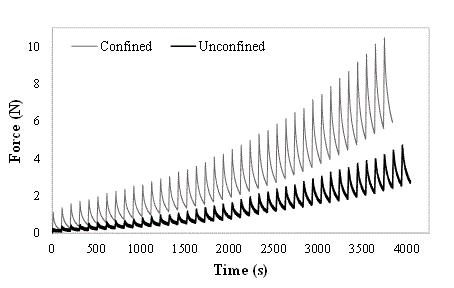
Figure 1. Typical behaviour of cartilage in confined and unconfined compression for a stress relaxation test (example from a sample with Ø=6mm)

Figure 2. Stress-strain response in the equilibrium for the stress-relaxation test
In a general way, all the samples showed a similar equilibrium response in the first 5-10% of strain. Increasing the strain (10-20%), the balance of stress-strain behavior is approximately linear, which allows the determination of the mechanical properties of interest (E and HA). The results from these tests are shown in Table 1, where Poisson’s ratio was determined according to Equation (1). The mechanical parameters obtained are dependent on the sample diameter. Although, and despite the different diameter, samples are from the same type of tissue (femoral cartilage). Due to this fact, a mean value of each mechanical property was calculated.
Table 1. Young's modulus and aggregate modulus determined by stress-relaxation tests and respective Poisson's ratio (mean ± sd, n=9)
Ø [mm] |
E [MPa] |
HA [MPa] |
v |
6 |
0.5464±0.1576 |
0.6421±0.1793 |
0.2392±0.0104 |
10 |
0.2307±0.0241 |
0.3133±0.1028 |
0.2712±0.0887 |
Mean |
0.3886 |
0.4777 |
0.2552 |
In the present study, confined and unconfined compression tests were conducted in samples of swine knee articular cartilage to determine its mechanical properties.
Compression stress-relaxation tests were performed to evaluate the mechanical properties of cartilage. In fact, this kind of tests is used because they closely resemble what actually happens in the tissue when physiological loads are imposed. The compression phase in stress-relaxation tests is associated with the exudation of the interstitial fluid, where the maximum stress is generated when the fluid passes through the solid matrix, compressing it. On the other hand, in the next phase (relaxation) the fluid is redistributed through the porous matrix, with a stress relief. The analysis of this process leads to the conclusion that under physiological loading conditions it is difficult to maintain excessive levels of stress in the cartilage, due to its natural process of stress‑relaxation, which permits to rapidly attenuate the high-generated stresses.
According to the definition of stress, a larger diameter corresponds to a lower stress, because the stress decreases with the increase of the sectional area of the sample. The diameters differentiation shows that the maximum force achieved is dependent on the diameter, and for a larger diameter, the force required for tissue’s failure is also higher.
Confined and unconfined compression tests allowed the determination of the values of aggregate and Young’s modulus, through the linear range of the stress-strain curves in the equilibrium (Figure 2). From this data, values of Poisson’s ratio can be calculated. A comparison with the results obtained from the present study with the ones found in the literature is present on Table 2.
Table 2. Comparison of the mechanical properties achieved in the present study with the mechanical properties of the literature
Reference |
Sample type |
E [MPa] |
HA [MPa] |
v |
Optic |
Indirect |
Jurvelin et al., 1997 |
Bovine |
0.677 |
0.754 |
0.185 |
0.174 |
Buschmann et al., 1998 |
Bovine |
- |
0.56 |
- |
- |
Korhonen et al., 2002 |
Bovine |
0.31 |
0.34 |
0.21 |
0.26 |
Jurvelin et al., 2003 |
Human |
0.581 |
0.845 |
- |
0.158 |
Present study |
Swine |
0.3886 |
0.4777 |
- |
0.2552 |
The results shows consistency with the literature, especially with the study of Korhonen et al., [7]. Note that most of the literature reports rely on bovine cartilage samples. The results of the present study demonstrate that swine cartilage can be equally valid when compared to human tissue.
The entire analysis of the cartilage equilibrium response was based on the assumption of isotropic material, which is a very common approach to the confined and unconfined compression tests. Although this assumption seems to satisfactorily meet the requirements, Korhonen et al., [7], reports that the elastic parameters depends on the technique used to determine a mechanical property. So, an isotropic model cannot be universally imposed in the characterization of the AC. Despite that equilibrium response in confined and unconfined compression are satisfactory described by an elastic isotropic model, Korhonen et al., [7], reports higher values of the mechanical properties for the indentation test, as well as dependency from indenter diameter.
In the present study indentation tests could not be realized, due to the fact that no intact cartilage samples (cartilage attached to subchondral bone) were extracted. The authors believe that a more accurate conclusion can be achieved if these tests were performed. Moreover, the present study results should be compared with a more realistic approach by viscoelastic models assumption that includes the permeability of the tissue. Additionally, a theoretical analysis focusing on the nonlinearity of the cartilage matrix could clarify the mechanism behind the mechanical response in continuous compression tests. Nevertheless, the authors have confidence in that the present study is a contribution to the mechanical characterization of a tissue (swine cartilage) that has not yet been much studied. This work is a first step of a bigger goal that intends the future study of osteoarthritis and its repair and reconstruction techniques.
The authors of this paper have no financial or personal relationships with other people or organizations that could inappropriately influence (bias) our work.
2021 Copyright OAT. All rights reserv
This research did not receive any specific grant from funding agencies in the public, commercial, or not‑for‑profit sectors.
- Mansour JM (2003) Biomechanics of Cartilage, in Kinesiology: The Mechanics and Pathomechanics of Human Movement 66-79.
- Buschmann MD, Soulhat J, Shirazi-adl A, Jurvelin JS, Hunziker EB (1998) Confined compression of articular cartilage: Linearity in ramp and sinusoidal tests and the importance of interdigitation and incomplete confinement. J Biomech 31: 171-178. [Crossref]
- Completo A, Fonseca F (2011) Fundamentos de Biomecânica Músculo-Esquelética e Ortopédica. Publindústria.
- Jurvelin JS, Buschmann MD, Hunziker EB (1997) Optical and mechanical determination of Poisson’s ratio of adult bovine humeral articular cartilage. J Biomech 30: 235-241. [Crossref]
- Jurvelin JS, Buschmann MD, Hunziker EB (2003) Mechanical anisotropy of the human knee articular cartilage in compression. Proc Inst Mech Eng H 217: 215-219. [Crossref]
- Hayes WC, Keer LM, Herrmann G, Mockros LF (1972) A mathematical analysis for indentation tests of articular cartilage. J Biomech 5: 541-551. [Crossref]
- Korhonen RK, Laasanen MS, Töyräs J, Rieppo J, Hirvonen J, et al. (2002) Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J Biomech 35: 903-909. [Crossref]
- Mow VC, Gibbs MC, Lai WM, Zhu WB, Athanasiou KA (1989) Biphasic Indentation of Articular Cartilage - II. A Numerical Algorithm and an Experimental Analysis. J Biomech 22: 853-861. [Crossref]
- Donzelli PS, Spilker RL, Ateshian GA, Mow VC (1999) Contact analysis of biphasic transversely isotropic cartilage layers and correlations with tissue failure. J Biomech 32:1037-1047. [Crossref]
- Guo H, Maher SA, Spilker RL (2013) Biphasic finite element contact analysis of the knee joint using an augmented Lagrangian method. Med Eng Physvol 35: 1313-1320. [Crossref]
- Wang CB, Hung CT, Mow VC (2001) An analysis of the efects of depth-dependent aggregate modulus on articular cartilage stress-relaxation behavior in compression. J Biomech 34: 75-84. [Crossref]
- Cortez S, Completo A, Alves JL (2015) Análise computacional da reorientação das fibras de colagénio na cartilagem articular,” in 6o Congresso Nacional de Biomecânica.
- Gu KB, Li LP (2011) A human knee joint model considering fluid pressure and fiber orientation in cartilages and menisci. Med Eng Phys vol. 33: 497-503. [Crossref]
- Halonen KS, Mononen ME, Jurvelin JS, Töyräs J, Korhonen RK (2013) Importance of depth-wise distribution of collagen and proteoglycans in articular cartilage - a 3D finite element study of stresses and strains in human knee joint. J Biomech 46: 1184-1192. [Crossref]
- LeRoux MA, Setton LA (2002) Experimental and biphasic FEM determinations of the material properties and hydraulic permeability of the meniscus in tension. J Biomech Eng 124: 315-321. [Crossref]
- Spilker RL, Donzelli PS, Mow VC (1992) A transversely isotropic biphasic finite element model of the meniscus. J Biomech 25: 1027-1045. [Crossref]
- Vaziri A, Nayeb-Hashemi H, Singh A, Tafti BA (2008) Influence of meniscectomy and meniscus replacement on the stress distribution in human knee joint. Ann Biomed Eng 36: 1335-1344. [Crossref]
- Wilson W, Van Rietbergen B, Van Donkelaar CC, Huiskes R (2003) Pathways of load-induced cartilage damage causing cartilage degeneration in the knee after meniscectomy. J Biomech 36: 845-851. [Crossref]
- Korhonen RK, Saarakkala S (2011) Biomechanics and Modeling of Skeletal Soft Tissues, in Theoretical Biomechanics, V. Klika, Ed. InTech 113-132.
- Sharpe WM (2008) Ed., Springer Handbook of Experimental Solid Mechanics.
- Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC (1991) Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res 9: 330-340. [Crossref]
- Ronken S, Arnold MP, Ardura García H, Jeger A, Daniels AU, et al. (2012) A comparison of healthy human and swine articular cartilage dynamic indentation mechanics. Biomech Model Mechanobiol 11: 631-639. [Crossref]

