Abstract
Background: Various biomaterials have been used for manufacturing dental implants with titanium being widely used. However, titanium has exhibited a few drawbacks such as metal ion release, corrosion of metals and poor compatibility with modern imaging techniques. To overcome these, alternative substitutes to titanium such as hydroxyapatite, zirconia, polyetheretherketone (PEEK) have been recently introduced.
Material and methods: Fifty edentulous sites were rehabilitated with PEEK implants out of which twenty-five were immediately loaded (day one post-op) while, the remaining 25 were loaded 30 days post-op after placement (early loading). Implants were evaluated clinically and radiographically at 1 week, 1, 3, 6 and 12 months after placement for success parameters like marginal bone loss, mobility, pain, infection, bleeding and implant survival rate.
Results: At the end of one-year observation period after implant placement, nine out of 25 immediately loaded PEEK implants failed scoring a failure rate of 36%. Six out of the other 25 early loaded implants failed, scoring a failure rate of 24%. After 12 months thirteen out of 25 immediately loaded implants and six out of other 25 early loading implants showed score 1 of bleeding on probing respectively.
Conclusions: Within the limitations of the present study, the immediately loaded PEEK implants exhibited higher failure rate compared to implants loaded with early loading protocol.
Key words
Poly ether ether ketone (PEEK), immediate loading, early loading
Introduction
Dental implants have been universally accepted as the best option for prosthetic rehabilitation of completely and partially edentulous patients, due to its biocompatibility, high resistance to fatigue, and osseointegration properties [1,2]. Titanium and its alloys have some drawbacks such as potential metal ion release and subsequent osteolysis, corrosion of metals and poor compatibility with modern imaging techniques [2].
Typically, metals used for implantation have large elastic modulus leading to impaired load force transmission at the implant tissue interface contributing to stress shielding and peri implant bone resorption. Whenever there is thin gingiva around a metallic implant, the metallic hue is shown through compromising aesthetics [2]. The varying density and iso-elasticity of titanium compared to cortical bone, makes titanium implants ideally to be functionally loaded after a healing period of 3-6 months [3]. The delay in early loading of the implant is usually unacceptable to the patient who demands aesthetically and functionally acceptable immediate prosthetic rehabilitation, especially in the anterior zone [4].
To overcome these limitations and to minimize post-implant placement biological complications, research has shifted focus towards evaluating alternative substitute to titanium. The most promising novel alternative is polyetheretherketone (PEEK) which is a partially crystalline poly aromatic linear thermoplastic substance. The PEEK (BIOPIK International Medical implant, France) has been used in a wide range of industrial applications and was highlighted as a potential biomaterial in the 1980s [5]. It has been introduced as a dental implantable quality grade material since last 8–10 years. PEEK offers superior characteristics such as excellent mechanical properties, versatile mass production, processing ability using plastic technology, natural radiolucency and MRI compatibility, lack of toxicity, good chemical and sterilization resistance, reproducible, pure and traceable supply route compared to titanium. The lighter weight, ‘‘natural’’ beige colour and reduced heat transfer are all beneficial for rehabilitating patients [5]. Contrary to metals and their alloys, PEEK combines high strength with a relatively low Young’s modulus which is closer to that of human bone than titanium. This property may minimize the stress by distributing it in more physiological manner thus supporting bone formation around the implant and reducing osteolysis [5]. The PEEK composite material is already a proven implant substitute in the load bearing long bones and has been used extensively in the field of Orthopaedics [6]. However, there is still lack of evidence as dental implant material. The present study was therefore undertaken to evaluate the clinical survival and success rate of PEEK composite dental implants using ‘immediate’ and ‘early’ loading protocols.
Material and Methods
After obtaining the institutional ethical committee clearance to carry out the study (Project No. 4225/12), 43 medically fit patients were selected for the study. The patients who were included in the study were above the age of 18 years, having at-least one tooth missing between premolars with acceptable oral hygiene after due mouth preparation and requiring an implant restoration. The inclusion criteria for the implant sites were that the edentulous sites should be completely healed, have adequate bone quality and quantity, be opposed by natural teeth or a fixed prosthesis, and the teeth in acceptable occlusal and interproximal relationships.
The exclusion criteria included patients with systemic conditions on chronic antibiotic or steroid therapy, renal failure, bisphosphonate therapy, severe or uncontrolled metabolic disorders, radiation therapy to head or neck, chronic alcoholism or drug abuse, HIV infection or smokers (more than 10 cigarettes per day), chronic tobacco chewers and patients with parafunctional habits such as severe bruxism or clenching.
Written informed, consent was obtained from each patient. A total of 50 implant sites in 43 patients were selected. Out of 50 implant sites 25 were selected for immediately loading protocol and other 25 were selected for early loading protocol. In the present study we have used Theta Implants with height of 12 mm. To eliminate the observer, bias all the assessments were done by three independent observers and mean value of observations were considered for each patient (Figure 1).
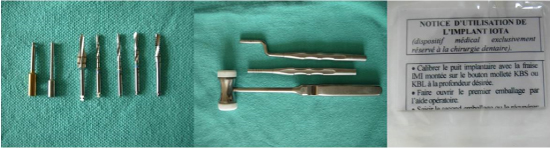
Figure 1. Osteotomy drills, Depth Gauge and Implant
Eligible patients were assessed by clinical and radiographic examination, and detailed medical and dental history was collected. Randomly selected twenty-five implant sites were loaded immediately on day 01 of surgery and the remaining twenty-five implant sites were loaded 30 days after implant placement. Surgical protocols were followed by a single operator (Figures 2-4) under local anaesthesia under aseptic conditions. Standard technique for raising the flap was used and the manufacturer’s recommendations on sequence of osteotomy procedures and implant placement were followed. A periotest was used to assess the primary implant stability and any implant having a value of +20 to +50 was excluded from further study participation. The day of surgery was defined as the baseline (day 01).
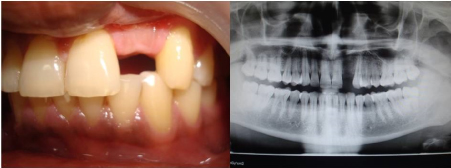
Figure 2. Pre-operative intraoral view and Orthopantomograph
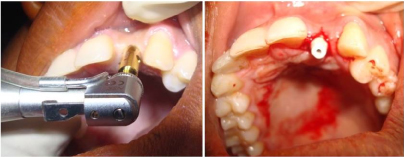
Figure 3. Osteotomy and implant placement
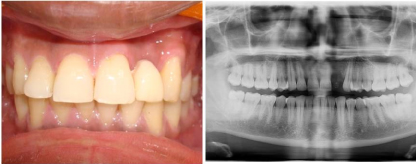
Figure 4. Post-operative intraoral view with finished restoration & Orthopantomograph
In the immediate loading group, the implants were provided with a provisional prosthesis on the day of surgery. In the early loading group, implants were placed single stage. These were loaded with a provisional prosthesis after one month (day 30). All provisional restorations were placed out of occlusal contact in centric and eccentric movements. In both groups, permanent fixed prostheses were placed 3 months after implant placement (Day 90).
The study subjects in both groups were evaluated clinically for mobility, peri-implant probing depths and bleeding on probing of implant site using periodontal probe and/or naked eye examination at baseline and thereafter 01-week, 01 month, 03 months, 06 months and 12 months (Table 1-3). Lack of clinical mobility, absence of peri-implant radiolucency, recurrent peri-implant infection and pain was the criteria used to assess success or failure of the implants. Recurrent pain and periapical infections were not observed in any of the cases. Periimplant radiolucency was assessed by the comparison of radiolucency of pre and post x ray of the PEEK implant. Implants with clinical mobility of score 3 and score 4 were considered as failures and replaced at the later stage (Table 4).
Table 1: Data on probing depth
|
Baseline Mean ± SD (mm) |
1 week Mean ± SD (mm) |
1 month Mean ± SD (mm) |
3 months Mean ± SD (mm) |
6 months Mean ± SD (mm) |
12 months Mean ± SD (mm) |
|
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Mesial |
2.23 ± 0.45 |
2.86 ± 0.41 |
2.37 ± 0.49 |
2.67 ± 0.53 |
2.67 ± 0.41 |
2.87 ± 0.13 |
2.73 ± 0.52 |
2.73 ± 0.62 |
2.92 ± 0.48 |
2.98 ± 0.41 |
3.98 ± 0.41 |
3.17 ± 0.46 |
Mid Buccal |
1.84 ± 0.41 |
1.67 ± 0.42 |
1.98 ± 0.52 |
1.83 ± 0.42 |
2.48 ± 0.32 |
2.83 ± 0.62 |
2.12 ± 0.43 |
1.96 ± 0.40 |
2.42 ± 0.45 |
2.67 ± 0.47 |
3.61 ± 0.48 |
2.96 ± 0.41 |
Distal |
2.58 ± 0.51 |
2.12 ± 0.52 |
2.69 ± 0.45 |
2.52 ± 0.51 |
2.64 ± 0.35 |
2.92 ± 0.61 |
2.98 ± 0.42 |
2.84 ± 0.54 |
3.36 ± 0.57 |
3.12 ± 0.58 |
4.37 ± 0.58 |
3.43 ± 0.50 |
Mid lingual |
1.61 ± 0.47 |
1.47 ± 0.41 |
1.81 ± 0.57 |
1.68 ± 0.45 |
2.11 ± 0.47 |
2.18 ± 0.55 |
2.21 ± 0.41 |
1.97 ± 0.49 |
2.52 ± 0.50 |
2.41 ± 0.49 |
3.67 ± 0.63 |
2.87 ± 0.48 |
Table 2. Data on bleeding on probing
|
Baseline |
1 week |
1 Month |
3 months |
6 months |
12 months |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Score 0 |
25 |
25 |
20 |
23 |
18 |
22 |
17 |
22 |
14 |
21 |
7 |
18 |
Score 1 |
0 |
0 |
4 |
2 |
5 |
3 |
7 |
3 |
9 |
4 |
13 |
6 |
Score 2 |
0 |
0 |
1 |
0 |
2 |
0 |
1 |
0 |
1 |
0 |
3 |
1 |
Score 3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
2 |
0 |
• Score 0 - No bleeding when a periodontal probe is passed along the mucosal margin adjacent to the implant
• Score 1 - Isolated bleeding spots visible
• Score 2 - Blood forms a confluent red line on mucosal margin
• Score 3 - Heavy or profuse bleeding
Table 3. Data on implant mobility
|
Baseline Mean |
1 week |
1 Month |
3 months |
6 months |
12 months |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Score 0 |
25 |
25 |
25 |
25 |
25 |
25 |
12 |
16 |
19 |
14 |
6 |
14 |
Score 1 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
3 |
6 |
3 |
6 |
3 |
Score 2 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
3 |
4 |
3 |
4 |
2 |
Score 3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
6 |
3 |
Score 4 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
3 |
Score 0 – Absence of clinical mobility in direction
Score1 – slight detectable horizontal movement
Score 2 – Moderate visible horizontal mobility up to 0.5 mm
Score 3 – Severe horizontal movement greater than 0.5 mm
Score 4 – Visible moderate to severe horizontal and any visible vertical movement
Table 4. Data on implant survival
|
Baseline |
1 Month |
3 Months |
6 months |
12 months |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
Immediate Loading Group |
Early Loading Group |
No of implants Evaluated |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
No of Implants Failed |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
09 |
6 |
Overall Failure |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
36% |
24% |
Overall Survival |
100% |
100% |
100% |
100% |
100% |
100% |
100% |
100% |
64% |
76% |
Kaplan-Meier Survival Probability Estimates with 0.95 Confidence Interval |
1
(0.834227-1) |
1
(0.834227-1) |
1
(0.834227-1) |
1
(0.834227-1) |
1
(0.834227-1) |
1
(0.834227-1) |
1
(0.834227– 1) |
1
(0.834227 -1) |
0.64
(0.426153 - 0.81288) |
0.76
(0.544792-0.898419) |
Table 5. Data on implant location and location of failed implants
|
Maxillary First premolars |
Maxillary centrals |
Maxillary laterals |
Maxillary canines |
Mandibular first premolars |
Mandibular centrals |
Mandibular laterals |
Mandibular canines |
Total |
No of Sites |
2 |
14 |
12 |
8 |
2 |
4 |
4 |
4 |
50 |
No of Failures |
0 |
5 |
4 |
3 |
0 |
2 |
1 |
0 |
15 |
The radiographic assessment was done by placing the intra oral periapical film with grid parallel to the implant and by directing the X-ray beam perpendicular to the implants. The distance between the cone and the implant was standardized for each patient using a long cone technique and a ‘Rinn’ x-ray holder. These standardized radiographs with grids were used to calculate changes in crestal bone level between the implant shoulder and the first bone to implant contact (BIC), measured at the mesial and distal aspects of each implant (Figure 5). Due to the radiolucent nature of the implant material, radiographic parameters could not be evaluated and hence the crestal bone loss and peri implant radiolucency were not ascertained.
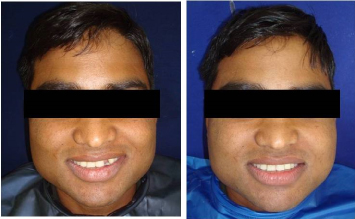
Figure 5. Pre- and Post-operative photographs
Results
In this study, all patients participated until the end of the study with no clinical dropout. At the end of one-year observation period, out of 25 PEEK implants loaded with immediate loading protocol, nine had to be replaced due to failure, therefore depicting a failure rate of 36%. Out of 25 implants loaded with early loading protocol, 6 had to be replaced thus a failure of 24%. There was 64% mean survival rate of immediately loaded and 76% mean survival rate of early loaded implants. Out of fifty PEEK implants evaluated at the end of one year 15 implants needed replacement making overall failure rate to be 30%.
Out of 50 implants evaluated, 15 implants exhibited score 3 or score 4 mobility requiring replacement. Clinical parameters were the main criteria for evaluation of success or failure of this system.
Table 1 depicts data on probing depth at base line, after one week, one month, three months, six months and one year. Table 2 depicts data on bleeding on probing according to criteria given by Mombelli et al. Table 3 depicts data on implant mobility. Table 4 depicts data on implant survival at the end of one month, three months, six months and one year of observation period with both immediate and early loading protocols which were statistically analysed with Kaplan-Meier Survival Probability Estimates (0.95 Confidence Interval).
Discussion
The field of implantology has experienced significant advancements over the past 40 years, which has revolutionized the field of dentistry. The implant shape, surface treatment, and the aesthetics of prosthetic components are of prime concern. The use of polymers to manufacture bone integrated implants as a substitute for conventional titanium components have been developed [5].
Polyether ether ketone (PEEK) is an injection molded non-metallic, non-ceramic, bioactive colorless organic polymer of calcium phosphate [7]. It is a dispersion of Beta tri calcium phosphate and titanium oxide in a PEEK matrix. PEEK (poly-ether-ether-ketone), is a dominant member of the PAEK (poly-aryl-ether-ketone) polymer family, was used as a main substitute for the metallic components in orthopaedic cases and trauma. Although pure poly-aromatic polymers exhibit elastic modulus that varies from 3 to 4 GPa, this value can be modified to achieve a tensile strength of 90-100 Mpa and elastic modulus as near to cortical bone (18 GPa) with the addition of composites, such as carbon fiber (CFR-PEEK) [8]. This biocompatible material exhibits a wide range of physical, mechanical, and surface properties and in several shapes. Based on the energy dissipation theory, a force applied to the implant supported crown is known to be transferred through the implant, with small alterations due to the energy conservation feature of the rigid implants. Metallic implants are at least eight times harder than the neighbouring bone. This gradient difference generates stresses in the bone-implant interface during load transfer. An implant with an elastic modulus similar to bone has the potential for a more homogenous stress distribution to the supporting tissues with a stress decrease in this interface [9].
Polyether-ether ketone (PEEK) is a hard-radiolucent plastic that is used in conjunction with carbon fibre reinforcement or as pure PEEK. PEEK is chemically inert and insoluble in all conventional solvents at room temperature, with the exception of 98% sulphuric acid [9].
The PEEK implants were from manufacturer BIOPIK International Medical implant, France. These are single piece and three types of Implants are available in this system i.e. Theta, Tau and Iota type [10]. Theta implants are of 4.8 mm diameter and with three heights of 10.5, 12, 15 mm which are inserted by gentle friction to ensure primary fixation. Tau and Iota implants are of 4.8 mm and 3.0 mm diameter respectively and with three heights of 10.5, 12, 15 mm and both are inserted as a cylindrical drill to ensure primary fixation. All the three implants can be adjusted in mouth for height and aesthetics.
The bone is considered as a composite material with the mineral part of hydroxyapatite (70%) and the organic portion of glycoproteins, proteoglycans and bone proteins (30 %). Implants used in orthopaedics and dentistry, to be effective in the bone, should simulate physical properties of bone. The PEEK implant materials seems better than the titanium since they have homogeneity, the composite texture opens the avenue to individualization of the implant allowing specific needs of individual cases [11].
An in vitro study by Berendsen AD et al. [12] demonstrated attachment of a collagen gel to PEEK by enzyme-induced mineral deposition. Such kind of coating could be used to anchor a PEEK implant in the alveolar bone by collagen fibers similar to that observed in a natural tooth, which could represent another advantage of PEEK over titanium, giving back the physiological tensile load to the bone.
There are many scientific publications which have confirmed that the PEEK material is suitable to be used for implantation for long term in the human body [5,7,13]. The pure PEEK OPTIMA is an excellent material, perfectly suited, in terms of biomechanics, able to support and transfer the usual constraints of masticatory forces acting on the jaws and facial bones during mastication. It also provide the basis for the integration of implants in bone reinforced by PEEK and as Oral basal implant altogether [14]. PEEK implants have shown many advantages over the titanium and other implant materials used such as radio-transparency, easy modification in the operating room and lack of corrosion [15]. The possibilities of strengthening and integration of the modulus of elasticity in individual cases, stimulation of bone formation by tight matching, which can be expected from PEEK OPTIMA composite material, is also an additional advantage to achieve good results [16,17].
All the failed fifteen implants, which showed clinical mobility with severe horizontal movement (greater than 0.5 mm) or visible vertical movement required replacement. Mobility and failure of the implants can be attributed to crestal bone loss seen as peri-implant radiolucency, but however this parameter could not be ascertained radiologically due to radiolucent nature of the material. Failure rate was more in implants loaded with immediate loading protocol compared to implants loaded with early loading protocol, suggesting that early loading protocol has more chances of survival compared to immediate loading of PEEK implants. All the failed implants had pocket depth more than 5 mm with heavy or profuse bleeding on probing. This could be attributed to the increased sulcus depth. As the sulcus depth increases, oxygen tension decreases. Oral hygiene procedures cannot maintain and clean a sulcus greater than 2 mm which could have led to greater incidence of anaerobic bacteria causing more bone loss and more incidences of failure.
Conclusion
PEEK implants have emerged as the most aesthetic biocompatible material for dental implants in contrast to the titanium implants with many advantages.
The immediately loaded PEEK implants exhibited higher failure rates compared to implants loaded with early loading protocol.
Long-term multi centric studies with various possible parameters to ascertain dependability of loaded PEEK implants in vitro and in vivo are necessary. The design of a two-piece implant made from PEEK, which allows the submerged healing method, has to be developed. PEEK material used for a dental implant should have a similar and different light translucency like a natural tooth to achieve much more favourable aesthetic results.
References
- Branemark PI, Adell R, Albrektsson T, Lekholm U, Lundkvist S, et al. (1983) Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials 4: 25–28. [Crossref]
- Tengvall P, Lundström I (1992) Physico-chemical considerations of titanium as a biomaterial.Clin Mater9: 115-134. [Crossref]
- Misch CE (2015) Dental implant Prosthetics 3rd ed. Elsevier: Mosby Co.
- Sagomonyants KB, Jarman-Smith ML, Devine JN, Aronow MS, Gronowicz GA. (2008) The in vitro response of human osteoblasts to polyetheretherketone (PEEK) substrates compared to commercially pure titanium. Biomaterials 29: 1563-72. [Crossref]
- Skirbutis G, Dzingutė A, Masiliūnaitė V, Šulcaitė G, Žilinskas J. (2017) A review of PEEK polymer's properties and its use in prosthodontics.Stomatologija19: 19-23. [Crossref]
- Kurtz SM, Devine JN (2007) PEEK biomaterials in trauma, orthopedic, and spinal implants.Biomaterials28: 4845-4869. [Crossref]
- Harmand MF, Cougoulic JP (2004) A new biocompatible biomaterial: PEEK/ß-TCP/TiO2 composite. Sydney 9WBC Congress.
- Williams DF, McNamara A, Turner RM (1987) Potential of polyetheretherketone (PEEK) and carbon-fiber reinforced PEEK in medical applications. J Mat Sci Letters 6: 188.
- Sarot JR, Contar CM, Cruz AC, de Souza Magini R (2010) Evaluation of the stress distribution in CFR-PEEK dental implants by the three-dimensional finite element method. J Mater Sci Mater Med 21: 2079–2085. [Crossref]
- Marya K, Dua JS, Chawla S, Sonoo PR, Aggarwal A, et al. (2011) Polyetheretherketone (PEEK) Dental implants: A case for immediate loading. Inter J Oral Implantol Clin Res 2: 97-103.
- Volpe S, Verrocchi D, Andersson P, Gottlow J, Sennerby L (2008) Comparison of early bacterial colonization of peek and titanium healing abutments using real-time PCR. Applied Osseointegration Research 6: 54-56.
- Berendsen AD, Smit TH, Hoeben KA, Walboomers XF, Bronckers AL, et al. (2007) Alkaline phosphatase induced mineral deposition to anchor collagen fibrils to a solid surface. Biomaterials 28: 3530-3536. [Crossref]
- Wenz LM, Merritt K, Brown SA, Moet A, Steffee AD (1990) In vitro biocompatibility of polyetheretherketone and polysulfone composites. J Biomed Mater Res 24: 207–215. [Crossref]
- Poulsson A (2010) Surface Modification of PEEK- parallel investigations of primary human osteoblast cytocompatibility and bacterial adhesion. Scadinavian society of biomaterials.
- Spahn F (2010) Metal as connector between the implant and prosthodontics called into question: PEEK a simple, durable new solution. International Scientific Society for Promotion of Knowledge about Isoelastic Materials in Intraosseous Surgery.
- Schmidlin PR, Stawarczyk B, Wieland M, Attin T, Hämmerle CH, et al. (2010) Effect of different surface pre-treatments and luting materials on shear bond strength to PEEK.Dent Mater26: 553-559. [Crossref]
- Koutouzis T, Richardson J, Lundgren T (2011) Comparative soft and hard tissue responses to titanium and polymer healing abutments.J Oral Implantol37 Spec No: 174-182. [Crossref]





