A study was carried out at the Ngaoundere municipal slaughterhouse (Adamawa-Cameroon) on 199 cycling zebu cows to evaluate the potential of ovaries preserved at 5°C for in vitro production of cultivable oocytes. Thus, two (02) experiments were performed. The first trial consisted in observing and classifying the oocytes according to their quality every hour (1-13 hours) in order to determine the optimal time of preservation of the ovaries at 5 °C. The second trial investigated the biochemical variations as a function of the preservation time of glucose, total cholesterol and calcium in the follicular fluid. The results indicated that the average weight of the ovaries did not change during 13 hours of preservation. The mean follicular population of the ovaries was 8.81± 5 (5-12) and it decreased significantly (P = 0.02) from 09 hours. After 11 hours of preservation, the mean oocyte yield was 10.24 ± 5.90 (3-12) and it decreased significantly (P = 0.00). The average of the cultivable oocytes (Q1 and Q2) was 7.30± 4.87 (2-8) and it decreased significantly after 12 hours. The mean concentration of glucose in the follicular fluid after 13 hours of preservation at 5°C was 29.25±16.19 mg/dl and it increased significantly (P = 0.00) after 10 hours. Total cholesterol decreased significantly (P = 0.00) after 10 hours and was 17.97±10.63 mg/dl. The average of calcium concentration was 2.86 ±2.01 mg /dl and increased significantly (P = 0.00) after 10 hours. This study indicates that the zebus cows' ovaries could be preserved at 5°C for 9 hours without any significant change in biochemistry, yield and quality of cultivable oocytes.
zebu, ovary, preservation time, cultivable oocyte, biochemistry, Adamawa, Cameroon
Cattle breeding is one of the major components of Cameroon's agricultural economy. With a herd estimated at 6 million heads in 2013, cattles contribute 398.4 billion CFA to the country's Gross Domestic Product (GDP). On the 10 regions in Cameroon, Adamawa remains the pole for zebu breeding (Bos indicus) [1]. However, despite the great diversity of cattle breeds, animal productivity remains low. Ebangi et al. [2] reported that this low productivity is due to poor farming conditions, health, nutritional and reproductive problems. The desire to increase productivity has led to the development of genetic improvement programs. Natural mating and Artificial Insemination (AI) are practiced anarchically, thus contributing to the dispersal and dilution of the local genotype. In Vitro embryo Production (IVP) is therefore positioned as a safe alternative to improving the performance of our breeds [1]. In addition, it allows the preservation of the genetic potential of sub-fertile or dead animals by creating a gene bank. Bracket et al. [3] reported the first success of IVF and embryo production in domestic cattle. Since then, despite considerable improvement in the processes or stages of gamete collection, oocyte maturation, fertilization, culture and embryo transfer, many problems persist. In particular, the capacity of the post-collection ovaries to produce fertilizable oocytes after a certain period of preservation. In fact, the ovaries collected and preserved in a normal saline solution at 35-37 °C lose their viability (fertile oocyte life) after 2 hours of transport [4]. The ovaries taken postmortem from animal carcasses in slaughterhouses are the largest source of primary oocytes obtained at low cost for large-scale production of bovine embryos in vitro [1]. However, the distance between research laboratories and slaughterhouses, the poor condition of roads, climatic problems, the poor state of the vehicles lengthen the transport time of the ovaries with consequent loss of important sources of genetic material represented by gametes [5]. The preservation of the ovaries at 5 °C can therefore be an alternative to maintain the ovarian potential of the Cameroonian herd and to a large extent preserve the genetic potential of endangered animals [6-8]. It is in this context that this study was conducted on the zebus of Adamawa which has for main objective the evaluation of the ovarian potential of the zebus of Adamawa-Cameroon preserved at 5 °C for the in vitro production of fertile oocytes. Specific objectives were to characterize the cows in the study; determine the optimal time of preservation of the ovaries at 5 °C for a better yield and oocyte quality; to study the biochemical variations of glucose, cholesterol and calcium in the follicular fluid as a function of the time of preservation of the ovaries.
Study area
This study was conducted during the period from February to October 2018 in the Adamawa region (Cameroon). Samples were taken at the Ngaoundere municipal slaughterhouse and analysed at the ADAM'S LABO. Adamawa is a region of national transition between North and South Cameroon. This geographical position (Latitude 7 ° 19'39N and Longitude 13 ° 35'4E) gives it a Sudano-Guinean climate characterized by a dry season (from November to March) and a rainy season (from April to October). The average annual temperature is 23°C with a minimum of 9°C often reached in January and a maximum of 31°C reached between the months of March and April. The average annual rainfall varies between 900 and 1500 mm.
Characteristics of animals
Of the cows examined, 199 zebus females of different breeds [Goudali (110), Red Fulani (52), White Fulani (37)] were selected for the study. Cycling cows with a BCS equal or greater to 3 and less than ten years were selected as recommended by Kouamo et al. [9]. The live weight of the animals was determined by measuring the thoracic perimeter by the formula (124.69 - 3.171 x THC + 0.0276 xTHC²) of Njoya et al. [10]. The Body Condition score (BCS) and the age of the animals were determined as described by Vall et al. [11] and Fassi Fihri [12], respectively. The distribution of cows as a function of time and temperature was random according to the following table 1.
Table 1. Distribution of cows as a function of time
Variation |
Control |
T1 |
T2 |
T3 |
T4 |
T5 |
T6 |
T7 |
T8 |
T9 |
T10 |
T11 |
T12 |
T13 |
N (199) |
14 |
19 |
17 |
17 |
17 |
17 |
18 |
18 |
17 |
13 |
10 |
9 |
7 |
6 |
T: time. N: number of cows. Control: ovaries were preserved classically (35-37°C).
Ovary collection and handling
After identification and slaughter of each cow, the ovaries were removed by section of the broad ligament using scissors. Then they were individually introduced into tapered tubes containing isotonic saline (0.9% NaCl) supplemented with penicillin-streptomycin (100 IU / ml penicillin and 100 μg / ml streptomycin) and labeled with identification. After, the sample was divided into 02 groups according to the preservation temperature. The first control group tubes were placed in an isothermal container at 35-37°C and transported to the laboratory for analysis within two hours of slaughter. The tubes of the second group were placed in an isothermal container at a temperature of 5°C, transported to the laboratory and kepted in the refrigerator at 5 °C according to a time.
Determination of the weight and follicular population of the ovary
In the laboratory, the ovaries were cleared of tissue debris (broad ligament attached to the ovary) and weighed using an SF-400 electronic scale with an accuracy of 0.1. The follicles visible on the surface of the ovary were counted.
First Experiment: collection and grading of the oocyte after 5°C preservation
The slicing technique was used to collect oocytes [1], using a stereoscope at the objective 10X. The oocytes were then classified into 4 grades (Figure 1) taking into account the homogeneity of the cytoplasm and the layers of cumulus oophorus cells according to Alves et al. [13].
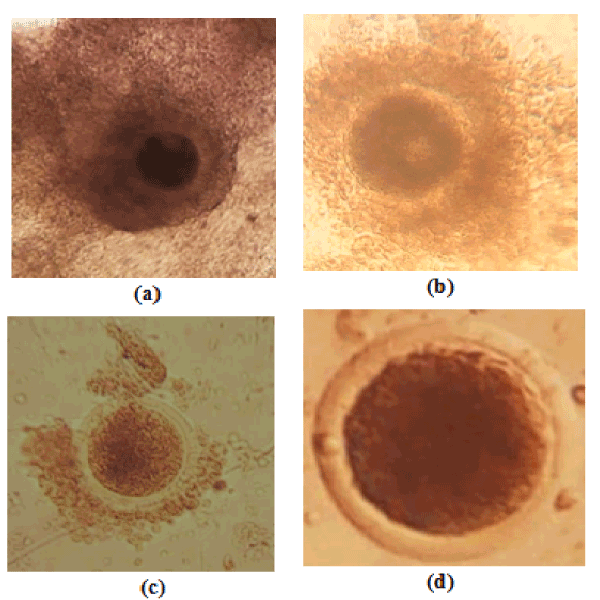
Figure 1. Oocyte quality, a= grade I; b= grade II; c= grade III; d= grade IV
Second experiment: study of biochemical variations of the follicular fluid of the ovaries preserved at 5°C
After cleaning and washing (0.9% NaCl, 5°C) of the ovaries previously stored at 5°C for a defined period of time, the follicular contents were aspirated using a sterile syringe mounted by an 18G needle. Then the follicular fluid was placed in 5ml dry tubes. To rid the cell debris, the follicular fluid was centrifuged at a rate of 3,000 rpm for 10 minutes and then the supernatant was recovered and stored at -20°C in Ependorf tubes until analysis.
Follicular fluid sample was evaluated for the concentration of glucose, total cholesterol and calcium. The three metabolites are involved in the growth and maturation of oocytes. All biochemical parameters were determined by a spectrophotometric (EMP-168 Biochemical analyzer) method using commercial kits supplied by SIGMA Diagnostic (glucose and total cholesterol) and Inmesco (calcium) laboratories.
Statistical analysis
The collected data was recorded in Microsoft Excel 2010 and the statistical analysis was performed by Statgraphic Plus version 5.0 software. The Shapiro-Wilk test was used for the normality test of the variables. One-way analysis of variance (ANOVA) was performed on normal quantitative variables. For non-standard quantitative variables, Fisher's LSD tests, Wilcoxon and Kruskal-Wallis tests were used for comparison of means. All data were represented as mean ± ESM (Standard Error of Mean) at the 5% threshold.
Characteristics of cows
BCS, age and weight (minimum-maximum) of slaughtered cows were 3.13 ± 0.33 (3-4), 6.33 ± 1.25 years (3-9) and 326.26 ± 29.90 kg (270-462), respectively.
Variation in ovarian weight, follicular population, yield and oocyte quality as a function of preservation time
From this study, it appears that after preservation of the ovaries at 5°C, the weight of the ovaries (8.5 ± 3.9 g) does not vary significantly (P = 0.224) for 13 hours (Table 2). Of a total of 398 ovaries harvested, 1872 follicles were counted. The average number of total follicles per cow was 9.40±6.22. Overall, the mean follicular population decreased significantly (p = 0.02) from 09 hours (Table 2). Of a total of 398 ovaries harvested, 1949 oocytes were counted. The oocyte yield per cow was 9.79 ± 5.90 and the average oocyte yield decreases significantly (p = 0.00) after 11 hours of preservation (Table 2). The optimal preservation time of the cultivable oocytes (grades I and II) was 12 hours. From 13 hours, there was a significant decrease (p = 0.032) of the average of the cultivable oocytes (Figure 1) which no longer guarantees the success of maturation.
Table 2. Variation in weight, follicular population, yield and oocyte quality as a function of preservation time (mean ± SEM)
Facteur |
N |
Ovaries weight (g) |
Follicular population |
Average oocyte yield |
Oocyte quality |
Cultivable oocytes grades I and II (%) |
|
I |
II |
III |
IV |
Control (T0) |
14 |
9.14±3.52 |
9.78±7.72 a, b, c |
9.85±5.39 b, c, d |
3.64±3.81 a, b, c |
2.42±1.78 a, b |
1.5±1.09 d, e, f |
2.28±1.54 f |
6.07±5.16 a, b, c, d (61.62) |
T1 |
19 |
9.10±2.33 |
10.84±7.52 b, c |
11.26±4.02 c, d |
4.57±2.09 a, b, c |
3±1.45 b, c |
1.68±1.41 e, f |
2±1.73 d, e, f |
7.57±2.52 b, c, d (67.22) |
T2 |
17 |
8.58±2.20 |
10±3.96 b, c |
10.82±5.15 b, c, d |
4.17±2.76 a, b, c |
3.17±2.81 b, c |
1.29±0.84 c, d, e |
2.17±1.62 e, f |
7.35±4.34 b, c, d (67.92) |
T3 |
17 |
8.94±3.03 |
10.41±6.69 b, c |
10.82±5.50 b, c, d |
5.47±3.08 b |
2.47±2.37 a, b |
0.82±0.88 a, b, c, d |
2.05±1.51 d, e, f |
7.94±4.46 c, d (73.38) |
T4 |
17 |
10.52±3.62 |
12.05±8.33 c |
12.41±8.13 d |
3.64±3.14 a, b, c |
4.35±4.30 c |
2.11±1.86 f |
2.29±1.68 f |
8±6.68 c, d (64.46) |
T5 |
17 |
9.58±3.18 |
10.17±4.65b, c |
8.23±4.25 a, b, c |
3.29±2.56 a, b |
2±2.03 a, b |
0.94±0.74 a, b, c, d |
2±1.17 d, e, f |
5.29±4.01 a, b, c (64.27) |
T6 |
18 |
8.72±2.44 |
8.83±5.27 a, b, c |
10.50±6.31 b, c, d |
5.55±3.97 b |
2.44±1.58 a, b |
0.94±1.05 a, b, c, d |
1.55±1.42 c, d, e, f |
8±5.14 c, d (76.19) |
T7 |
18 |
10.61±5.16 |
11.55±9.43 c |
10.88±7.72 b, c, d |
5.50±4.56 b |
2.94±2.94 b, c |
1.16±1.29 b, c, d, e |
1.27±1.07 b, c, d, e |
8.44±7.26 c, d (77.57) |
T8 |
17 |
10.05±3.39 |
8.64±4.70 a, b, c |
11.05±6.03 c, d |
5.58±3.39 b |
3.05±1.95 b, c |
1.11±1.16 b, c, d, e |
1.29±1.31 b, c, d, e |
8.64±4.75 d (78.19) |
T9 |
13 |
8.23±2.08 |
5.15±1.90 a |
7.38±4.68 a, b, c |
3.84±3.02 a, b, c |
1.76±1.73 a, b |
0.61±0.65 a, b, c |
1.15±1.06 a, b, c, d |
5.61±3.88 a, b, c, d (76.01) |
T10 |
10 |
10.3±4.39 |
7.80±3.82 a, b, c |
10.30±6.18 b, c, d |
5.50±2.83 b, c |
2.70±2.58 a, b, c |
0.8±1.03 a, b, c, d |
1.30±1.05 a, b, c, d, e, f |
8.20±4.87 b, c, d (79.61) |
T11 |
9 |
11.22±4.26 |
8.11±3.01 a, b, c |
6.33±3.27 a, b |
4.44±2.92 a, b, c |
1.44±1.33 a, b |
0.11±0.33 a |
0.33±0.70 a, b |
5.88±3.10 a, b, c, d (92.89) |
T12 |
7 |
6.85±2.11 |
5.71±2.36 a, b |
4.42±29a |
2.85±1.95 a, b, c |
0.71±0.75 a |
0.28±0.48 a, b |
0.57±0.78 a, b, c |
3.57±1.81 a, b (80.76) |
T13 |
6 |
8.5±3.93 |
5.33±3.32 a, b |
3±2.52a |
1.83±1.16 a |
1±1.26 a, b |
0.16±0.40 a, b |
00±00 a |
2.83±2.31 a (94.33) |
P-Value |
0.224 |
0.020 |
0.002 |
0.047 |
0.018 |
0.000 |
0.000 |
0.032 |
a, b, c, d : The averages in a column with different exponents are significant (P<0.05)
Variation of glucose, cholesterol and calcium concentration in follicular fluid as a function of ovarian preservation time
Glucose concentration increased significantly (p = 0.00) in the follicular fluid from 10 hours. On the other hand, the concentration of total cholesterol decreases significantly (p = 0.00) from 10 hours of preservation. The concentration of calcium in the follicular fluid increases significantly (p = 0.00) from 09 hours (Table 3).
Table 3. Variation of the concentration of glucose, total cholesterol and calcium in the follicular fluid as a function of the preservation time (mean ± SEM)
Factor |
N |
Glucose (mg/dl) |
Total Cholestérol (mg/dl) |
Calcium (mg/dl) |
Control (T0) |
14 |
9.89±4.71 a |
34.76±29,58 c |
5.95±2.34 e |
T1 |
19 |
34.18±7.47 b, c, d, e |
14.18±1,91 a, b |
4.12±1.44 c, d, e |
T2 |
17 |
21.00±8.81 a, b |
20.7±12,87 a, b |
4.79±1.19 d, e |
T3 |
17 |
37.08±22.68 d, e, f |
26.58±16,49 b, c |
2.80±0.66 a, b, c |
T4 |
17 |
21.92±11.26 a, b, c |
22.47±19,08 a, b, c |
2.32±2.40 a, b |
T5 |
17 |
29.74±17.59 b, c, d, e |
14.48±2,76 a, b |
2.96±2.70 a, b, c, d |
T6 |
18 |
25.01±15.79 b, c, d |
16.75±5,77 a, b |
1.45±0.83 b, c, d |
T7 |
18 |
42.18±24.17 e, f |
10.83±3,26 a |
3.43±2.78 b, c, d, e |
T8 |
17 |
27.95±9.88 b, c, d, e |
19.38±7,11 a, b |
2.14±1.56 a, b |
T9 |
13 |
22.35±7.57 a, b, c |
16.31±4,57 a, b |
1.40±0.88 a |
T10 |
10 |
49.91±11.76 f |
12.04±6,44 a |
11.58±2.14 f |
T11 |
9 |
38.28±8.34 d, e, f |
9.49±5,94 a |
11.67±2.59 f |
T12 |
7 |
37.16±8.34 c, d, e, f |
9.91±3,42 a |
10.15±0.74 f |
T13 |
6 |
36.15±17.62 c, d, e, f |
10.33±2,97 a |
10.04±1.64 f |
P-Value |
|
0.000 |
0.000 |
0.000 |
a, b, c, d : The averages in a column with different exponents are significant (P<0.05)
Relationship between yield and quality of oocytes and concentration of glucose, total cholesterol, calcium as a function of temperature and preservation time
In the ovaries preserved at 5°C, the yield of cultivable oocytes (red curve) decreases when the preservation period (blue right) increases, unlike glucose concentration (green curve) which increases with time. The curve showing the concentration of total cholesterol (purple curve) evolves in the same direction as the yield of cultivable oocytes. The concentration of calcium (black curve) evolves in the same direction as the yield of oocytes cultivable up to 9 hours. From 10 hours, the concentration increases in the follicular fluid. These results indicate that the ovaries could be preserved at 5°C for at least 9 hours with no significant change in glucose, total calcium and cholesterol concentration, and oocyte quality (Figure 2).
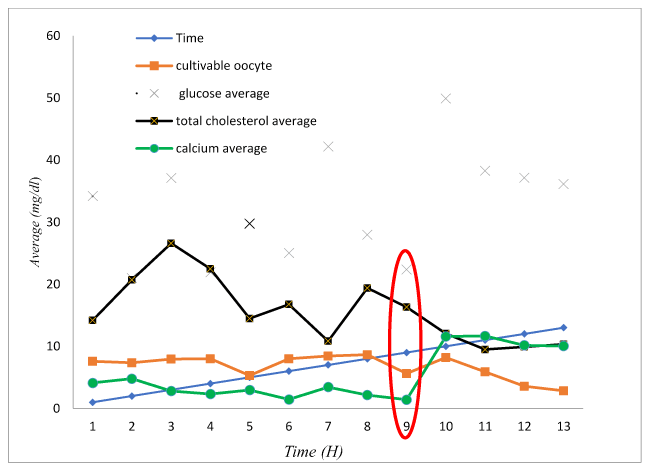
Figure 2. Relationship between oocyte yield and mean concentration of glucose, total cholesterol and calcium preserved at 5°C
The average weight per ovary preserved at 5°C for 13 h was similar to that reported by Kouamo et al. [1] (4.60 ± 1.82 g) and Natumanya et al. [14] (4.6 ± 2.3g) on the ovary of Cameroonian and Ankole zebus preserved at 35-37°C, in Cameroon and Uganda, respectively. The ovaries can be preserved at 5°C for more than 13 hours without a significant variation in their weight.
The follicular population of the ovaries preserved at 5°C varied significantly from 9 hours. This result is different from that obtained by Santos et al. [15] and Lucci et al. [6] in Brazil who reported that the preanal follicles of goats and zebus can be preserved at 4°C at most 12 and 18 hours, respectively, without morphological alteration of the follicle. These differences are likely related to the specific choice of preantral follicles, breed and / or the environment.
The ovaries could be preserved at 5°C for 11 hours without significant variation in average overall oocyte yield. In addition, grade I and II cultivatable oocytes could also be preserved at this temperature for 12 hours without significant morphology and intrinsic potential. This result is comparable to those of Ita [8] and García-Álvarez et al. [5], working on goats and Iberian deer, respectively, demonstrated that ovaries preserved for 12 hours at 5°C and 4°C, produce oocytes able of successfully undergoing oocyte maturation. Similarly, Febretrisiana et al. [16] in Indonesia have also shown that sheep ovaries preserved at 4°C for 10 hours maintain good developmental abilities that ovaries preserved at 28 ° C. In bovine ovaries preserved at 4°C for more than 12 hours, there is apoptosis of cumulus cells with negative effects on in vitro maturation [17]. On the other hand, Solano et al. [18] reported that intrafollicular oocytes can be preserved at 4°C for 12-24 hours without impairing their ability to mature, fertilize, and develop in vitro. These differences in the duration of ovarian preservation could be explained by the method of determining the time and the storage temperature. The time determination method used by Solano et al. [18] does not take into account the oocyte kinetics for each hour of preservation and does not evaluate the morphological and physiological changes of oocytes over time. In addition, at more than 12 hours of preservation at 5°C, there are still good quality oocytes but their yield at maturation and fertilization will be lower.
The concentration of glucose in the follicular fluid of the ovaries preserved at 5°C increases significantly (p = 0.00) in the follicular fluid from 10 hours. Yokoo et al. [19] and Isachenko et al. [20] explain that this increase is due to the decrease in the number of cumulus cells following cell apoptosis. According to Sutton-McDowall et al. [21], this cellular destruction would result in the release of the glucose stored by the cumulus oophorus cells in charge of glycolysis. This change in concentration shows that the temperature (5°C) decreases the ability of the oocyte and cumulus cells to consume glucose as the main energy fuel. This is in line with the work of Woods et al. [22] who reported that preservation at low temperatures leads to irreversible structural changes in cumulus cells as well as in the oocyte itself, increasing the risk of irreversible disruption of the fiber bundles constituting the nucleus structure.
Unlike the change in glucose concentration in the follicular fluid, that in total cholesterol decreases as time increases. It decreases significantly (p = 0.00) from 10 hours. Cetica et al. [23] explain that this cholesterol drop in follicular fluid is due to increased lipolysis in large follicles. Yokoo et al. [19], Ferguson and Leese [24] and Rahman et al. [25] report that cellular apoptosis following low temperatures would release growth factors (EGF) thereby stimulating beta-oxidation, responsible for drop of total cholesterol in the follicular fluid. In the absence of glucose, the oocyte solicits lipids for its energy production [26].
At the temperature of 5°C, the concentration of calcium varies significantly (p = 0.00) in the follicular fluid from 10 hours. It decreases until 09 hours, then increases sharply to 10 hours. Kobayashi et al. [27] and Homa et al. [28] demonstrate that the oocyte prepares for its maturation by storing the calcium present in the follicular fluid in cortical granules. The sudden increase in calcium concentration in the follicular fluid would probably be due to the degradation of poor-quality oocytes, with release of calcium contained in the granules.
This study demonstrates that cultivable oocytes can be preserved at 5°C for at least 12 hours without altering the morphology of grades I and II oocytes. This time seems to have passed at 9 hours when the influence of temperature on the oocyte metabolism is taken into account. Indeed, at more than 10 hours, metabolites such as glucose, total cholesterol and calcium exhibit concentration variations that no longer guarantee the success of maturation and in vitro fertilization.
In conclusion, this study indicates that the zebu cows' ovaries could be preserved at 5°C for 9 hours without any significant change in biochemistry, yield and quality of cultivable oocytes.
- Kouamo J, Dawaye SM, Zoli AP, Bah GS (2014) Evaluation of bovine (Bos indicus) ovarian potential for in vitro embryo production in the Adamawa plateau (Cameroon). Open Vet J 4: 128-136. [Crossref]
- Ebangi AL, Erasmus GJ, Mbah DA, Tawah CL, Ndofor-Foleng HM (2011) Evaluation of level of inheritance in the growth traits in the Gudali and Wakwa beef cattle breeds of Adamawa, Cameroon. Lives Res Rural Dev 23: 111-130.
- Brackett BG, Bousqet D, Boice ML, Donawick WS, Evans JF, et al. (1982) Normal development following in vitro fertilization in the cow. Biol Reprod 27: 147-158. [Crossref]
- Arlotto T, Schwartz JL, First NL, Leibfried Rutledge ML (1996) Aspect of follicle and oocyte stage that affect in vitro maturation and development of bovine oocytes. Theriogenology 45: 943-956. [Crossref]
- García-Álvarez O, Maroto-Morales A, Berlinguer F, Fernández-Santos MR, Esteso MC, et al. (2011) Effect of storage temperature during transport of ovaries on in vitro embryo production in Iberian red deer (Cervus elaphus hispanicus). Theriogenology 75:65-72. [Crossref]
- Lucci CM, Kacinskis MA, Rumpf R, Bao SN (2004) Effects of lowered temperatures and media on short-term preservation of zebu (Bos indicus) preantral ovarian follicles. Theriogenology 61: 461-472. [Crossref]
- Tas M, Evecen M, Ozdas BO, Cirit U, Demir K, et al. (2006) Effect of transport and storage temperature of ovaries on in vitro maturation of bitch oocytes. Anim Reprod Sci 96: 30-34. [Crossref]
- Ita D (2010) Developmental capacity of goat oocytes collected from 5°C preserved ovaries. Indonesian J Vet Sci Med 2: 43-48.
- Kouamo J, Nono Fambo SM, Zoli AP (2017) Effect of the Stage of Sexual Cycle, Harvesting Technique and Season on Follicular Dynamics and Oocyte Quality of Zebu Cattle under Sudano-Sahelian Climate. Integr J Vet Biosci 1: 1-7.
- Njoya A, Bouchel D, Ngo Ntama AC, Moussa CA, Martrenchar A, et al. (1997) Systèmes d’élevage et productivité des bovins en milieu paysan au nord-Cameroun. World Animal Review 89: 12-23.
- Vall C, Abakar O, Dongmo Ngoutsop AL (2002) Note d’état corporel des zébus de trait dans les savanes d’Afrique centrale. N’Djamena, Tchad, fiches techniques du Prasac n° 13, 4.
- Fassi Fihri A (2006) Collecte et maturation des ovocytes bovins : effet de l’état nutritionnel sur le rendement et la qualité des ovocytes. Thèse de PhD Université de Rabbat Maroc : 163p.
- Alves BG, Alves KA, Lucio AC, Martins MC, Silvas TH (2014) Ovarian activity and oocyte quality associated with the biochemical profil of serum and follicular fluid from girolando dairy cows postpartum. Anim Reprod Sci 146: 117-125. [Crossref]
- Natumanya R, Owiny D, Kugonza R (2008) The potential of Ankole cattle abattoir ovaries for in vitro embryo production. African J Ani Biomed Sci 3: 1-5.
- Santos RR, Silva JRV, Costa SHF, Rodrigues APR, Lôbo RNB, et al. (2002) Effect of 0.9% Saline Solution and Phosphate Buffer Saline at different temperatures and incubation times on the morphology of goat preantral follicles. Braz J Vet Res Anim Sci 39: 254-259.
- Febretrisiana A, Setiadi MA, Karja NWK (2015) Nuclear maturation rate of sheep oocystes in vitro : Effect of storage duration and ovary temperature. Indonesian J Trop Anim Agri 40: 93-99.
- Nakao H, Nakatsuji N (1992) Effect of storage conditions of bovine ovaries and oocytes on the success rate of in vitro fertilization and culture. J Reprod Dev 38: 11-13.
- Solano R, Armas R, Pupo CA, Castro FO (1994) Short-term preservation of intrafollicular oocytes at 4°C. Theriogenology 41: 299.
- Yokoo M, Sato E (2004) Cumulus-oocyte complex interactions during oocyte maturation. Int Rev Cytol 235: 251-291. [Crossref]
- Isachenko E, Isachenko V, Nawroth F, Rahimi G, Weiss MJ (2009) Effect of long-term exposure at suprazero temperatures on activity and viability of human ovarian cortex. Fertil Steril 91: 1556-9. [Crossref]
- Sutton-McDowall ML, Gilchrist RB, Thompson JG (2010) The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 139: 685-695. [Crossref]
- Woods EJ, Benson JD, Agca Y, Critser JK (2004) Fundamental cryobiology of reproductive cells and tissues. Cryobiology 48 : 146–156. [Crossref]
- Cetica P, Pintos L, Dalvit G, Beconi M (2002) Activity of key enzymes involved in glucose and triglyceride catabolism during bovine oocyte maturation in vitro. Reproduction 124: 675-681. [Crossref]
- Ferguson EM, Leese HJ (2006) A potential role for tryglyceride as an energy sources during bovine oocyte maturation and early embryo development. Mol Reprod Dev73 : 1195-1201. [Crossref]
- Zia-Ur-Rahman, Bukhari SA, Ahmad N, Akhtar N, Ijaz A, et al. (2008) Dynamics of follicular fluid in one-humped camel (Camelus dromedarius). Reprod Domest Anim 43: 664-671. [Crossref]
- McEvoy TG, Coull GD, Broadbent PJ, Hutchinson JS, Speake BK (2000) Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J Reprod Fertil 118 : 163-170. [Crossref]
- Kobayashi Y, Kitai H, Santulli R, Karen HW, Wallach EE (1984) Influence of Calcium and Magnesium Deprivation on Ovulation and Ovum Maturation in the Perfused Rabbit Ovary. Biol Reprod 31: 287-295. [Crossref]
- Homa ST, Carroll J, Swann K (1993) The role of calcium in mammalian oocyte maturation and egg activation. Hum Reprod 8: 1274-1281. [Crossref]


