Abstract
Objectives: The precise epidemiology of community acquired childhoodpneumoniais crucial for timely and adequate treatment. The objective of this study was to determine the current etiological profile of pneumonia in children after introduction of pneumococcal vaccine in Bulgaria.
Methods: We evaluated prospectively from December 2015 through November 2018, 285 immunocompetentchildrenhospitalized with radiographically confirmed pneumonia. We specifically looked for prior antibiotic use and immunization status. The laboratory data included - CRP, full blood count, sputum culture examination, PCR and/or serology for respiratory viruses, Chlamydia and Mycoplasma.
Results: In 44.9% of the cases we couldn’t prove etiological agent, bacteria were confirmed in 41.6% and viruses in 16.2% (in 4.6% - co-infection). For the prior antibiotic use, we couldn’t find any significance for etiological confirmation (p=0.36) but for Streptococcus pneumoniae (p=0.015). As expected, Mycoplasma was isolated in older children and mainly in summer/autum season, while Streptococus was mainly isolated in younger ones with no specific seasonal peak. Mean age for children with bacterial pneumonia was 6.54 years, for the ones with viral pneumonia 4.34 yrs., for combined (viral+bacterial) 3,08 yrs. and for fungal one was 10.42 yrs (p=0.000). Almost 2/3 of the children have been immunized with pneumococcal vaccine (66%). The immunized patient had higher numbers of viral and lower of bacterial isolates 25.7% and 37.23% vs. non-immunized ones – 4.12% and 48.45% respectively (p=0.002). There was no difference in Streptococcus pneumoniae isolation and vaccination status, but there was a major drop in Mycoplasma isolates in vaccinated ones (6.9% vs. 32.98%, p=0.000).
Conclusion: In future we could expect more viral pneumonia with increasing of the vaccination coverage and maybe we should reevaluate our treatment guidelines.
Key words
pneumococcal vaccine, atypical pneumonia, children
Abbreviations
CAP: Community-Acquired Pneumonia, RSV: Respiratory Syncytial Virus, RV: Rhinovirus, hMPV: human Metapneumovirus, M.pneumoniae: Mycoplasma pneumoniae, S.pneumoniae: Streptococcus pneuoniae, S. aureus: Staphylococcus aureus, C. pneumoniae: Chlamydia pneumoniae
Introduction
Over the last twenty years, the global incidence of pneumonia in young children (aged <5 years) reduced by 30% and the number of pneumonia deaths in young children almost halved in that time [1]. One big measure in this positive tendency was introduction of pneumococcal vaccine [2]. However, pneumonia remains a leading cause of preventable illness and death in this age group and overcoming this will require considerable effort [3]. The precise epidemiology of childhoodpneumoniachanges with the patient’s age, time of the study, as well as the medical policy and treatment guidelines in different countries [4-6].
Back in 2004 Michelow et al. have described the the epidemiology and morbidity of Community-Acquired Pneumonia (CAP) in hospitalized children with identified bacteria in 60% of the cases (73% for Streptococcus pneumoniae), viruses in 45%, and mixed bacterial/viral infections in 23%. Atypical bacterial pneumonia was found in 14% for Mycoplasma pneumoniae and 9% for Chlamydia pneumoniae [7]. Various publications later on however stated drastic drop of CAP caused by invasive S. pneumoniae after introduction of pneumococcal vaccine instead of the rise in non-vaccine serotypes [8,9]. In 2010 10-valent Pneumococcal Vaccine (PCV10) was introduced as an obligatory vaccination in Bulgaria [10].
The accumulated knowledge for the last 15 years, led us to the objective of this study, which was to determine the current etiological profile of pneumonia in children after introduction of pneumococcal vaccine in Bulgaria.
Material and methods
Patients
The study material comprised the data from 285 patients. The inclusion criteria that were met by all patients were: children with a clinical and X-ray confirmed pneumonia aged from 1 to 17,9 years, requiring in hospital treatment. We included all patients with CAP, hospitalized in the ward for acute respiratory infection during a period of 3 years (December 2015 - November 2018) with the exclusion of the patients with known immunocompromised condition (such as cystic fibrosis, immune-deficiency or bronchiectasis) or patients transferred from other hospitals that were on intravenous antibiotics before admission. All of the tests were performed after obtaining a signed informed consent of the legal guardian. There was no personal patient information in the database. The Hospital’s Ethics Committee granted study approval. The therapy prescribed was as all the clinical practice and guidelines approved, according the isolated pathogen.
Clinical, imaging and laboratory data methods
Clinical information, including demographic characteristics, immunization record, history of illness, symptoms, initial diagnosis, and comorbid illnesses, was documented on case report forms.
X-ray confirmation
A senior specialist in imaging diagnosis reviewed all chest X-rays and confirmed presence of pneumonia. The radiologist assigned the type of pneumonia on X-ray as - focal or segmental consolidation with or without pleural effusion or interstitial pneumonia.
Microbiology
Blood cultures were performed with the BacT/Alert 3D system (Organon Teknika, Boxtel, Netherlands) before initiation of parenteral antibiotic therapy in children in severe clinical condition (24% of all) following the standardized guidelines [11].
At the admission, before we collected sputum samples and deep nasopharyngeal swab (in cases where sputum was not obtained at the admission). All the samples were obtained by a pediatrician and then right away sent to the laboratory in a transport medium. Sputum samples were plated and incubated at 37 °C for 24-48 h. The standard protocol for microbiological diagnostic follow-up and tests was followed in the laboratory. For isolation and detection of the rigorous etiologic bacterial agents we used routine nutrition media such as Blood agar base supplemented with 5% sheep blood, MacConkey agar, Candida chrome agar and developed and implemented by our team selective media chocolate agar with vancomycin for Moraxellae and Haemophilae, for their identification, as well as a quantitative method for correct evaluation of the clinical significance of isolates [12]. The cultivation of inoculated samples on different media was performed in aerobic and in microaerophilic atmosphere respectively for various pathogens. For identification of the isolated clinical strains we used mainly the products and systems by Crystal BD BBL (BBL; Becton, Dickinson, Germany) and RapID System Remel Thermo Fisher Scientific (Remel, Thermo Fisher Scientific Remel Products; Santa Fe, USA). All bacterial isolates in a microbial number over the critical one for an infectious process (>100 000 CFU/mL), were considered as significant [12]. Susceptibilities to investigated pathogens were determined by Kirby-Bauer disk-diffusion method. Due to the necessity for some peculiar cases and for a representative sample strain, minimal inhibitory concentration according the criteria of CLSI were measured [13]. Isolated Streptococcus pneumoniae strains were preserved for serotyping for a future research in regards immunization status and the type of isolated S.pneumoniae.
For viral detection combined nasal and pharyngeal specimens were collected from the enrolled patients by means of commercial polyester collection swabs (Deltalab, Spain). Following collection, swabs were stored at 4°C for up to 72 hrs. Specimens were processed immediately or stored at -80°C prior to analysis. Viral nucleic acids were extracted automatically from 500μl of each respiratory specimen in a final eluate volume of 75μl using an ExiPrep Dx Viral DNA/RNA kit (BioNeer, Korea) in accordance with the manufacturer’s instructions. Detection and typing/subtyping of influenza viruses were carried out by a real time RT-PCR method and the SuperScript III Platinum ® One-Step qRT-PCR System (Invitrogen, ThermoFisher Scientific, USA) as previously described [14]. The detection of RSV, hMPV, PIV 1/2/3, RV and AdV was performed using singleplex real time PCR assays and an AgPath-ID One Step RT-PCR kit (Applied Biosystems, ThermoFisher Scientific, USA). The primers, probes and PCR conditions used in the study were identical to those previously described [15].
If indicated some patients were screened for pulmonary tuberculosis with intradermal Mantoux skin tests by using 5TU purified protein derivative.
Serology
Serum samples were stored at -70°C and subsequently analyzed. Serologic tests for M. pneumoniae, Chlamydia pneumoniae and Chlamydia trachomatis was performed with enzyme-linked immunosorbent assays with ready IgG and IgM kits (Abcam, UK). Additionaly to serology for the patients form the 2018 we used nasopharyngeal swabs for Real-Time Amplification test for the qualitative detection of Mycoplasma pneumoniae and Chlamydia pneumoniae - (Kit Mycoplasma pneumoniae / Chlamydophila pneumoniae Real-TM, Sacace Biotechnologies Srl) following the manufacturer instructions.
Inflammatory indices
Complete blood count with with manually verified differential count as well as CRP was performed in all children. For CRP detection (positive test 6 mg/L or higher) we used serums collected by standard procedures and slide agglutination test C-Reactive protein (CRP) latex (BioSystems S.A.; Costa Brava, Barcelona, Spain).
Treatment
All patients were treated with a sequential parenteral and oral antibiotic regimen for presumed bacterial LRI. The antimicrobial agent of choice for uncomplicated pneumonia was 3rd generation cefalosporine for a duration of 5 to 14 days. If atypical bacteria was confirmed the therapy was modified with macrolide. Alternative or additional agents were used at the discretion of the attending physician.
Statistical analysis
The data were analyzed using using Statistical Package for the Social Sciences (SPSS) for Windows, Version 19.0. (SPSS Inc.; Chicago, IL, USA). For nonnormally distributed results, comparisons were made by the Kruskal-Wallis test, Fisher exact test, or Wilcoxon signed-rank test as appropriate. For normally distributed results, comparisons were made by analysis of variance (ANOVA) and the paired t test. Chi Square was used for testing relationships between categorical variables. We considered p values of ≤0.05 to indicate statistical significance.
2021 Copyright OAT. All rights reserv
Results and discussion
A total of 285 children met the inclusion and had no exclusion criteria for enrollment. Of these patients, 135 (47.3%) were male and 150 females. Ages ranged from 1 year to 17.8 years, 47.0% less than 5 years old. One hundred and five children (36.8%) had received oral antibiotic therapy prior the hospitalization, most commonly cephalosporin 2nd generation or amoxicillin. Forty-one (14.4%) children had additionally asthma and four had neuromuscular disease. The median duration of hospitalization was 5.9 days (from 3 to 14). Only 17 (5.9%) received supplemental oxygen therapy for a median of 3 days, but 126 (44.2%) were with bronchial obstruction, requiring corticosteroid and salbutamol treatment. Thirty-six (12.6%) had pleural effusion, and only 3 of them required surgical intervention. All patients but the mentioned 3 were discharged at home with appropriate therapy. On X-ray studies 138 patients (48.4%) had consolidations while the rest had interstitial changes. Thirty percent (87 children) were hospitalized during winter season, 28.4% during spring, 26.6% during autumn and only 17% during summer. Almost 2/3 of the patients (65.9% or 188 children) had at least 3 shots of PCV10, the rest were not immunized. For this three-year period none of the included children required mechanical ventilation and none died. This also supports the publications regarding the positive impact of PCV10 on hospitalization rates and severity of the pneumonia [2,6,16].
Etiology
In 128 of the children (44.9% of the cases) we couldn’t prove etiological agent. In 117 (41.6%) children we confirmed bacteria. The most prevalent bacteria expectedly was S. pneumoniae (52.4%), followed by M. pneumoniae (44.4%), Staphylococcus aureus (9.4%), Haemophilus parainfuenzae (8.5%), Haemophilus infuenzae (3.2%). In 3 patients we found Klebsiela pneumoniae and in other 3 Pseudomonas aeruginosa, in 2 we found Stenotrophomonas maltophilia other pathogens found only in single cases included Moraxella catarrhalis, Chlamydia pneumoniae, Acinetobacter baumani, Streptococcus pyogenes, beta hemolytic Streptococcus, Mycobacterium tuberculosis, Flavobacterium meningosepticum and Enterobacter aerogenes. We found 100% concordance of the results from serology and PCR from nasopharyngeal swabs for the tested with both methods patients with 18 positive on both tests, and no false positive or false negative on the PCR compared with ELISA. In 17 cases we confirmed combined bacterial flora mainly with S. aureus, S.pneumonia and. M.pneumonia.
In 46 patients (16.2%) we confirmed viral infection (in 4.6% of the patients there was co-infection of virus and bacteria). The leading viral pathogen was Respiratory Syncytial Virus (RSV) in 28.3%, rhinovirus (RV) in 21.7%, human metapneumovirus (hMPV) and influenza virus A were isolated in 15.21%, adenovirus in 13.0%, influenza virus B and bocavirus were found in 2 patients and parainfluenza, parainfluenza virus in only one. In seven patients we found only Candida spp.
Despite PCV10 introduction in Bulgaria for the last 8 years, and relatively high coverage reported by the authorities [17], S.pneumonia is still the predominant bacterial pathogen for CAP in Bulgaria. Future studies are planned to confirm the possible seroconversion and the need for evaluation weather more valent pneumococcal vaccine is needed [18]. Surprisingly we couldn’t confirm the expected higher rate for C.pneumoniae, reported between 9 and 17% for we found only one case [7,19,20].
Etiology and demographic data
We couldn’t find any significant difference between isolated pathogen and the gender of the patients, there is however higher probability for pleural effusions in male patients (p=0.000). We couldn’t identify any correlation between effusion and etiological agent (p=0.35). There was a significant difference in the mean age and distribution of the patients the patients according the type of isolated agent. Mean age for children with bacterial pneumonia was 6.54 years, for the ones with viral pneumonia 4.34 yrs., for combined (viral + bacterial) 3,08 yrs. and for fungal one was 10.42 yrs (p=0.000) (Figure 1). As expected, M. pneumoniae was isolated in older children, while S. pneumoniae was mainly isolated in younger ones but the differences were not statistically significant (p=0.14), but the results reflect in the choice of antibiotic therapy – cephalosporin for children under age of 5 (p=0.036) and macrolide for older kids (p=0.000). There was no significant correlation between age and viral isolate. Our results are in concordance with the previously published data [7,19]
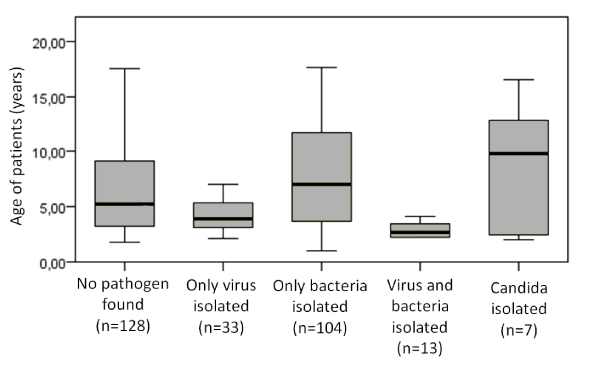
Figure 1. Box-plot for age distribution of patients according the microbiology/virology finding
Etiology and seasonal data
As expected, the lowest number of hospitalized patients with CAP is during summer, and for 3 years we found viral pathogen (adenovirus) only in one patient. RSV is more often isolated during autumn, while influenza and hMPV more during winter (p=0.02). More co-infected patients (bacteria and virus) were found during winter and spring (p=0.01). For S.pneumoniae we couldn’t find any seasonal prevalence (p=0.5), while for M.pneumoniae we found main prevalence during spring and early summer (p=0.000). Maybe these seasonal fluctuations of viruses and M.pneumoniae are behind the finding that there is no viral co-infection with M.pneumonia. More combined infections were found during winter (p=0.04). Our data is similar to previously published data [7], but different winter percentages are reported this year by Korean group, but the authors there state that there was specific M.pneumonia epidemy [6].
Prior antibiotic use and etiology
From 285 studied children 105 children have been taken antibiotics prior hospitalization the rest 180 patients were antibiotic naïve. When we divided the patients according this parameter the etiological distribution was: 38.8% no pathogen found, 48.3% bacteria confirmed, 15% virus isolated and 2.2% Candida found for antibiotic naïve vs. 55.23%, 28.5%,18.9% and 2.8% for the ones with prior use respectively (p=0.002). When we looked at the bacterial isolates and prior antibiotic use, we couldn’t find any significant difference regarding M.pneumoniae isolation (p=0.36) but there is one for S. pneumoniae (p=0.015), mainly due to the standard guidelines to GPs to start with penicillin antibiotic for pneumonia [21]. There was no significant effect on the prior antibiotic use and type of the isolated virus (p=0.06). Surprisingly the candida isolation was not significantly higher in the group that have used antibiotics prior the tests (p=0.08).
Etiology and immunization status
Almost 2/3 of the children have been immunized with PCV10 (66%). The immunized patient had higher numbers of viral and lower of bacterial isolates 25.7% and 37.23% vs. non-immunized ones - 4.12% and 48.45% respectively (p=0.002) (Figure 2). Only in vaccinated children there were co-infection found (p=0.000). There was no difference in S. pneumoniae isolation and vaccination status, but there is a major drop in M.pneumoniae isolates in vaccinated ones (6.9% vs. 32.98%, p=0.000). Our data corroborated findings of other investigators, claiming that PCV10 results in lowering the levels of S. pneumoniae and thus creating space for increasing M. pneumoniae. [6,7,22]
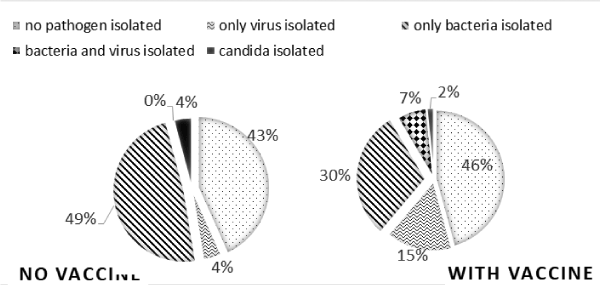
Figure 2. Percentage of isolated pathogen groups (virus, bacteria, combined, candida or without isolates) according immunization status with PCV10
Etiology and comorbidities
Due to low number of patients with neuro-muscular diseases we couldn’t perform a proper statistic for them. For the patients with asthma only in 14% we couldn’t identify microorganism, while in 48.5% and 51.2% we found viruses and bacteria respectively (in 23.26% we found co- infection). The bacterial profile was similar to the whole group with prevalence S. pneumoniae, followed by M. pneumoniae. In 50% of children without asthma we didn’t isolate etiological agent and only in one case there was co-infection, in 10.6% viral and 39.3% bacterial pneumonia was confirmed (p=0.008). We didn’t find the expected higher rates of M.pneumoniae in asthmatic children, as it was noted previously that M.pneumoniae infection is associated with both the initiation and persistence of asthma in children [23]. Since asthma exacerbation due to viral infection can mimic pneumonia, some studies that skip the imaging diagnosis can make improper conclusions. In previously published papers it was confirmed that less than 2% of children with asthma exacerbation have really pneumonic changes on X-ray, thus the routine uses of radiography for children with asthma who are not febrile or hypoxic should be discouraged [24]. To avoid some misunderstandings, all included in our study patients with asthma had X-ray confirmed pneumonia.
Etiology and inflammatory markers and X-ray changes
The values for CRP were lower in cases with Mycoplasma and in cases with non-compact infiltrate changes on X-rays (p=0.002 and p=0.000). The CRP couldn’t be used as a marker for differentiation of atypical from viral pneumonia (p=0.12). The higher CRP couldn’t be a marker of only bacterial pneumonia, for some viral cases also had extremely elevated values (p=0.09). We found that children with bronchial obstruction have elevated but not extremely high CRP values, the highest values were in cases without bronchial obstruction (p=0.000). Children with the highest values of CRP required longer inhospital stay (p=0.020). We couldn’t find any correlation between the etiologic agent and the differential blood count (including lymphocytes and neutrophil number) or wheezing episodes. As other authors have found almost half of the CAP cases with X-ray seen infiltration and confirmed pathogen are caused by M.pneumoniae or viruses [25]. We also agree that it is difficult to distinguish clinically between bacterial and viral aetiologies. Fever and tachypnoea are confirmed early features of pneumococcal pneumonia. Cough is not always apparent or required for diagnosis, and may be absent in the early stages of illness. M. pneumonia presents with cough and chest pain and is often associated with wheeze, general malaise, arthralgia, sore throat, and headache [4,5].
Conclusion
Our study through expanded diagnostic investigations aimed to define the etiological profile of the pathogens that cause pneumonia in hospitalized children in Bulgaria. Our data confirm the frequent occurrence of co-infection with bacteria and viruses in children with pneumonia. Following our findings in future we could expect more viral pneumonia with increasing of the vaccination coverage and maybe we should reevaluate our treatment guidelines. Even the most rigorous efforts to define the epidemiology of pneumonia in children are limited by the paucity of validated data. Therefore, accurate characterization of the causes of CAP is essential to guide appropriate antibiotic utilization.
Acknowledgements
The authors would like to express our gratitude to dr. E. Elencheva for her expert review of the X-rays of all children, and to prof. N. Korsun- for the viral detection. This work was supported by a grant from the Medical University of Sofia (Council of Medical Science, project no. 7771/2017, grant no. 107/2018).
Ethics committee approval
Ethics committee approval for this study was received from the ethics committee of Medical University of Sofia School of Medicine.
Informed consent
Written informed consent was obtained from parents of the patients who participated in this study.
Funding
This work was supported by supported by a grant from the Medical University of Sofia (Council of Medical Science, project no. 7771/2017, grant no. 107/2018).
References
- McAllister DA, Liu L, Shi T, Chu y, Reed C, et al. (2018) Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health 7: E47-E57.
- Vieira ILV, Kupek E (2018) The impact of pneumococcal vaccine in reducing pneumonia hospitalizations in children under 5 years old, in Santa Catarina, Brazil, 2006 a 2014. Epidemiol Serv Saude 27: e2017378. [Crossref]
- Howie SRC, Murdoch DR (2019) Global childhood pneumonia: the good news, the bad news, and the way ahead. Lancet Glob Health 7: e4-4e5. [Crossref]
- Harris M, Clark J, Coote N, Fletcher P, Harnden A, et al. (2011) British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 66 (Suppl 2): ii1-23. [Crossref]
- Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, et al. (2011) The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 53: e25-76. [Crossref]
- Shin EJ, Kim Y, Jeong JY, Jung YM, Lee MH, et al. (2018) The changes of prevalence and etiology of pediatric pneumonia from National Emergency Department Information System in Korea, between 2007 and 2014. Korean J Pediatr 61: 291-300. [Crossref]
- Michelow IC, Olsen K, Lozano J, Rollins NK, Duffy LB, et al. (2004) Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics 113: 701-707. [Crossref]
- Clarke SC (2006) Control of pneumococcal disease in the United Kingdom--the start of a new era. J Med Microbiol 55: 975-980. [Crossref]
- Pandya GA, McEllistrem MC, Venepally P, Holmes MH, Jarrahi B, et al. (2011) Monitoring the Long-Term Molecular Epidemiology of the Pneumococcus and Detection of Potential ‘Vaccine Escape’ Strains. PLoS ONE 6: 15950. [Crossref]
- Bulgarian national immunisation programme 2011
- Neuman MI, Hall M, Lipsett SC, Hersh AL, Williams DJ, et al. (2017) Utility of Blood Culture Among Children Hospitalized with Community-Acquired Pneumonia. Pediatrics 140: e20171013. [Crossref]
- Gergova RT, Petrova G, Gergov S, Minchev P, Mitov I, et al. (2016) Microbiological Features of Upper Respiratory Tract Infections in Bulgarian Children for the Period 1998-2014. Balkan Med J 33: 675-680. [Crossref]
- Clinical and Laboratory Standards Institute (2010) Performance standards for antimicrobial susceptibility testing: 20th informational supplement. Wayne, Pa M100-S20.
- CDC protocol of real-time RTPCR for influenza A(H1N1). Geneva: World Health, Organization 2009.
- Kodani M, Yang G, Conklin LM, Travis TC, Whitney CG, et al. (2011) Application of Taqman low-density arrays for simultaneous detection of multiple respiratory pathogens. J Clin Microbiol 49: 2175-2182. [Crossref]
- Dunne EM, Satzke C, Ratu FT, Neal EFG, Boelsen LK, et al. (2018) Effect of ten-valent pneumococcal conjugate vaccine introduction on pneumococcal carriage in Fiji: results from four annual cross-sectional carriage surveys. Lancet Glob Health 6: e1375-1385.
- O’Brien K (2017) Current Status of PCV Use and WHO Recommendations, WHO report from 18 October 2017.
- Setchanova L, Murdjeva M, Stancheva I, Alexandrova A, Sredkova M, et al. (2017) Serotype changes and antimicrobial nonsusceptibility rates of invasive and non-invasive Streptococcus pneumoniae isolates after implementation of 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Bulgaria. Braz J Infect Dis 21: 433-440. [Crossref]
- El Seify MY, Fouda EM, Ibrahim HM, Fathy MM, Husseiny Ahmed AA, et al. (2016) Microbial Etiology of Community-Acquired Pneumonia Among Infants and Children Admitted to the Pediatric Hospital, Ain Shams University. Eur J Microbiol Immunol (Bp) 6: 206-214. [Crossref]
- del Valle-Mendoza J, Orellana-Peralta F, Marcelo-Rodroaguez A, Verne E, Esquivel-Vizcarra M, et al. (2017) High Prevalence of Mycoplasma pneumoniae and Chlamydia pneumoniae in Children with Acute Respiratory Infections from Lima, Peru. PLoS One 12: e0170787. [Crossref]
- World Health Organization (2014) Revised WHO classification and treatment of pneumonia in children at health facilities: evidence summaries.
- National Infectious disease register Finland 2012.
- Biscardi S, Lorrot M, Marc E, Moulin F, Boutonnat-Faucher B, et al. (2004) Mycoplasma pneumoniae and asthma in children. Clinical Infectious Diseases 38: 1341-1346.
- Florin TA, Carron H, Huang G, Shah SS, Ruddy R, et al. (2016) Pneumonia in Children Presenting to the Emergency Department with an Asthma Exacerbation. JAMA Pediatr 170: 803-805. [Crossref]
- Guo W-liang, Wang J, Zhu L-yuan, et al. Differentiation between mycoplasma and viral community-acquired pneumonia in children with lobe or multi foci infiltration: a retrospective case study. BMJ Open 5: e006766. [Crossref]


