Abstract
Background: Helicobacter pylori is considered the most common bacteria worldwide with a prevalence about 50% in developed countries and can reach up to 80-90% in developing countries. The first line agent for eradication of H. pylori has always been the classic triple therapy. However, recent data has shown the decline of the efficacy of this regimen due to antibiotics resistance. In Lebanon, due to the scarcity of data over the resistance rate of antibiotics and the efficacy of triple therapy, our study proposed a retrospective analysis of the eradication rate of H. pylori by classic triple therapy in the South of Lebanon between 2007 and 2014.
Materials and methods: A total of 168 patients were found eligible for the inclusion criteria in an 8-year data retrieval. The factors studied were (obesity, age, gender and smoking). The included patients received either one of the classic triple therapy regimens for 7, 10, or 14 days with no previous eradication, and followed up by at least one month after treatment with Urea breath test.
Results: 61.9% of patients were found to be sensitive to triple therapy, whereas 38.1% were resistant. There was no statistical significance between gender, age, and smoking history, method of diagnosis or type of triple therapy with p > 0.05. Adherence to treatment was good. No adverse effects were reported.
Conclusions: Our results showed suboptimal eradication rate by triple therapy. Thus, triple therapy should no more be considered the first line therapy for H. pylori eradication in Lebanon.
key words
eradication, Helicobacter pylori, resistance, triple therapy
Introduction
Helicobacter pylori (H. Pylori) is a gram-negative spirally-shaped bacterium, equipped with 3 to 5 flagella that are responsible for its motility feature. Lingering with the host, it constitutes a lifetime infection, as it specifically colonizes the stomach, and, being ureases as well as oxidase positive, has the ability to survive in the gastric epithelium which is considered a remarked characteristic [1].
Colonizing about half of the world’s population, H. pylori infection has been the culprit in a number of gastric diseases, the most described falls under the description of chronic, often asymptomatic, gastritis, in addition to causing peptic ulcer disease, mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric adenocarcinoma [2]. Alongside being implicated in a variety of extra-gastric diseases, such as Idiopathic thrombocytopenic purpura, iron-deficiency anemia, sideropenic anemia, and vitamin B12 deficiency, the role of H. pylori in cardiovascular, neurologic, hematologic, dermatologic, head and neck, uro-gynecologic as well as diabetes mellitus and metabolic syndrome, is being constantly investigated [3].
The carcinogenic role of H. pylori has been established as a class I carcinogen, in 1994 by the International Agency for Research on Cancers (IARC) [4].
Wide differences have been reported concerning the prevalence rate of H. pylori between developed and developing countries, and between Western and Asian countries [5]. In a 2007-study the worldwide prevalence was found to be 50% and could increase to 80–90% in developing countries [6]. A recent review [7] in 2016, constructed on 44 articles regarding prevalence rates of H. pylori across Europe covering 22 countries reported the prevalence rate to be lowest in northern and western countries: United Kingdom-London 33% [8], UK-Scotland 66% [9], Sweden-Stockholm 25% [10], Switzerland 19% [11], Denmark 17% [12], Germany 41% [13], to highest in southern and eastern countries: Croatia 60% [14], Italy 30% [15], Greece 49% [16], Spain-Ourense 71% [17], and Russia 88% [18]. In Asia, the general prevalence of H. pylori was around 60% in 2007 [19].
In a systematic review article in 2014 concerning the epidemiology of H. pylori in Arab countries, 26 articles were reviewed thus reporting a rate of 78% in 2006 in the United Arab Emirates, 70% in 2013 in Oman, 28.3% in 2012 in Kingdom of Saudi Arabia which decreased down from 66% in 1989, 64% rate in 2010 in Tunisia, and 76% in Libya, whereas data from Egypt reported the highest rates of infection with H. pylori (from 88% in 1992 to 72.38% in 2007) [20].
In Lebanon, not many recent studies exist that address the prevalence rate of H. Pylori. A study published in 2000 addressing H. pylori infection in North Lebanon by endoscopic biopsy and reported the prevalence rates to be 25.9% in subjects with normal endoscopy, 60.8% in patients with duodenal ulcer, and 72% in patients with congestive antritis [21]. Another study in 2007 conducted on children aged between 1 month and 17 years of variant socioeconomic states used the stool antigen to determine the prevalence rate of infection. The overall prevalence was 21% [22]. In 2012, a study designed by Naja et al. [23] to investigate the link between H. pylori infection and insulin resistance among Lebanese adults calculated the prevalence rate to be 52%, in a population of 308 individuals.
The Maastricht IV/Florence Consensus Report recommendations have been updated in 2017 concerning the management of H. Pylori. The triple therapy which includes proton pump inhibitors, clarithromycin, and amoxicillin or metronidazole has been the standard first-line regimen globally; however, recent data have shown that the success rate of this combination has been declining over the years, which has been directly linked to the increasing resistance to antibiotics most importantly to clarithromycin [24].
In 2008-2009 the overall resistance rate was reported to be 17.5% for clarithromycin, and 34.9% for metronidazole, according to a study designed on 2204 patients [25].
In Lebanon, a study conducted by Sharara et al. [26] in 2002 on 54 H. pylori isolates extracted by routine endoscopy in Beirut yielded results similar to those of Europe and USA concluding a low clarithromycin resistance rate of 4%, and a metronidazole resistance rate of 29.5%.
Objective
In countries with strong prevalence of resistance to macrolide, sequential therapy or bismuth quadruple therapy is being preferred over triple therapy. However, in Lebanon, due to the scarcity of data concerning resistance to antibiotics, no change in regimens has been proposed. Therefore, the purpose of this research is to determine the eradication rate of H. pylori in patients initially treated with classic triple therapy. The secondary objectives are to determine the association of the eradication rate with multiple demographic variables such as gender, smoking, obesity, past medical history and medication history. To identify a relation between the eradication rate and either the different types of triple therapy or the methods of diagnosis and finally to assess the results of the eradication rate yearly and on a four- year scale.
Materials and methods
Our study covers the areas of Nabatieh and Tyre. The main approach is to check the eradication rate of H. pylori in the past 8 years from the year 2007 till 2014 by classic triple therapy in a centered university hospital located in the south of Lebanon.
No formal sample size will be used in this research. Sample size depends on the number of eligible patients. All eligible patients will be included in this study.
The inclusion criteria include; patients aged between 10-80 years old, patients with first line treatment, patients who took triple therapy for 7, 10, or 14 days, patients who had Urea Breath Test after one month of treatment. The exclusion criteria the study will consider the following: patients under 10 and older than 80, patients who took previous triple therapy as a first line treatment.
Diagnosis was made by either serology, stool antigen, endoscopy: (pathology, urease rapid test) or urea breath test. Follow-up was done by Urea Breath Test (UBT) at least one month following treatment.
A total of 814 patients were found to be H. pylori positive, of which 168 were eligible for inclusion. The medical records were reviewed individually to determine the eradication status for each patient. Chart analysis was filled according to the medical charts only and no patient interrogation occurred.
Results
A total of 816 patients were screened for entry into the study, of which 648 patients were not included. The most common reasons for these exclusions were failure to follow-up with a UBT (336 patients), first-line treatment with quadruple bismuth therapy (124 patients) and receiving no treatment at all (100 patients).
One hundred sixty-eight patients were enrolled in the study, where 16.1%, 14.3%, 16.7%, 13.7%, 10.7%, 18.5%, 6% and 4.2% of patients were diagnosed in 2007, 2008, 2009, 2010, 2011, 2012, 2013 and 2014, respectively.
Mean age of the patients at inclusion was 42.15 (± 15.18) years. 39.3% of the patients were males and 60.7% were females. The majority of the patients (94.5%) were non-smokers. All the enrolled patients were not obese.
The most reported reasons of initial visit were epigastric pain in 137 patients (81.6%), constipation in 22 patients (13.1%), nausea in 19 patients (11.3%), anemia in 13 patients (7.7%) and diarrhea in 12 patients (7.1%). 89.3% of the patients were not on any medication. Eighteen patients (10.7%) out of 168 patients were on medication. Of whom, only 3 patients (1.8%) received proton pump inhibitors (PPIs).
Table 1 shows the different diagnostic methods used to detect the H. pylori infection. Of whom Urease rapid test (70.8%) is found to be the most preferred test followed by serology (23.8%). The number of patients treated with different triple therapy regimens are listed in Table 1.
Table 1. Method of diagnosis and types of triple therapy
Diagnosis and type of triple therapy |
N (%) |
Method of diagnosis |
|
Serology |
40 (23.8%) |
Stool antigen |
0 (0%) |
Endoscopy: Pathology |
5 (3%) |
Endoscopy: Urease rapid test |
119 (70.8%) |
Urea Breath test |
4 (2.4%) |
First line treatment |
|
Triple therapy: Amoxicillin, PPI, Clarithromycin |
159 (94.6%) |
Triple therapy: Amoxicillin, PPI, Metronidazole |
7 (4.2%) |
Triple therapy: Clarithromycin, PPI, Metronidazole |
2 (1.2%) |
The overall results showed a 61.9% eradication rate with classic triple therapy confirmed by urea breath test (Table 2) with a 38.1% resistance rate as seen in Figure 1.
Table 2. Follow up and final result
Initial follow-up by Urea Breath test |
N (%) |
Follow-up test results |
Negative |
104 (61.9%) |
Positive |
64 (38.1%) |
Compliant to medication |
Compliant |
148 (100%) |
Missing |
20 |
Patient is |
Sensitive |
104 (61.9%) |
Resistant |
64 (38.1%) |
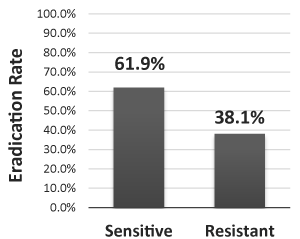
Figure 1: Percentage of sensitive and resistant patients
Figure 2 shows that the eradication rate per year varied significantly from a 74%, 46%, 64%, 74%, 50%, 61%, 70%, and 43% in years 2007, 2008, 2009, 2010, 2011, 2012, 2013, and 2014 respectively.
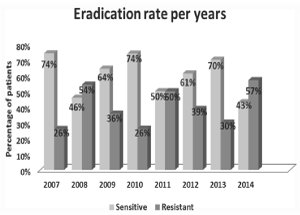
Figure 2. Eradication rate per year
The 4-year eradication rates from 2007 to 2010 and from 2011 till 2014 were 65% and 58% consecutively (Figure 3).
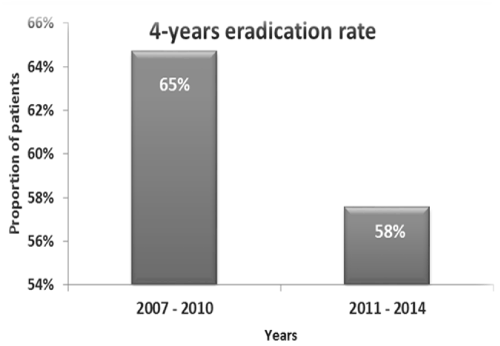
Figure 3. Four-years eradication rate
In Figure 4, the eradication rate was 63.5% in the amoxicillin, PPI, clarithromycin regimen, 42.9% in the amoxicillin, PPI, metronidazole regimen and 0% in the clarithromycin, PPI, metronidazole regimen, with no statistically significant differences (p-value = 0.105 > 0.05). Regimens including amoxicillin showed better eradication rates.
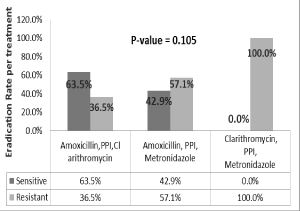
Figure 4. Eradication rate by types of triple therapy
Discussion
Various studies have demonstrated the importance of H. pylori eradication in improving the outcomes of its related gastric diseases [12,27-29]. However, recent data has been signaling the decline in the efficacy of the first line triple therapy [24], as worldwide reported eradication rates have been reported to be less than the targeted 80%-cure rate for an infectious disease [30]. The results in our study have aligned with this current statement as they have demonstrated an eradication rate of less than 80% over the span of 8 years.
In our study 104/168 (61.9%) have witnessed a successful eradication with the triple therapy, whereas 64/168 (38.1%) have reported treatment failure. Studies in Kuwait and Kingdom of Saudi Arabia have also shown similar low-success rates of 68.6% [31], and 67.6% [32], respectively. Our four-year eradication rate between 2007 and 2010 was 65%, with a decrease to 58% between 2011 and 2014, despite offering no statistical significance (p > 0.05) this decrease can be considered recognizable, as a study in Turkey also reported drops in the efficacy of triple therapy similar to ours from a rate of 68.8% substantiated from 1999 till 2005 [33], to 57% in 2011 [34]. In years 2013 and 2014 the number of included patients is 6% and 4.2% respectively, this decline in included patients is mostly due to physicians switching to either quadruple or sequential therapy as first line agents following the international recommended guidelines for developing countries as a reference. The majority of our included patients (159/168) have received amoxicillin, clarithromycin, and a PPI of whom 101 (63.5%) were sensitive, and 58 (36.5%) were resistant. Of the remaining nine patients, seven have received amoxicillin, metronidazole, and a PPI of whom three (42.9%) were sensitive and four (57.1%) were resistant, and the remaining two patients received clarithromycin, metronidazole, and a PPI, of which both (100%) were resistant, this high resistance rate to the clarithromycin and metronidazole combination shows that amoxicillin-based regimen is mandatory for efficient eradication.
Epigastric pain has been observed as the number one reason of initial visit (81.6%), but many lower gastrointestinal symptoms are seen such as constipation, rectorrhagia, melena or anal pain. In these cases, H. pylori was discovered accidentally during a regular endoscopy exam. No significant statistical variations (p > 0.05) were noted between gender distributions, age, obesity, smoking history, method of diagnosis, and reason of initial visit. A study by Silva et al. [35] in 2001 assessing the effects of the variables: age, tobacco, gender, alcohol, non-steroidal inflammatory drugs and previous treatment, has also shown no statistical significance with p > 0.05 with an exception for previously treated patients where the eradication rate was 53% in comparison with 76% for treatment-naïve patients [35]. The majority of our patients 94.5% were non-smokers. This has contributed in a positive aspect to our study, since smoking is one of the factors that lead to treatment failure as mentioned in a study by Namiot et al. [36] in 2000, where 142 H. pylori positive patients were treated with two regimens, both of which smoking has decreased the efficacy of eradication to 53.7% [36].
Researches have shown a growing concern for the declining success of the triple therapy, and worldwide studies have been published to study the triple therapy eradication rates of H. pylori. Unsatisfactory results have been reported nationwide across south Europe and South Asia [37-39]. In the United States of America, a decline of 56%, and 50% in the success rates of clarithromycin-containing, and metronidazole-containing regimens, respectively, has been determined in a systemic review article [40].
To our knowledge, in Lebanon, only one study has been recently published comparing the efficacy of bismuth-containing quadruple therapy to 14-days sequential therapy with eradication rates favoring the sequential treatment (80% vs 50%). However, the number of patients included in the study was too small, in our opinion, to result in a significant difference [41].
Conclusion
The results have shown suboptimal eradication rate by triple therapy. The demonstrated decreasing efficacy of the triple therapy, compatible with numerous global results, calls for a similar change in the first-line regimens for the proper eradication of this pathogen. This necessitates carrying out more research, which addresses the extent of this collapsing efficacy of the triple therapy in different areas throughout Lebanon, and on different ethnicities and age groups. The cause of the high resistance rate has not been identified and could be from overconsumption, self-medication, or uncontrolled prescription of clarithromycin and/or metronidazole in Lebanon. Furthermore, patient education about H. pylori infection and the importance of adhering to medications, combined with a thorough follow up is recommended to ensure complete eradications and diminish resistance to antibiotics, as these are of vital importance to proper treatment. Moreover, larger prospective randomized controlled studies are needed in Lebanon to compare the different first line treatments. Thus, establishing our own guidelines regarding H. pylori infection treatment.
References
- Wroblewski LE, Peek RM Jr, Wilson KT (2010) Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev 23: 713-739. [Crossref]
- Tegtmeyer N, Moodley Y, Yamaoka Y, Pernitzsch SR, Schmidt V, et al. (2016) Characterisation of worldwide Helicobacter pylori strains reveals genetic conservation and essentiality of serine protease HtrA. Mol Microbiol 99: 925-944. [Crossref]
- Goni E, Franceschi F (2016) Helicobacter pylori and extragastric diseases. Helicobacter 21: 45-48. [Crossref]
- Ozaydin N, Turkyilmaz SA, Cali S (2013) Prevalence and risk factors of Helicobacter pylori in Turkey: a nationally-representative, cross-sectional, screening with the 13C-Urea breath test. BMC Public Health 13: 1215. [Crossref]
- Breckan RK, Paulsse2021 Copyright OAT. All rights reserv B, et al. (2016) The All-Age Prevalence of Helicobacter pylori Infection and Potential Transmission Routes. A Population-Based Study. Helicobacter 21: 586-595. [Crossref]
- Rafeey M, Ghotaslou R, Nikvash S, Hafez AA (2007) Primary resistance in Helicobacter pylori isolated in children from Iran. J Infect Chemother 13: 291-295. [Crossref]
- Roberts SE, Morrison-Rees S, Samuel DG, Thorne K, Akbari A, et al. (2016) Review article: the prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe. Aliment Pharmacol Ther 43: 334-345. [Crossref]
- Mendall MA, Goggin PM, Molineaux N, Levy J, Toosy T, et al. (1992) Childhood living conditions and Helicobacter pylori seropositivity in adult life. Lancet 339: 896-897. [Crossref]
- McDonagh TA, Woodward M, Morrison CE, McMurray JJ, Tunstall-Pedoe H, et al. (1997) Helicobacter pylori infection and coronary heart disease in the North Glasgow MONICA population. Eur Heart J 18: 1257-1260.
- Sörberg M, Nyrén O, Granström M (2003) Unexpected decrease with age of Helicobacter pylori seroprevalence among Swedish blood donors. J Clin Microbiol 41: 4038-4042. [Crossref]
- Lehmann FS, Renner EL, Meyer-Wyss B, Wilder-Smith CH, Mazzucchelli L, et al. (2000) Helicobacter pylori and gastric erosions. Results of a prevalence study in asymptomatic volunteers. Digestion 62: 82-86. [Crossref]
- Dahlerup S, Andersen RC, Nielsen BS, Schjødt I, Christensen LA, et al. (2011) First-time urea breath tests performed at home by 36,629 patients: a study of Helicobacter pylori prevalence in primary care. Helicobacter 16: 468-474. [Crossref]
- Kuepper-Nybelen J, Thefeld W, Rothenbacher D, Brenner H (2005) Patterns of alcohol consumption and Helicobacter pylori infection: results of a population-based study from Germany among 6545 adults. Aliment Pharmacol Ther 21: 57-64. [Crossref]
- Babus V, Presecki V, Katicic M, Balija M, Zoric I, et al. (1997) Distribution of Helicobacter pylori infection in the adult population of Croatia. Lijec Vjesn 119: 139-142. [Crossref]
- Shapira Y, Poratkatz B-S, Gilburd B, Barzilai O, Ram M, et al. (2012) Geographical differences in autoantibodies and anti-infectious agents antibodies among healthy adults. Clin Rev Allergy Immunol 42: 154-163. [Crossref]
- Apostolopoulos P, Vafiadis-Zouboulis I, Tzivras M, Kourtessas D, Katsilambros N, et al. (2002) Helicobacter pylori (H. pylori) infection in Greece: the changing prevalence during a ten-year period and its antigenic profile. BMC Gastroenterol 2: 11. [Crossref]
- Macenlle García R, Gayoso Diz P, Sueiro Benavides RA, Fernández Seara J (2006) Risk factors associated with Helicobacter pylori infection. A population-based study conducted in the province of Ourense. Rev Esp Enferm Dig 98: 330-340. [Crossref]
- Malaty HM, Paykov V, Bykova O, Ross A, Graham DP, et al. (1996) Helicobacter pylori and socioeconomic factors in Russia. Helicobacter 1: 82-87. [Crossref]
- De Vries AC, Kuipers EJ (2007) Epidemiology of premalignant gastric lesions: Implications for the development of screening and surveillance strategies. Helicobacter 12: 22-31. [Crossref]
- Holcombe C (1992) Helicobacter pylori: the African enigma. Gut 33: 429-431. [Crossref]
- Kalaajieh WK, Chbani-Rima A, Kassab TF, Baghdadi FM (2000) [Helicobacter pylori infection in North Lebanon]. Santé 10: 31-35. [Crossref]
- Naous A, Al-Tannir M, Naja Z, Ziade F, El-Rajab M (2007) Fecoprevalence and determinants of Helicobacter Pylori infection among asymptomatic children in Lebanon. J Med Liban 55: 138-144. [Crossref]
- Naja F, Nasreddine L, Hwalla N, Moghames P, Shoaib H, et al. (2012) Association of H. pylori Infection with Insulin Resistance and Metabolic Syndrome among Lebanese Adults. Helicobacter 17: 444-451. [Crossref]
- Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, et al. (2017) Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 66: 6-30. [Crossref]
- Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, et al. (2013) Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 62: 34-42. [Crossref]
- Sharara AI, Chedid M, Araj GF, Barada KA, Mourad FH (2002) Prevalence of Helicobacter pylori resistance to metronidazole, clarithromycin, amoxycillin and tetracycline in Lebanon. Int J Antimicrob Agents 19: 155-158. [Crossref]
- Graham DY, Lee Y-C, Wu M-S. (2014) Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence (revision 2). Clin Gastroenterol Hepatol 12: 177-186.
- Ito M, Haruma K, Kamada T, Mihara M, Kim S, et al. (2002) Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: a 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol Ther 16: 1449-1456. [Crossref]
- Jin X, Li Y (2007) Systematic review and meta-analysis from Chinese literature: the association between Helicobacter pylori eradication and improvement of functional dyspepsia. Helicobacter 12: 541-546. [Crossref]
- Graham DY, Lu H, Yamaoka Y (2007) A report card to grade Helicobacter pylori therapy. Helicobacter 12: 275-278. [Crossref]
- Alboraie M, Saad M, Al-Ali J, Malik M, Asem N, et al. (2015) Quadruple therapy versus standard triple therapy for eradication of Helicobacter pylori in Kuwait. Arab J Gastroenterol 16: 131-135. [Crossref]
- Alsohaibani F, Al Ashgar H, Al Kahtani K, Kagevi I, Peedikayil M, et al. (2015) Prospective trial in Saudi Arabia comparing the 14-day standard triple therapy with the 10-day sequential therapy for treatment of Helicobacter pylori infection. Saudi J Gastroenterol 21: 220-225. [Crossref]
- Kadayifci A, Buyukhatipoglu H, Cemil Savas M, Simsek I (2006) Eradication of Helicobacter pylori with triple therapy: an epidemiologic analysis of trends in Turkey over 10 years. Clin Ther 28: 1960-1966. [Crossref]
- Polat Z, Kadayifci A, Kantarcioglu M, Ozcan A, Emer O, et al. (2012) Comparison of levofloxacin-containing sequential and standard triple therapies for the eradication of Helicobacter pylori. Eur J Intern Med 23: 165-168. [Crossref]
- Silva FM, Zaterka S, Eisig JN, Chehter EZ, Chinzon D, et al. (2001) Factors affecting Helicobacter pylori eradication using a seven-day triple therapy with a proton pump inhibitor, tinidazole and clarithromycin, in Brazilian patients with peptic ulcer. Rev Hosp Clin Fac Med Sao Paulo 56: 11-16. [Crossref]
- Namiot Z, Namiot DB, Kemona A, Golebiewska M, Bucki R (2000) The effect of cigarette smoking and alcohol consumption on efficacy of Helicobacter pylori eradication. Pol Arch Med Wewn 104: 569-574. [Crossref]
- Gatta L, Vakil N, Vaira D, Scarpignato C (2013) Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ 347: f4587.
- Almeida N, Donato MM, Romaozinho JM, Luxo C, Cardoso O, et al. (2015) Beyond Maastricht IV: Are standard empiric triple therapies for helicobacter pylori still useful in a South-European country? BMC Gastroenterol 15: 23. [Crossref]
- Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, et al. (2007) Sequential Therapy versus Standard Triple-Drug Therapy for Helicobacter pylori Eradication. Ann Intern Med 146: 556-563. [Crossref]
- Houben MHMG, Van De Beek D, Hensen EF, De Craen AJM, Rauws EAJ, et al. (1999) A systematic review of Helicobacter pylori eradication therapy-the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther 13: 1047-1055. [Crossref]
- Tarhini M, Fayyad-Kazan M, Fayyad-Kazan H, Mokbel M, Nasreddine M, et al. (2018) First-line treatment of Helicobacter pylori in Lebanon: Comparison of bismuth-containing quadruple therapy versus 14-days sequential therapy. Microbial Pathogenesis 117: 23-26. [Crossref]




