Coccidioidomycosis and Strongyloidiasis are fungal and parasitic diseases, respectively, which typically cause disease in immunocompromised individuals. In the United States, cases are seen in patients who either live in or have traveled to endemic areas. With no prior documented cases to our knowledge, we present two cases of overwhelming eosinophilia due to co-infection with Coccidioides and Strongyloides. Co-infection was suspected in these patients after presenting with complicated Coccidioidomycosis, with persistent eosinophilia, and a suspicious travel history, which prompted additional diagnostic testing. Severe, acute coccidioidomycosis likely caused a temporary immune suppression, allowing for the emergence of an underlying dormant Strongyloides infection.
Coccidioides, Strongyloides, co-infection, eosinophilia
Inhalation of spores of the dimorphic fungi Coccidioides immitis or Coccidioides posadasii causes coccidioidomycosis, and the immunocompromised are usually most susceptible to severe disease and dissemination. Endemic to the Southwestern United States, cases of Coccidioides immitis are most commonly found in southern California, while Coccidioides posadasii is distributed across Arizona, Utah, New Mexico, Texas, and even further south in Mexico and South America [1,2]. The Centers for Disease Control and Prevention reported 14,364 cases of coccidioidomycosis in 2017 with approximately 48% of cases occurring in California [3]. Coccidioides grows as a mold with septate hyphae in soil, and the hyphae fragment into arthroconidia, which are easily aerosolized when dry desert soil is disturbed by winds or digging. A susceptible host inhales the arthroconidia, and the new moist warm environment of the lungs triggers a morphological change into spherules, filled with endospores over 48-72 hours. Spherules rupture, releasing hundreds to thousands of endospores that are capable of disseminating throughout the host and the process repeats [1,4].
Pulmonary disease, more commonly known as Valley fever, manifests as a community acquired pneumonia with pleuritic chest pain and cough with possible hemoptysis, dyspnea, and fever [5]. An estimated less than 1% to 4.7% of cases are disseminated. Dissemination may be cutaneous, with granulomatous lesions or abscesses, bone and joint involvement with osteomyelitis and arthritis, and/or meningitis [5,6]. Rarely, disease can spread to the thyroid and reproductive organs [5,7]. Diagnostic confirmation with multiple methods includes identification of endospore containing spherules from a tissue sample, culture, PCR, and serology with IgM and IgG antibodies [1]. First line treatment is with triazole antifungals for both pulmonary and disseminated disease, followed by amphotericin B in cases that do not respond to triazoles [8,9].
Strongyloides stercoralis is a soil-transmitted nematode that causes strongyloidiasis and a more commonly known cause of infectious eosinophilia [10]. Like coccidioidomycosis, it usually affects immunocompromised individuals, but is largely asymptomatic in competent hosts and can remain dormant for decades [11]. Strongyloides is endemic to regions where climate conditions are warm and humid, such as Central and South America, sub-Saharan Africa, and South and Southeast Asia. In the United States, infection often occurs in immigrants, refugees, travelers, and military personnel who have lived in endemic areas or the Southeastern United States [10,12].
Infection begins through skin contact with filariform larvae from fecal contaminated soil, and the larvae travel through the bloodstream and lymphatics to the lungs. Larvae ascend the tracheobronchial tree or are coughed up and then swallowed, where they enter the digestive system. Filariform larvae develop into adults, and eggs released from females become rhabditiform larvae, which pass in stool with the potential to become free-living adult males and females in soil or can enter into a cycle of autoinfection within a host [12,13]. Symptoms occur in conjunction with the life cycle, resulting in dyspepsia with pain or diarrhea; respiratory complaints of cough, dyspnea, or wheezing; and cases of autoinfection can result in hyperinfection syndrome due to high parasite burden. Disease confirmation is through stool analysis with positive identification of larvae or serology with IgG [13]. Treatment of choice is with ivermectin, or second line with albendazole.
Here we present two cases of co-infection with Coccidioides and Strongyloides, which to our knowledge per literature review are the first reported cases of persistent eosinophilia associated with co-infection.
In 2017, a 25-year old Caucasian male with a past medical history of asthma and seasonal allergies presented to an emergency department in Northern Arizona with complaints of 5-6 weeks of progressively worsening dyspnea, with associated productive cough with green-yellow sputum, and fatigue. On the day of presentation, the patient had been moving heavy objects at home, when he developed a sudden worsening of cough, pleuritic chest pain, and thought he had injured his back. He denied fevers, chills, weight loss, diarrhea, rashes, or joint pain. The patient admitted to one day of abdominal upset, with nausea and non-bloody vomiting, about one week prior to admission. In the emergency department, initial vital signs were stable, and labs revealed a CBC with hemoglobin 6.1 g/dL, white blood cell count of 11.9 × 103/µL, platelets 357 × 103/µL, with a differential of 31% lymphocytes, 53% neutrophils, 6% monocytes, and 7% eosinophils. C-reactive protein was 5.9 mg/L and complete metabolic panel was within normal range. Initial chest x-ray showed a large right pneumothorax and right pleural effusion, requiring emergent chest tube placement (Figure 1). Follow-up CT scan of the chest with contrast showed improvement of pneumothorax and pleural effusion but revealed a thick-walled 3.9 cm cavitary mass in the right lower lobe with satellite nodules, which were concerning for an infectious process (Figure 2). Physical exam after chest tube placement was grossly benign, except for noted white purulent drainage from the tube. Social history was significant for recent travel to northern Mexico and Jamaica (at age 18). The patient was admitted to the ICU for spontaneous pneumothorax and a cavitary lung lesion with suspected infectious etiology, and empirically started on broad-spectrum antibiotics with piperacillin-tazobactam and clindamycin.
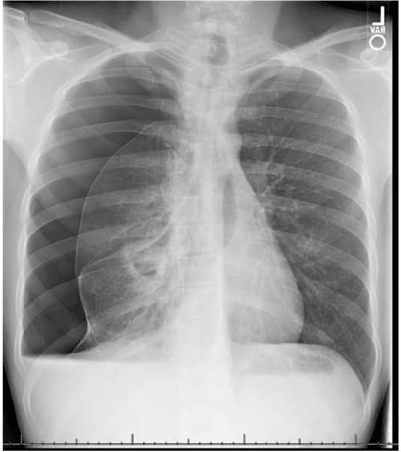
Figure 1. Chest X-ray demonstrating large right pneumothorax and a cavitary lesion
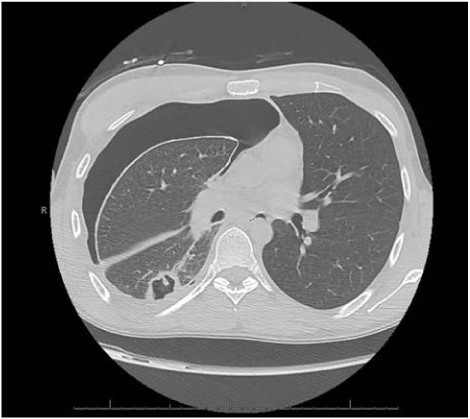
Figure 2. CT chest without contrast demonstrating a right lower lobe cavitary lesion
A negative TB QuantiFERON gold test and AFB smears and cultures ruled out tuberculosis. Pleural fluid analysis was consistent with an exudate and cytopathology revealed septated hyphae and rare, budding, consistent with fungal organisms. Pleural fluid cultures grew Coccidioides. Serum studies yielded a positive cocci serology for IgM and IgG, and titer by complement fixation of 1:32. Serology was negative for Aspergillus. Fluconazole 400 mg IV daily was started, and antibiotics were discontinued once pleural fluid culture was finalized, as there was low suspicion for a secondary bacterial process.
Due to persistent drainage from the chest tube and air leaks, the patient went to surgery for wedge resection of the right lower lobe cavitary mass and decortication. Histopathology demonstrated necrotizing cavitary granulomatous inflammation and numerous fungal hyphae. The remainder of his hospitalization was generally unremarkable, except for a rising eosinophilia, which peaked at 33%. Eosinophilia was suspected to be due to allergic response, but the patient had no systemic signs and was asymptomatic. Serial chest x-rays ensured resolution of pneumothorax, chest tubes were removed, and the patient was discharged home with instructions to complete a long-term course of oral Fluconazole 400 mg for at least 6 months.
Three months later, the patient again presented with complaints of dyspnea and pleuritic chest pain for 2 weeks and was readmitted for recurrence of right pneumothorax as confirmed on chest CT. Physical exam was significant for dullness of breath sounds. At time of admission, initial vitals were stable, and complete blood count and metabolic panel were unremarkable, except for a persistent eosinophilia at 28.8%. A pigtail drain was placed, but he did not have full re-expansion of the lung and had a persistent air leak. Given the persistent pneumothorax with air leak, the patient was taken to the operating room for right thoracotomy with partial decortication of the right lower lobe, partial parietal pleurectomy with creation of intercostal muscle flap for reinforcement of repair of a bronchopleural fistula, and chemical pleurodesis with doxycycline. Cultures taken during the procedure again grew Coccidioides. Given the patient’s persistent illness, fluconazole was changed to a loading dose of voriconazole 400 mg PO BID with a maintenance dose of 200 mg PO BID due to concerns for an unknown secondary fungal infection.
Histopathology showed fungal hyphae and budding yeast, and an unclear fibrinopurulent material with organization, which raised suspicion for a secondary etiology that may explain the eosinophilia and treatment failure on Fluconazole. Additional infectious work up, including HIV and Paragonimus serology, and galactomannan for Aspergillus were negative. Ova and parasite stool analysis was negative. The patient tested positive for Strongyloides IgG in serum and received Ivermectin 200 mcg/kg PO, with immediate resolution of eosinophilia. The fibrinopurulent material seen on histopathology were likely the filariform larvae of Strongyloides stercoralis (Figures 3,4). The patient was discharged home in stable and improved condition once chest tubes were removed. The patient continued treatment for coccidioidomycosis with a planned 6-12-month course.
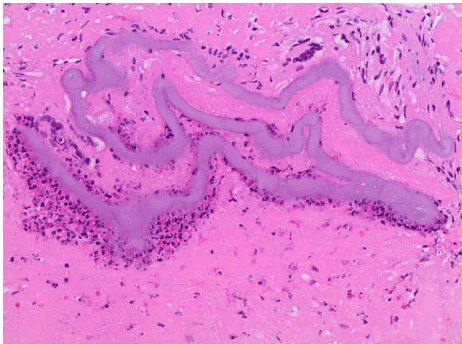
Figure 3. Light microscopy with 40× magnification: H&E stain of lung tissue with filariform larvae of Strongyloides stercoralis
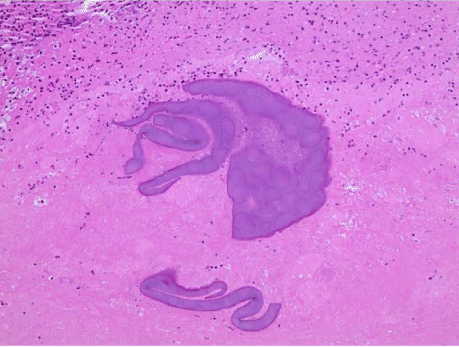
Figure 4. Light microscopy with 40× magnification: H&E stain of lung tissue with filariform structures, presumptively associated with larvae of Strongyloides stercoralis
In 2018, a 46-year-old Caucasian male with medical history of hyperlipidemia and recurrent pneumonia and bronchitis, presented to the emergency department in Flagstaff, Arizona with complaints of right sided pleuritic chest pain for 5 days, with associated cough and fever with a maximum temperature of 104°F. The patient was initially evaluated at his primary care provider’s office, where he received clarithromycin for pneumonia after a chest x-ray revealed the presence of a right upper and middle lobe consolidation. His symptoms did not improve after 3 days, with worsening pleuritic pain. He admitted to productive cough with yellow sputum, and denied any weight loss, chills, night sweats, nausea, or vomiting. In the emergency department, the patient was afebrile, and oxygen saturation decreased to 86% on room air with improvement to above 90% with 2L of supplemental oxygen. Physical exam was unremarkable, and the lungs were clear to auscultation. However, subsequently became severely dyspneic with nasal flaring with removal of supplemental oxygen. Initial labs revealed hemoglobin of 15.1 g/dL, white blood cell count of 10.4 × 103/µL, platelets 208 × 103/µL, with differential of 15% lymphocytes, 75.3% neutrophils, 6.3% monocytes, 2.9% eosinophils, and 0.5% basophils. A CT angiogram of the chest ruled out pulmonary embolism and was significant for a large dense consolidation of the right upper lobe, airspace disease in the right middle and lower lobes, and right hilar mediastinal adenopathy (Figure 5). The patient was admitted to the telemetry unit for acute hypoxic respiratory failure secondary to community-acquired pneumonia with pleurisy, and empirically treated with ceftriaxone and azithromycin.
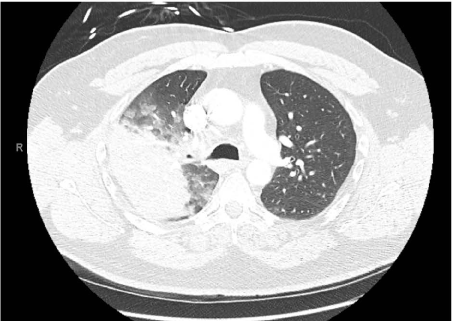
Figure 5. CT angiogram of chest: Large dense consolidation in the right upper lobe apical posterior
Infectious pneumonia work-up including viral respiratory panel, urinary antigens for legionella and pneumococcus, blood cultures, and sputum cultures were all negative. He was discharged home 3 days later on oral doxycycline and azithromycin, and supplemental oxygen.
The patient followed up with his primary care provider 3 weeks after discharge as he still had complaints of dry cough and a daily low-grade fever. A repeat chest x-ray showed worsening consolidation of a large portion of the right lung and persistent reticulonodular densities in the left mid-chest, and his primary care provider referred the patient to the emergency department. Complete blood count showed no significant leukocytosis but was concerning for an eosinophilia of 30%. CT angiogram done in the emergency department, again demonstrated consolidative opacities in the right upper lobe with diffuse nodular opacities and bronchiolitis throughout the right lung with less pronounced nodular opacities of bronchiolitis in the left upper greater than lower lobes. Mediastinal and right hilar lymphadenopathy were present (Figure 6). The patient declined hospitalization but agreed to follow up in the infectious disease’s outpatient clinic.
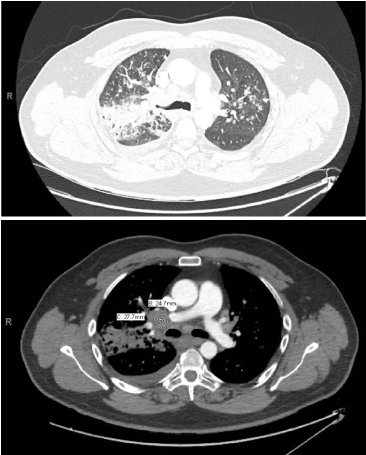
Figure 6. (a) CT angiogram of chest: Consolidative opacities in the right upper lobe with diffuse nodular opacities and bronchiolitis through the right lung with less pronounced nodular opacities of bronchiolitis in the left upper lobe (b) CT angiogram of chest: Multiple enlarged right hilar lymph nodes, with largest demarcated as 2.8 × 2.5 cm
During his visit at the infectious disease office, patient reported frequent travel to Needles, California for work and travel to the Caribbean and Belize 10 years prior. Due to persistent eosinophilia, the patient was evaluated for fungal pneumonia, and coccidioidomycosis serology was positive for IgM and IgG, with titer by complement fixation of 1:32. Strongyloides serology was also positive for IgG. He was treated with fluconazole 400 mg daily and weight-dosed ivermectin, with complete resolution of eosinophilia.
We have presented two cases of strongyloidiasis that emerged in the setting of complicated coccidioidomycosis and subsequent persistent eosinophilia. In the first case, the patient presented with Valley Fever complicated by recurrent pneumothorax and bronchopleural fistula. The patient likely acquired coccidioidal infection by living in the endemic state of Arizona in the setting of previous infection with Strongyloides due to his international travel. In the second case, the patient presented with a history of recurrent and persistent pneumonia due to Coccidioides, which he acquired after living in endemic areas of Arizona and traveling to California. The patient likely became infected with Strongyloides from travel to the Caribbean or Belize. In both cases, infection with Coccidioides and Strongyloides was suspected due to worsening eosinophilia after an initial diagnosis of severe primary pulmonary coccidioidomycosis; Strongyloides infection was confirmed through serology. The first case had additional evidence of infection with Strongyloides via histopathology of the lung, revealing presence of filariform larvae. The two patients initially received treatment with first-line fluconazole and ivermectin.
Based on extensive literature review these appear to be the first two cases of documented co-infection with Coccidioides and Strongyloides. The patients complicated clinical courses and persistent eosinophilia raised concern for co-infection. Additional travel history and residence in areas endemic for Strongyloides and Coccidioides prompted testing and evaluation for disease in these patients.
A unique characteristic of Strongyloides stercoralis is the ability to cause autoinfection, followed by a possible hyperinfection syndrome. Hyperinfection syndrome results in extensive and excessive worm burden within areas of the traditional reproductive cycle of Strongyloides, such as the skin, gastrointestinal, and respiratory systems of hosts [14]. Hyperinfection syndrome is more likely to occur after changes in immune status within a host, most commonly with transplant patients, patients receiving glucocorticoids or other immunosuppressants, or patients infected with human-T-lymphotropic virus type 1 or Human immunodeficiency virus [14,15]. We suspect that the presentation with severe, acute Coccidioides infection resulted in a temporary immune suppression in these two patients, allowing for the unmasking of an underlying Strongyloides infection. This lead to a high suspicion of hyperinfection syndrome given the patients complicated respiratory presentations. Based on these case reports, we support maintaining a low threshold to evaluate for Coccidioides and Strongyloides infections through stool analysis, serology, culture, or direct tissue analysis with microscopy, in patients who present with an overwhelming eosinophilia after the diagnosis of coccidioidomycosis despite appropriate treatment and with suspicious travel history.
There are no financial conflicts of interest to disclose.
We thank the microbiology laboratory staff at Flagstaff Medical Center and Drs. Darlene Lee, Harold E. Sightler and Elaina A. Pirruccello, pathology department for their support on the diagnosis of these cases. We would also like to acknowledge Dr. Nicole Sydow, cardiothoracic surgeon, Andrea Boyce, Infectious disease pharmacist and Emilie Bowers, Infectious disease nurse practitioner for their involvement on these cases.
- Saubolle MA, McKellar PP, Sussland D (2006) Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol 45: 26-30. [Crossref]
- Brown J, Benedict K, Park BJ, Thompson GR (2013) Coccidioidomycosis: epidemiology. Clin Epidemiol 5: 185-197. [Crossref]
- Centers for Disease Control and Prevention (2019) Valley Fever (Coccidioidomycosis) statistics. Available at: https://www.cdc.gov/fungal/diseases/coccidioidomycosis/statistics.html [Accessed February 13, 2019].
- Kirkland TN, Fierer J (1996) Coccidioidomycosis: a reemerging infectious disease. Emerg Infect Dis 2: 192-199. [Crossref]
- Crum NF, Lederman ER, Stafford CM, Parrish SJ, Wallace MR (2004) Coccidioidomycosis: A descriptive survey of a reemerging disease. Clinical Characteristics and current controversies. Medicine 83: 149-175. [Crossref]
- Odio CD, Marciano BE, Galgiani JN, Holland SM (2017) Risk factors for disseminated coccidioidomycosis, United States. Emerg Infect Dis 23: 311. [Crossref]
- Adarm RD, Elliott SP, Taljanovic MS (2009) The spectrum and presentation of disseminated coccidioidomycosis. Am J Med 122: 770-777. [Crossref]
- Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, et al. (2005) Coccidioidomycosis. Clin Infect Dis 41: 1217-1223. [Crossref]
- Stockamp NW, Thompson GR 3rd (2016) Coccidioidomycosis. Infect Dis Clin North Am 30: 229-246. [Crossref]
- Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, et al. (2013) Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl Trop Dis 7: e2288. [Crossref]
- Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O (2011) Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis 17: 931-932. [Crossref]
- Centers for Disease Control and Prevention (2019) Parasites-Strongyloides. Available at: https://www.cdc.gov/parasites/strongyloides [Accessed February 17, 2019].
- Ericsson CD, Steffen R, Siddiqui AA, Berk SL (2001) Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis 33: 1040-1047. [Crossref]
- Kassalik M, Mönkemüller K (2011) Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol 7: 766-768. [Crossref]
- Keiser PB, Nutman TB (2004) Strongyloides stercoralis in the Immunocompromised Population. Clin Microbiol Rev 17: 208-217. [Crossref]






