Elevated low-density lipoprotein (LDL) cholesterol has been established as one of the major risk factors for adverse cardiovascular events [1], and accumulating evidence has indicated LDL-lowering with statins is the most essential therapeutic component for primary and secondary prevention in coronary artery disease (CAD) [2,3]. Despite a large body of evidence regarding a higher intensity LDL-lowering strategy with the notion “the lower, the better” and recommendations by U.S. and European guidelines of lipid management [4,5], the efficacy of higher intensity statin therapy in Asian populations has been rarely investigated, although there was one large-scale placebo-controlled study of pravastatin involving 7,832 Japanese patients without a history of cardiovascular disease that examined primary prevention of cardiovascular disease with pravastatin in Japan (MEGA) trial [6]. A guideline by the American College of Cardiology and American Heart Association (ACC/AHA) indicated that doses lower than those used in Western countries may be appropriate in Asians, which is a recommendation similar to that for patients who have a history of hemorrhagic stroke [4,5]. However, as a consequence of obviously insufficient evidence, discussion regarding Asians in ACC/AHA and European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) lipid management guidelines is very limited. In fact, only one sentence addresses this issue in each guideline, despite the recent growth in Asian populations in these areas (https://www.census.gov/main/www/cprs.html). Furthermore, although “high intensity” statin therapy is recommended in the ACC/AHA guideline for secondary prevention based on findings in previous studies, including the safety and efficacy of enoxaparin vs unfractionated heparin in patients with non-ST-segment elevation acute coronary syndromes (A to Z) [7], treating new targets (TNT) [8], incremental decrease in end points through aggressive lipid lowering (IDEAL) [9], and pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction 22 (PROVE-IT TIMI22) [10], the doses of statins in “high intensity” in these trials, such as atorvastatin 40 mg or 80 mg/day or rosuvastatin 20 mg or 40 mg/day, are excessively higher than those approved and covered by insurance in Japan. In contrast, “high-intensity or high-dose statins” in Japan, such as pravastatin 40-80 mg, atorvastatin 10-20 mg, rosuvastatin 5-10 mg, and pitavastatin 2-4 mg are listed within the “moderate-intensity” statin group in the ACC/AHA guideline [5]. Since no previous large-scale randomized trial anywhere in the world has demonstrated the superiority of such moderate-intensity statin therapy compared to low-intensity statin therapy, no sufficient evidence has been obtained for the treatment. Therefore, prospective endpoint trials for the prognostic benefit of higher-intensity statin therapy, although it has been previously considered as “moderate-intensity”, have been warranted to establish evidence particularly for Asians, who may need lower doses of statins compared to non-Asian patients in Western countries.
Two large-scale randomized trials enrolling Japanese patients of less- and more-intensive statin therapy have recently been published. One is a primary study and the other is a secondary prevention study. The EMPATHY study is an intention-to-treat analysis evaluating the efficacy of “intensive” LDL-C lowering therapy targeting less than 70 mg/dL (n=2518) in serum LDL-C, compared to the “standard” one targeting 100-120 mg/dL (n=2524) in high-risk primary prevention. Patients with diabetic retinopathy in addition to dyslipidemia, but without a history of cardiovascular disease, were enrolled [11]. The REAL-CAD study addressed the prognostic superiority of higher-dose pitavastatin (4 mg/day, “moderate intensity” in U.S. guideline) (n=6526) compared to low-dose (1 mg/day) (n=6528) in secondary prevention [12]. The protocols, study demographics and results in these two studies are summarized in Figure 1 and Table 1 [13,14]. In the EMPATHY study, the primary endpoint was a “cardiovascular (CV) event”, a composite of wider range cardiovascular, cerebral and renal events than other endpoint studies in this field, such as CV death, myocardial infarction (MI), unstable angina (UA) requiring hospitalization or coronary revascularization, ischemic stroke, cerebral revascularization, deterioration of renal function including initiation of hemodialysis, and aortic and peripheral diseases including aortic dissection, critical limb ischemia and peripheral revascularization. In contrast, the primary endpoint in the REAL-CAD study was a composite of rather focused events, which is generally defined as 4P-MACE, including CV death, non-fetal MI and stroke and hospitalization for UA. In the EMPATHY study, intensive LDL-C lowering therapy by statins was indicated to achieve LDL-C<70 mg/dl, but the mean LDL-C level was 76.5 mg/dl at 3 years after randomization, indicating a substantial population did not achieve the target LDL-C level. As a result, intension to treat analysis showed intensive statin therapy did not reduce the cumulative incidence of the primary endpoint. However, in post-hoc analysis limited to patients who actually achieved the target LDL-C level, a significantly lower cumulative incidence of primary events in the intensive group was demonstrated. As key secondary endpoints, the incidence of a cerebral event was suppressed by intensive statin therapy targeting <70 mg/dl, as compared to standard statin therapy targeting 100-120 mg/dl. In contrast, in the REAL-CAD study, pitavastatin 4 mg/day, as compared to 1 mg/day, significantly reduced the primary endpoint. As the secondary endpoint, 4 mg/day pitavastatin reduced the risk of all-cause death, myocardial infarction and any coronary revascularization.
Table 1. The protocols and results in EMPATHY study and REAL-CAD study
|
EMPATHY study |
REAL-CAD study |
Primary, Secondary prevention |
Primary |
Secondary |
Disease of subjects |
Diabetic retinopathy |
Stable coronary artery disease |
Intervention |
Intensive lipid lowering aiming at LDL-C <70 mg/dl |
Pitavastatin 4 mg/day |
Control |
Standard: LDL-C 100-120 mg/dl |
Pitavastatin 1 mg/day |
Intention-to-treat |
Yes |
No (dose fixed) |
Number of patients (Intervention) |
2518 |
6199 |
Number of patients (Control) |
2524 |
6214 |
Run-in period |
4-8 weeks |
4 weeks or more |
Follow-up period |
>2 years (Mean 3.1 years) |
2021 Copyright OAT. All rights reserv
>3 years (Median 3.9 years) |
Final LDL-C, mg/dl (Intervention) |
76.5 |
76.6 |
Final LDL-C, mg/dl (Control) |
104.1 |
91 |
Primary endpoint |
CV event |
CV death + MI +Stroke + Hospitalization for unstable angina |
Efficacy of intervention on primary endpoint |
No difference |
Significant lower incidence of primary endpoint in 4 mg/day pitavastatin |
Hazard ratio, 95% confidential interval, p-value |
0.84, 0.67-1.07, p=0.15 |
0.81, 0.69-0.95, p=0.01 |
Note |
Significantly lower CV event rate in post-hoc analysis of patients who reached target range |
High dose pitavastatin reduced secondary endpoint, coronary revascularization plus primary endpoint |
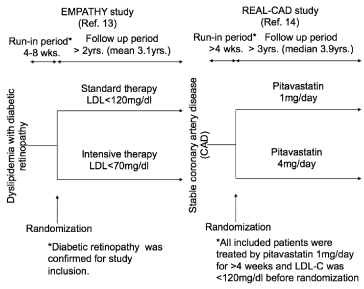
Figure 1. Study scheme of EMPATHY study and REAL-CAD study.
These two studies provide important evidence. First, they, the REAL-CAD study in particular, demonstrated moderate-intensity statin therapy to be effective for better outcomes as compared to low-intensity statin therapy, which have rarely been investigated in previous large-scale randomized trials [15]. Furthermore, these studies were the first to establish evidence of higher intensity statin therapy in high-risk primary and secondary prevention for Asian populations. The incidence of CV events in Japanese was substantially lower than that in Western countries. For instance, the overall primary endpoint rate in the TNT study, the study design of which was similar to that of the REAL-CAD study, was 9.8%, while 4.6% in the REAL-CAD study, although the definition of the primary endpoint is slightly different between the two studies. Nevertheless, higher intensity statin therapy, even though it was “moderate intensity” in Western guidelines, was associated with a reduced risk of adverse cardiovascular events. The findings in these two studies indicate the need for moderate intensity statin therapy in Asians as secondary and high-risk primary prevention, and may have an impact on future recommendations in the guidelines of not only Asian countries, but also those of the United States and European countries.
References
- Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, et al. (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97: 1837-1847. [Crossref]
- [No authors listed]. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344: 1383-1389. [Crossref]
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, et al. (1996) The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 335: 1001-1009. [Crossref]
- Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, et al. (2016) 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J 37: 2999-3058. [Crossref]
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, et al. (2014) 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129: S1-45. [Crossref]
- Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, et al. (2006) Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 368: 1155-1163. [Crossref]
- Blazing MA, de Lemos JA, White HD, Fox KA, Verheugt FW, et al. (2004) Safety and efficacy of enoxaparin vs unfractionated heparin in patients with non-ST-segment elevation acute coronary syndromes who receive tirofiban and aspirin: a randomized controlled trial. JAMA 292: 55-64. [Crossref]
- LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, et al. (2005) Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 352: 1425-1435. [Crossref]
- Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, et al. (2005) High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 294: 2437-2445. [Crossref]
- Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, et al. (2004) Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 350: 1495-1504. [Crossref]
- Itoh H, Komuro I, Takeuchi M, Akasaka T, Daida H, et al. (2018) Intensive Treat-to-Target Statin Therapy in High-Risk Japanese Patients With Hypercholesterolemia and Diabetic Retinopathy: Report of a Randomized Study. Diabetes Care 41: 1275-1284.
- Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, et al. (2018) High-Dose Versus Low-Dose Pitavastatin in Japanese Patients With Stable Coronary Artery Disease (REAL-CAD): A Randomized Superiority Trial. Circulation 137: 1997-2009. [Crossref]
- Ueshima K, Itoh H, Kanazawa N, Komuro I, Nagai R, et al.(2016) Rationale and Design of the Standard Versus Intensive Statin Therapy for Hypercholesterolemic Patients with Diabetic Retinopathy (EMPATHY) Study: a Randomized Controlled Trial. J Atheroscler Thromb 23: 976-990. [Crossref]
- Miyauchi K, Kimura T, Shimokawa H, Daida H, Iimuro S, et al. (2018) Rationale and Design of Randomized Evaluation of Aggressive or Moderate Lipid Lowering Therapy with Pitavastatin in Coronary Artery Disease (REAL-CAD) Trial. Int Heart J 59: 315-320. [Crossref]
- Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, et al. (2017) Association Between Intensity of Statin Therapy and Mortality in Patients With Atherosclerotic Cardiovascular Disease. JAMA Cardiol 2: 47-54. [Crossref]

