Introduction: While it is known that training improves Olfactory function, this paper aims to discuss whether gustatory training using taste strips as a therapeutic tool, along with olfactory training, leads to improvements in patients with hyposmia.
Methods: The study included 38 patients with hyposmia, in two groups. Using the taste training set of 4 different strips (TST), the patients were asked to train with it twice daily over a period of 6 weeks. One group was given an additional set of 4 sniffing sticks for smell training while the other group was not. Both groups were screened using a TDI (Threshold-Discrimination-Identification) test battery, TST-nasal endoscopy, and visual analogue scale. The first follow up was performed after 6 weeks of training and the second was 4 months after the first screening.
Results: The test–retest reliability of TDI highlighted the effectiveness of therapy with taste strips (p = 0.04) along with sniffing sticks (p = 0.002). Furthermore, results of TST (p = 0.015) are significantly better in the combination of olfactory and gustatory training.
Conclusion: Taste strips are easy to use and are a strong training tool. Offering hyposmia patients the training option of taste strips and sniffing sticks in a training set was beneficial, and significantly enhanced subjective smell improvement. Female and older patients had better results. Additional detailed studies with a larger number of patients would be required to further validate the results.
Threshold-Discrimination-Identification, hyposmia, visual analogue scale, nasal endoscopy
Smell and taste disorders have a major adverse impact on the quality of life for those they impact [1]. The olfactory function plays a crucial role in memory, mood, emotion, and forms a major part of many of life’s pleasurable experiences. Sudden loss of olfactory function is usually noticed (e.g., post-infectious or posttraumatic), whereas gradual deterioration of smell functions may go unnoticed (e.g., in relation to age or neurodegenerative diseases) [2,3]. The loss of olfactory function increases with age; Individuals over 50, forming a quarter of the population, have an impaired sense of smell (presbyosmia) [4]. Exposure to specific odors (olfactory training) may increase olfactory sensitivity in patients with postinfectious and post-traumatic olfactory dysfunction [5]. Taste strips (“Burghart, Medizin Technik”, Germany) are strips of paper that can be purchased and come flavored with 4 taste qualities: sweet, sour, salty, and bitter [6]. They are used in medical practice as a tool for hypogeusia and hyposmia diagnosis. The aim of this study was to investigate the efficacy of gustatory training with taste strips as a therapeutic tool in hyposmia patients.
Subjects
Between January 2017 and April 2017, 38 patients (28 female, 10 male) diagnosed with hyposmia of different etiologies were screened and investigated over a 4-month period (postiviral n = 21, posttraumatic n = 4, sinonasal n = 8, idiopathic n = 3). Predefined exclusion criteria were: Age below 18 or over 80, pregnancy, olfactory disorder with neurodegenerative or congenital etiology. Patients were randomly divided in two groups. Each group was asked to train twice daily over a 6 week period. The first group (19 patients) was given a set of 4 taste strips (sweet, sour, bitter and salty - Burghart Medizin Technik, Germany), whereas the second group was offered a set of four sniffing sticks, (Coffee, orange, rose, eucalyptus (Burghart, Germany)) in addition to the taste strips.
On baseline and follow up visits, all patients had to complete a questionnaire and underwent an otorhinolaryngological (ORL) examination. The patients had a follow up 6 weeks post screening, then again after 4 months.
Olfactory testing
The Threshold Discrimination Identification (TDI) score was used for orthonasal olfactory testing (7, 8). The olfactory threshold (T) was measured using 48 “Sniffin’ Sticks” with a 16 stage dilution series of Butanol. The discrimination test (D) was performed with 48 “Sniffin’ Sticks” of different smell qualities. Everyday odors were identified with the Identification test (I) which consist of 16 “Sniffin’ Sticks”. The TDI values were determined as followed: Less than 16 points was rated as anosmia, up to 30.5 points as hyposmia and a value above 31 points as normosmia, thus excluded. The patients also estimated their smell function on a visual analogue scale (VAS), ranging from 0 to 10 points.
Gustatory testing
The TST taste strip test (TST) was applied. This 16-part test evaluated four different qualities of taste (sweet, sour, bitter and salty) each presented in four different concentrations. A test value of less than 9 was considered as hypogeusia.
Statistical analysis
Statistical analyses were performed using SPSS 24 (SPSS Inc., Chicago, Illinois, USA). Data was presented as mean, ± standard deviation (SD), or median and quartile. Mixed-Design ANOVA was used for a combination of ordinary and variance analysis with measurement repetitions. This method was chosen because there are no non-parametric methods for this experimental design. Effect sizes are also considered using eta-square after Cohen (1988). A value of more than 0.01 represents small effects, more than 0.06 represents average effects, and more than 0.14 represents large effects. A probability value of less than 0.05 was considered statistically significant.
All 38 patients performed the follow-up visits and testing. Test-retest reliability of TDI showed effectiveness of the therapy in both groups offered the taste strips. The group that received the additional “Sniffin’ Sticks” showed a slightly higher benefit. Interestingly, females and older patients showed a faster and better recovery rates. Smoking habits and etiology have not shown a significant effect on the results of this study.
All parameters are presented in mean±standard deviation. Differences between the baseline and follow-up visits (6 weeks later) assume normal distribution of the data with the paired t-test. The significance level was defined at p≤0.05 (Table 1). TDI improved significantly (p = 0,002) after gustatory training, and VAS also showed a significant improvement (p = 0,003). Likewise, the TST also showed a positive improvement (p = 0,002). Baseline visits showing the correlation between VAS and TDI are demonstrated in Figure 1 and follow-up visits with a significant correlation are shown in Figure 2. Finally, the significant improvement of the TDI score over a 6 week period is shown in Figure 3. Additionally, differences between male and female in both groups over six weeks training period is shown in Figure 4.
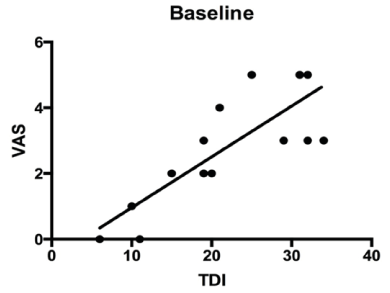
Figure 1. Correlation between VAS and TDI.
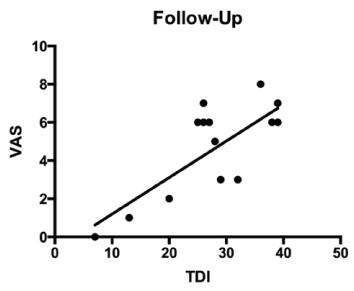
Figure 2. Follow-up visits.
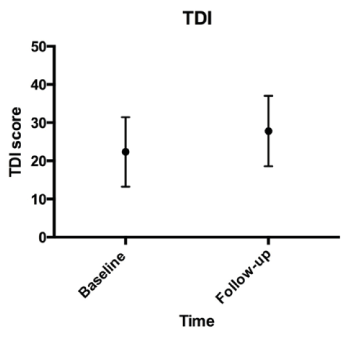
Figure 3. TDI score over a 6 week period.
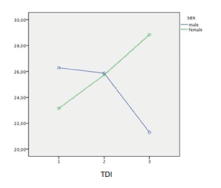
Figure 4. Differences between male and female in both groups over six weeks training period.
Table 1. Group 1 with (olfactory and gustatory training).
Patients with declining olfactory functions often report a diminished quality of life, negative effects on interpersonal relations [9], worries about not identifying toxic substances, poor control over personal hygiene, difficulties with daily routines, depression [10,11] and even suicidal thoughts [12]. There is no treatment of choice for all types and degrees of hyposmia, but the etiology of hyposmia could offer a remedy. Patients with chronic rhinosinusitis are shown to experience different degrees of hyposmia [13]. Therefore, sinus surgery has been proven to be helpful in regaining the sense of smell, like in septoplasty for obstructive deviated septum [14], diode laser inferior turbinate reduction (ITR) in hypertophic turbinate [15], endoscopic polypectomy surgery in polyposis nasi [16], or revision endoscopic sinunasal surgery (RESS) in chronic sinusitis [17].
In cases of a post viral [18], neurodegenerative, [19,20] or idiopathic hyposmia, olfactory training has proven its effectivity and reliability. The application of smell training through the orthonasal olfaction is in many hyposmia cases, a therapy option that showed to be beneficial in regenerating the ability of the sense of smell [21,22,23]. Orthonasal olfaction occurs during sniffing [24] as opposed to retronasal olfaction, which is the perception of odors emanating from the oral cavity during eating and drinking. Orthonasal and retronasal olfactory information has been shown to be processed differently on a cerebral level [25]. This seems to suggest that the structures responsible for orthonasal and retronasal olfaction are functionally different but may also be structurally different at the OE or olfactory bulb levels, and even at the cerebral level [26,27].
The aim of this study was to investigate the effectivity of the olfactory training concept [28] through the retronasal olfaction way by using taste strips of four different tastes (sweet, sour, salty and bitter) in order to stimulate the olfactory bulb function in patients with hyposmia. The 38 patients were randomly separated in two groups. The group of patients who were asked to train twice daily with the four taste strips over a six weeks period showed a significant recovery rate (p = 0.04). The second group was asked to train with “sniffin’ sticks” additionally to the taste strips the first group had received. In this group, the significance of recovery rates was higher (p = 0.005). Females and older patients showed a faster and better recovery rate. One explanation could be the superiority of olfaction perception in females compared to males [29]. Though not highly significant, older patients mentioned being very disciplined in following the twice- daily- training schema of the study. Following these results, we can say that training the olfactory bulb and its receptors stimulates the recovery of hyposmia. The gustatory pathway showed to be a reliable method to add an intensity to the olfactory training. Therefore, it is suggested that the olfactory training could be classified as orthonasal and retronasal training. In this study, training the olfaction through both pathways showed to be significantly more efficient than training either ortho or retonasal alone. Further studies comparing results of retronasal versus orthonasal training and comparing the combination of both trainings versus only the orthonasal one is to be followed. Retronasal olfaction method is being studied as a diagnostic tool to evaluate the sense of smell using taste powders [30]. In future research, it may also be useful as a therapeutic option for treating patients with olfactory loss.
The combination of gustatory and olfactory training showed a relevant effectiveness in the treatment of olfactory dysfunction. Gustatory training added a richness and intensity to the smell stimulation. A radiologic translation of these results with the help of a functional MRI conducted study could be helpful, with further studies are to come.
- Croy I, Nordin S, Hummel T (2014) Olfactory Disorders and Quality of Life—An Updated Review. Chem Senses 39: 185-194. [Crossref]
- Stevenson RJ (2010) An initial evaluation of the functions of human olfaction. Chem Senses 35: 3-20. [Crossref]
- Miwa T, Furukawa M, Tsukatan T, Costanzo RM, DiNardo LJ, et al. (2001) Impact of olfactory impairment on quality of life and disability. Arch Otolaryngol Head Neck Surg 127: 497-503. [Crossref]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BEK, Klein R, et al. (2002) Prevalence of olfactory impairment in older adults. JAMA 288: 2307-2312. [Crossref]
- Konstantinidis I, Tsakiropoulou E, Bekiaridou P, Kazantzidou C, Constantinidis J (2013) Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope 123: E85-E90. [Crossref]
- Landis B, Welge-Luessen A, Brämerson A, Bende M, Mueller CA, et al. (2009) "Taste Strips" - a rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J Neurol 256: 242-248. [Crossref]
- Hummel T, Kobal G, Gudziol H, Mackay-Sim A (2007) Normative data for the "Sniffin' Sticks" including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol 264: 237-243.
- Hummel T, Sekinger B, Wol SR, Pauli E, Kobal G (1977) Sniffin' sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22: 39-52. [Crossref]
- Corwin J, Loury M, Gilbert AN (1995) Workplace, Age, and Sex as Mediators of Olfactory Function: Data from the National Geographic Smell Survey. J Gerontol 50B: 179-186.
- Koros Ch, Stamelou M, Simits A (2018) Selective cognitive impairment and hyposmia in p.A53T SNCA PD vs typical PD. Neurology 90: e864-e869. [Crossref]
- Rochet M, El-Hage W, Rich S, Kazour F, Atanasova B (2018) Depression, Olfaction, and Quality of Life: A Mutual Relationship. Brain Sci 8: 80. [Crossref]
- Joo YH, Hwang SH, Han KD (2015) Relationship between Olfactory Dysfunction and Suicidal Ideation: The Korea National Health and Nutrition Examination Survey. Am J Rhinol Allergy 29: 268-272. [Crossref]
- Morse JC, Shilts MH, Ely KA, Li P, Sheng Q, et al. (2019) Patterns of olfactory dysfunction in chronic rhinosinusitis identified by hierarchical cluster analysis and machine learning algorithms. Int Forum Allergy Rhinol 9: 255-264. [Crossref]
- .Valsamidis K, Printza A, Titelis K, Constantinidis J, Triaridis S (2019) Olfaction and quality of life in patients with nasal septal deviation treated with septoplasty. Am J of Otolaryngology 40: 747-754. [Crossref]
- Göktas Ö, Lau L, Fleiner F (2014) Effect of Laser Treatment on Olfactory Dysfunction. Indian J Otolaryngol Head Neck Surg 66: 173. [Crossref]
- Kuperan A, Lieberman S, Jourdy D (2015) The effect of endoscopic olfactory cleft polyp removal on olfaction. Am J Rhinol Allergy 29: 309-313.
- Hsu CY, Wang YP, She PH, Weitzel EK, Lai JT, Wormald PJ (2013) Objective olfactory outcomes after revision endoscopic sinus surgery. Am J Rhinol Allergy 27: e96-e100. [Crossref]
- Li KY, Liu J, Xia W, Ren YY, Wei YX (2016) Characteristics of post viral olfactory disorder. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2016 51: 838-841. [Crossref]
- Fleiner F, Dahlslett SB, Schmidt F, Harms L, Goektas O (2010) Olfactory and gustatory function in patients with multiple sclerosis. Am J Rhinol Allergy 24: e93-97. [Crossref]
- Godoy MDCL, Godoy CL, Voegels RL, de Rezende Pinna F, Imamura R, et al. (2015) Olfaction in Neurologic and Neurodegenerative Diseases: A Literature Review. Int Arch Otorhinolaryngol 19: 176-179. [Crossref]
- Konstantinidis I, Tsakiropoulou E, Bekiaridou P, Kazantzidou C, Constantinidis J (2013) Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope 123: E85-E89. [Crossref]
- Damm M, Pikart L, Reimann H, Burkert S, Göktas O, et al. (2014) Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope 124: 826-831. [Crossref]
- Jensine M, Soler Z, Schlosse R (2019) A Pilot Study of Olfactory Training in Older Hyposmic Adults. Am J Rhinol Allergy 33: 650-656. [Crossref]
- Heilmann S, Hummel T (2004) A new method for comparing orthonasal and retronasal olfaction. Behav Neurosci 118: 412-419. [Crossref]
- Rozin P (1982) Taste-smell confusions and the duality of the olfactory sense. Percept Psychophys 31: 397-401. [Crossref]
- Landis B, Frasnelli J, Reden J, Lacroix JS, Hummel T (2005) Differences Between Orthonasal and Retronasal Olfactory Functions in Patients With Loss of the Sense of Smell. Arch Otolaryngol Head Neck Surg 131: 977-981. [Crossref]
- Small DM, Gerber JC, Mak YE, Hummel T (2005) Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron 47: 593- 605.
- Fleiner F, Lau L, Göktas Ö (2012) Active Olfactory Training for the treatment of Smelling Disorders. Ear Nose Throat J 91: 198-203, 215. [Crossref]
- Sorokowski P, Karwowski M, Misiak M, Marczak MK, Dziekan M, et al. (2019) Sex Differences in Human Olfaction: A Meta-Analysis. Front Psychol 10: 242. [Crossref]
- Yoshino A, Göktas G, Mahmut MK, Zhu Y, Goektas O, et al. (2020) A New Method for assessing Retronasal Olfactory Function. Laryngoscope 131: E324-E330. [Crossref]
=




