Objective: To observe the efficacy and safety of post-operative long-term low-dose oral administration of clarithromycin in patients with refractory chronic rhinosinusitis (RCRS). Explore the characteristics of post-operative microbiota in the nasal cavity in patients with RCRS and compare the differences and changes in microbiota in the nasal cavity before and after medication.
Method: This was a prospective, self-controlled study. 18 RCRS patients who had persistent symptoms after endoscopic sinus surgery (ESS) and standard therapy with normal IgE and eosinophil level were included. Low dose (250 mg, QD) clarithromycin was orally administrated for 12 weeks, Symptom severity and endoscopic findings were evaluated before, after 4-week and 12-week of treatment, and nasal cavity microbiota was analyzed simultaneously.
Results: A total of 18 RCRS patients were enrolled, and 17 patients completed the study. Four weeks after oral administration of clarithromycin, significant improvement was observed in subjective symptoms including nasal congestion, rhinorrhoea, postnasal drip and general discomfort, as well as endoscopic findings including general surgical cavity condition, rhinedema and rhinorrhoea (P<0.05). After continuous treatment to the 12th week, the improvements of symptoms showed significant improvement compared with baseline, and endoscopic score showed significant improvement compared with both baseline and 4 weeks after treatment. Analysis of middle nasal meatus flora revealed a significant decrease of Streptococcus pneumoniae after 12 weeks of clarithromycin treatment (P<0.05), while the richness, composition, and diversity similar before and after treatment. Patients enrolled experienced no adverse drug reaction or allergic reaction, nor clinical significant liver function impairment observed.
Conclusion: Post-operative low-dose long-term oral administration of clarithromycin in RCRS patients can improve the clinical symptoms and facilitate the mucosal epithelization, with good tolerance and safety. The efficacy of clarithromycin in RCRS patients may be related to its regulatory effect on nasal cavity microbiota.
Clarithromycin, refractory chronic rhinosinusitis, clinical efficacy, safety, microbiota in nasal cavity
Chronic Rhinosinusitis (CRS) is a chronic inflammatory disease involving the nasal cavity and nasal sinus and affecting 8% of Chinese people according to a latest multi-center cross-sectional study in China [1]. The current treatment for CRS, endoscopic sinus surgery (ESS) and standard post-operative medication including antibiotic, intranasal corticosteroids mucigogues, oral hormone, and nasal irrigation, could achieve a clinical cure rate up to 75-98% [2-3], but there are still nearly 10% CRS patients failing to obtain the ideal clinical outcomes [4]. These patients show no obvious improvements in their symptoms and local signs after ESS and standard post-operative treatment, hence can be classified as "refractory chronic rhinosinusitis (RCRS)" [5-7]. In RCRS patients and part of CRS patients who preferred non-surgical treatment, anti-inflammatory treatment is essential to improve efficacy.
In recent years, accumulating evidence have shown that 14-membered ring macrolides have anti-inflammatory, immunoregulatory [8,9], and anti-biofilm [10] effects. Moreover, many randomized controlled trails in China and abroad have confirmed the clinical efficacy of post-operative long-term low-dose oral administration of macrolides in CRS patients. However, controversy remains between results from different studies. For example, one randomized controlled trial by Amin Amali et al. [11] enrolled 66 post-operative CRS patients reported significant remissions of subjective symptoms including nasal congestion and postnasal drip after 12 weeks of azithromycin treatment, while another randomized controlled trial by Boris R Haxel et al. [12] enrolled 67 post-operative CRS patients only observed decrease in endoscopic score after 12 weeks of erythromycin treatment. Moreover, CRS patients enrolled in these studies were all routinely treated with macrolides after operation, without the examination of IgE or eosinophil level, nor the distinguish of RCRS patients according to the efficacy of post-operative standard therapies. Therefore, it is still necessary to further verify the efficacy of long-term low-dose macrolides in RCRS patients with normal IgE and eosinophil level. In addition, recent studies have shown that there is colonization of commensal bacteria in human nasal cavity [13], and the disturbance its balance by pathogen or antibiotics may contribute to the pathogenesis and progression of CRS [14-15]. As a result, the effect of post-operative long-term low-dose 14-membered ring macrolides on the nasal commensal microbiota of CRS patients needs to be further studied.
In order to evaluate the clinical efficacy of 14-membered ring macrolides in the treatment of RCRS, we designed this prospective self-controlled study. The study evaluated the efficacy and safety of long-term low-dose oral administration of clarithromycin in RCRS patients with normal IgE and eosinophil level by dynamical follow-up of symptom scores, endoscopic scores and adverse events during treatment, and analyzed its impact on nasal microbiota by comparing the 16S rDNA sequencing results of middle nasal meatus flora secretion before and after treatment.
Study design and case selection:
This study is a prospective, single-arm, single-center and self-controlled trial designed in compliance with the Declaration of Helsinki, ICH-GCP and relevant laws and regulations of China authority. The registered ethical number for this study is TRECKY2017-030, and the Chinese clinical trial registration number is ChiCTR1800014382. Study enrolled adult RCRS patients with normal IgE and eosinophil level treated at the Rhinology Department, Beijing Tongren Hospital, Capital Medical University during the period from June 2016 to June 2017. RCRS patient is defined as CRS patient who received ESS and standard post-operative treatment including antibiotic, intranasal corticosteroids, mucogogues, oral hormone and nasal irrigation, but subsequently experienced no obvious improvement or had persistent symptoms lasted for more than one month. Exclusion criteria: 1) patients with immunodeficiency syndromes, such as primary ciliary dyskinesia (PCD); 2) patients with bronchial asthma or concomitant severe allergic rhinitis and/or fungal sinusitis; 3) pregnant and breastfeeding women; 4) Patients allergic to macrolide drugs; 5) Patients with organic diseases in heart, liver, kidney and/or digestive tract or patients who had obvious elevated liver enzymes level and/or severe gastrointestinal adverse reactions after administration of clarithromycin. Patients who met the inclusion/exclusion criteria are well-informed of the study and signed the informed consent form before enrolment.
Treatment schedule and follow-up:
All the patients enrolled were orally administrated with 14-membered ring macrolides clarithromycin (Trade name: Klacid, Abbott Laboratories), 250 mg, qd for continuous 12 weeks on top of standard post-operative treatment such as intranasal corticosteroids, mucogogues and nasal irrigation. Follow-ups were conducted at baseline, 4 weeks, and 12 weeks after treatment.
Clinical data collection:
Collection of efficacy data: All the patients should complete VAS scoring and endoscopic scoring before treatment (baseline), after 4 weeks and 12 weeks of treatment.
Collection of safety data: 1. Liver function: Liver function was examined after 4 weeks and 12 weeks of treatment. 2. Adverse drug reactions: Symptoms occurred during therapy including nausea, vomiting, diarrhea and/or dyspepsia were collected by questionnaires at each follow up. 3. Allergic reactions to treatment were asked and recorded at each follow up.
Sample collection:
The secretion from the middle nasal meatus area was collected with nasopharyngeal swab through sheathing canal under the guidance of nasal endoscope, through brushing with a sheath canal. Sampling was conducted by brushing 6 cycles clockwise and 6 cycles anticlockwise. After sampling, the brush head was cut off, placed in sterile tube, rapidly frozen in liquid nitrogen, and stored in a -80- refrigerator for further usage.
DNA extraction and 16S rDNA sequencing:
CTAB method was used to extract the genomic DNA from middle nasal meatus secretion. After quantification, DNA was diluted to 1ng/ul and 16S rDNA V4 region was amplified and purified using primers 515F and 806R. Library construction was performed using the library construction kit TruSeq® DNA PCR-Free Sample Preparation Kit. Libraries constructed were proved qualified through Qubit and Q-PCR quantification, and second generation high-throughput sequencing was performed using HiSeq2500 PE250.
Sequencing data analysis:
Based on valid data, sequences satisfying 97% consistency were clustered into OTUs (Operational Taxonomic Units) following the default, and sequences with the highest frequency within OTUs was selected as the representative sequence of OTUs and annotated. The richness, diversity and composition of nasal flora were compared among groups.
Statistical analysis:
Quantitative data were described with (median, interquartile range) or (mean ±standard deviation). T test or non-parametric test was conducted for data analysis using SPSS 24 for Mac statistical software. A statistically significant difference was considered to be observed when P<0.05, and a strong statistically significant difference was considered to be observed when P<0.01.
Basic characteristics:
A total of 18 patients were enrolled in this study, including 12 males and 6 females, with a median age of 42 years old (age ranging from 19 to 63 years old). After 12 weeks of treatment, 1 patient were lost to follow-up. Finally, the efficacy and safety data of 17 patients were integrated and included in the subsequent analysis. The basic characteristics are shown in (Table 1). No patients experienced clinically significant changes in liver function after 4 and 12 weeks of clarithromycin treatment. No patients complained of discomforts such as nausea, vomiting, diarrhea and dyspepsia; no allergic reactions to clarithromycin were observed during the study. Middle nasal meatus secretion samples before treatment, after 4 weeks and 12 weeks of treatment were collected from 16 patients.
Table 1. Basic characteristics of subjects.
Basic characteristics |
Result (N=17) |
Age (year) |
|
Median age |
42 |
Quartiles |
35,56 |
Male/Female |
12/5 |
EOS% |
2.62%±1.55% |
Smoker (%) |
6 (35.3%) |
Concomitant asthma |
0 |
Average operations |
2 |
Interval between last surgery and the first medication of Clarithromycin (Month) |
2.45 |
Efficacy evaluation:
The VAS scoring and endoscopic scoring data showed a skewed distribution, and Friedman rank sum test was used for statistical analysis.
VAS scoring: As shown in Figures 1 and 2, symptoms including nasal congestion, rhinorrhea, postnasal drip and general discomfort improved significantly after 4-week and 12-week oral administration of clarithromycin compared with baseline according to VAS scoring results. However, further significant relief of symptoms was not observed after comparison of VAS scores after 4 weeks and 12 weeks of treatment. In addition, no significant improvement of hyposmia was found after 4 weeks and 12 weeks of treatment.
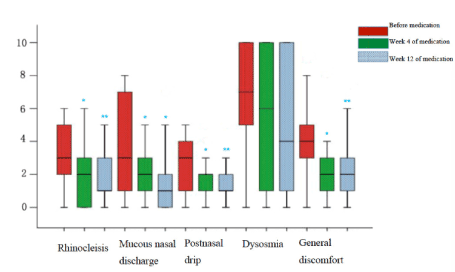
Figure 1. VAS scores box chart.
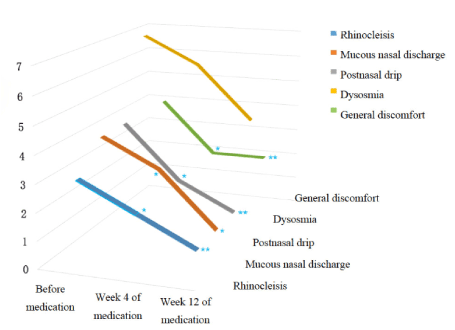
Figure 2. VAS scores 3D variation trend chart.
Endoscopic scoring: Four weeks after oral administration of clarithromycin, significant improvement in endoscopic scoring including general surgical cavity condition, rhinorrhea and rhinedema (P<0.05) were shown compared with baseline. Furthermore, when continued clarithromycin treatment till the 12th week, the endoscopic scoring in general surgical cavity condition (P<0.01), rhinorrhea (P<0.05) and rhinedema (P<0.01) showed significant improvement compared with baseline and the 4th week. Data mentioned above were summarized in Figures 3 and 4. Representative pictures of surgical cavity mucosa taken under endoscopy before and after clarithromycin treatment could be found in Figures 5 and 6.
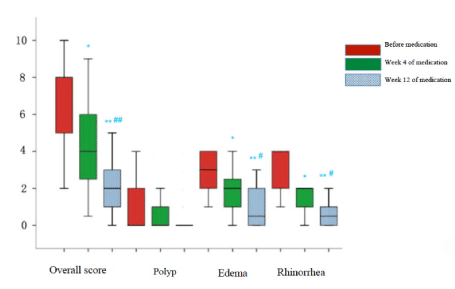
Figure 3. Endoscopic scores box chart.
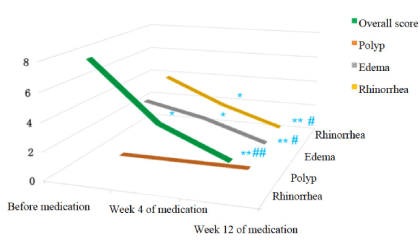
Figure 4. Endoscopic scores 3D variation trend chart.
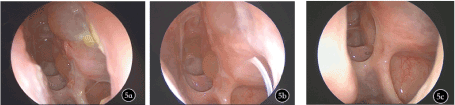
Figure 5. Comparison of endoscopy before and after treatment a: Before treatment, the mucosa in the surgical cavity showed obvious rhinedema, vesiculation, with a large volume of viscous secretions; b: In the 4th week of medication, rhinedema of mucosa in the surgical cavity was obviously mitigated, with small volume of viscous secretions; c: In the 12th week of medication, the mucosal epithelialization in the surgical cavity was good, the sinus ostia were unobstructed and no obvious secretion was observed.
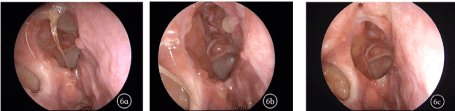
Figure 6. Comparison of endoscopy before and after treatment a: Before treatment, the mucosa in the surgical cavity showed obvious rhinedema, vesicle and granulation, with a large volume of viscous secretions; b: In the 4th week of treatment, the mucosa in the surgical cavity was still edematous with a large volume of viscous secretions; c: In the 12th week of treatment, the mucosal epithelialization in the surgical cavity was good, and the sinus ostia were unobstructed and no obvious secretion and neoplasm were observed.
Flora analysis:
Nasal flora sequencing data showed a skewed distribution, so Friedman rank sum test was used for statistical analysis.
Richness: The total number of OTUs detected was 14,437 before treatment, then 14,177 after 4 weeks of treatment, and then 14,977 after 12 weeks of treatment. No statistical difference was found in the multiple comparisons among these 3 groups.
Abundance: Generally speaking, the composition of nasal flora was similar before and after clarithromycin administration. The most abundant bacteria generas before treatment, 4 weeks and 12 weeks after treatment were all Staphylococcus (9.34% before treatment, 12.46% 4 weeks after treatment, 19.80% 12 weeks after treatment), Pseudomonas (2.93%, 2.33%, 3.20%), Streptococcus (2.26%, 1.04%, 0.25%), Corynebacterium (1.22%, 0.72%, 0.41%), Haemophilus (2.46%, 0.37%, 0.57%), Delftia (0.03%, 0.05%,0.04 %) and Fusobacterium (0.06%, 0.07%, 0.03%), with slightly differences in proportion. Among which, the proportion of Streptococcus decreased significantly after 12 weeks of treatment (P<0.05), and the proportion of Haemophilus decreased significantly after 4 weeks of treatment (P<0.05), both compared with baseline. Similarly, among the top four abundant bacteria species, namely Staphylococcus aureus, Pseudomonas veronii, Streptococcus pneumoniae, and Pseudomonas geniculate, only the proportion of Streptococcus pneumoniae decreased significantly after 12 weeks of treatment compared with baseline(P<0.05). (Figures 7 and 8).
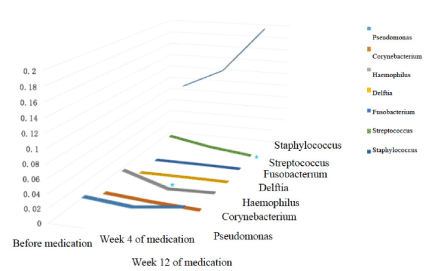
Figure 7. 3D variation trend chart of the top 7 generas in terms of relative abundance at genera level.
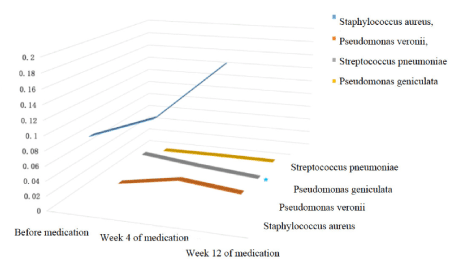
Figure 8. 3D variation trend chart of the top 4 strains in terms of relative abundance at species level.
Diversity: Shannon index was used to measure the diversity of the microbiota in the nasal cavity before and after treatment. As shown in Figure 9, no statistical difference was observed in the multiple comparisons among these 3 groups.
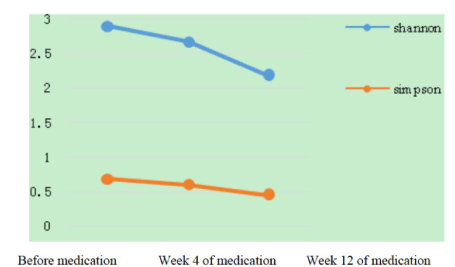
Figure 9. Diversity index variation trend chart.
There are many studies on the long-term low-dose medication of macrolides for treatment of CRS, with an overall effective rate of 60% -80% [16]. However, there are relatively few literatures analyzing the curative effects of macrolide drugs in RCRS patients. According to the results of a self-controlled study on 13 post-operative patients with RCRS by Xu Geng et al. [17], the decreases in the scores of subjective symptoms and endoscopic scores after oral treatment with clarithromycin 250mg/d, for continuous 12-28 weeks had statistical significance. According to the results of a prospective open-label study on 17 post-operative patients with RCRS by Cervin et al. [18], the decreases in the scores of nasal congestions, rhinorrhea and viscous nasal discharge among subjective symptoms and the decreases in endoscopic score after 12 weeks of oral treatment with clarithromycin 250mg/d or erythromycin ethyl succinate 250mg bid for continuous 12 weeks had statistical significance. However, in these two studies, eosinophils and/or IgE levels before treatment were not evaluated, and dynamic evaluation of efficacy and safety at different time points during treatment was lacking.
Liu Zheng et al. [19] reported a randomized controlled trial in 43 cases for dynamic observation of the clinical efficacy of macrolide drugs. According to the results, compared with baseline, after 4 weeks of oral administration of clarithromycin, symptoms including nasal congestion, rhinorrhea and general discomfort relieved, and endoscopic scores including rhinedema in the surgical cavity and rhinorrhea improved. After continuous treatment till the 12th week, subjective symptoms and endoscopic scores showed further improvement. However, this study was conducted in CRS patients without nasal polyps. In the present study, 17 CRS patients with normal IgE and eosinophil level who responded poorly to ESS and standard post-operative therapies were treated with long-term low-dose clarithromycin and followed up for 12 weeks. The main symptoms in enrolled patients were nasal congestion, rhinorrhea, postnasal drip, hyposmia and rhinedema in the surgical cavity. It was reasonable to classify these patients as RCRS from clinical perspective. After 4 weeks of oral administration of clarithromycin, subjective symptoms including nasal congestion, rhinorrhea, postnasal drip and general discomfort significantly relieved. Endoscopic score improved in terms of general surgical cavity condition, rhinorrhea and rhinedema, while inflammatory signs like rhinedema and vesiculation of surgical cavity mucosa still not fully recovered. After continued administration of clarithromycin for 12 weeks, the subjective symptoms showed no further significant improvement, indicating symptoms stabilized after 4 weeks of treatment. Noteworthily, according to endoscopic observation, surgical cavity mucosa was better epithelized, and the endoscopic scores were significantly improved compared with baseline as well as 4 weeks after treatment. These findings suggested that the subjective symptoms severity in RCRS patients do not necessarily synchronize with mucosal inflammation and recovery status of nasal cavity. Also, in this case, although subjective symptoms had been improved significantly after 4 weeks of oral clarithromycin therapy, 12 weeks of treatment could achieve more significant and more stable therapeutic effect regarding to mucosal recovery and epithelialization extent.
It has been reported that biofilm formation could be found in 30-82% of CRS patients [20-21] and is one of the important reasons for delayed recovery from mucosal inflammation after ESS [22]. Increased abundancy of various bacteria strains including Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus, and Pseudomonas aeruginosa was reported to be related to the formation of bacterial biofilm [23-25]. Previous studies have shown that administration of clarithromycin may have inhibitory effect on biofilms formed of Streptococcus pneumoniae [26]. In this study, the proportion of Streptococcus pneumoniae in the nasal flora of RCRS patients decreased significantly after 12 weeks of clarithromycin administration(P<0.05), indicating that clarithromycin may promote the improvement of clinical symptoms and recovery of surgical cavity mucosa by regulating nasal cavity microbiota and inhibiting bacterial biofilm formation. The safety of the long-term oral administration of macrolides has always been controversial. The safety concern of macrolides in most studies mainly focused on gastrointestinal reactions, allergic reactions and liver function impairment, while there are relatively few evidence about its effect on the diversity of nasal flora. The results of this study showed that there was no significant change in the richness and diversity of nasal flora after long-term low-dose oral administration of clarithromycin for up to 12 weeks in the post-operative RCRS patients, suggesting that long-term low-dose oral administration of clarithromycin has low risk to cause nasal flora imbalance. It is worth noting that in this study, the nasal flora of the post-operative RCRS patients was mainly composed of Staphylococcus, Pseudomonas, Corynebacterium, Haemophilus, Delftia, Fusobacterium and Streptococcus at the genera level, and of Staphylococcus aureus, Pseudomonas veronii, Streptococcus pneumoniae and Pseudomonas geniculate at the species level, which is not fully consistent with previous literature [27]. The different composition may be related to various factors such as different races, differences inclusion/exclusion criteria, different antibiotic exposure frequency, et al. This also indicated the necessity to conduct similar studies in other races.
In summary, post-operative long-term low-dose oral administration of clarithromycin (250mg/d) in RCRS patients can significantly improve the clinical symptoms and promote mucosal epithelialization; the efficacy is more significant and more stable after 12 weeks of treatment, with good tolerability and safety. The efficacy of clarithromycin in RCRS patients may be related to its regulatory effect on nasal flora. Due to the small sample size and short observation time in this study, long-term studies with large sample size are needed to make solid conclusions.
- Shi JB, Fu QL, Zhang H, Cheng L, Wang YJ, et al. (2015) Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy 70: 533-539. [Crossref]
- Senior BA, Kennedy DW, Tanabodee J, Kroger H, Hassab M, et al. (1998) Long-term Results of Functional Endoscopic Sinus Surgery. Laryngoscope 108: 151-157. [Crossref]
- Ragab SM, Lund VJ, Scadding G, Saleh HA, Khalifa MA (2010) Impact of chronic rhinosinusitis therapy on quality of life: a prospective randomized controlled trial. Rhinology 48: 305-311. [Crossref]
- Muneif AS, Zuo KJ, Shi JB, Xu G (2010) Investigation of relevant factors on refractory chronic rhinosinusitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 45: 1003-1007. [Crossref]
- Wreesmann VB, Fokkens WJ, Knegt PP (2001) Refractory chronic sinusitis: Evaluation of symptom improvement after Denker's procedure. Otolaryngology Head Neck Surgery 125: 495-500. [Crossref]
- Bhattacharyya N (2006) Surgical treatment of chronic recurrent rhinosinusitis: a preliminary report. Laryngoscope 116: 1805-1808. [Crossref]
- Gosepath J, Pogodsky T, Mann WJ (2008) Characteristics of recurrent chronic rhinosinusitis after previous surgical therapy. Acta Otolaryngol 128: 778-784. [Crossref]
- Tamaoki J (2004) The Effects of Macrolides on Inflammatory Cells. Chest 125: 41S-51S. [Crossref]
- Khair OA, Devalia JL, Abdelaziz MM, Sapsford RJ, Davies RJ (1995) Effect of erythromycin on Haemophilus influenzae endotoxin-induced release of IL-6, IL-8 and sICAM-1 by cultured human bronchial epithelial cells. Eur Respir J 8: 1451-1457. [Crossref]
- Wozniak DJ, Keyser R (2004) Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa. Chest 125: 62S-69S. [Crossref]
- Amali A, Saedi B, Rahaviezabadi S, Ghazavi H, Hassanpoor N (2015) Long-term post-operative azithromycin in patients with chronic rhinosinusitis: A randomized clinical trial. Am J Rhinol Allergy 29: 421-424. [Crossref]
- Haxel BR, Clemens M, Karaiskaki N, Dippold U, Kettern L, et al. (2015) Controlled trial for long-term low-dose erythromycin after sinus surgery for chronic rhinosinusitis. Laryngoscope 125: 1048-1055. [Crossref]
- Human Microbiome Project Consortium (2012) Structure, function and diversity of the healthy human microbiome. Nature 486: 207-214. [Crossref]
- Abreu NA, Nagalingam NA, Song Y, Roediger FC, Pletcher SD, et al. (2012) Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Trans Med 4: 151ra124. [Crossref]
- Feazel LM, Robertson CE, Ramakrishnan VR, Frank DN (2012) Microbiome complexity and Staphylococcus aureus in chronic rhinosinusitis. Laryngoscope 122: 467-472. [Crossref]
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, et al. (2012) EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50: 1-12. [Crossref]
- Zuo K J, Xu G (2011) Macrolide therapy on nasal mucosal persistent refractory inflammation after endoscopic sinus surgery. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 46: 718-722. [Crossref]
- Cervin A, Kalm O, Sandkull P, Lindberg S (2002) One-year low-dose erythromycin treatment of persistent chronic sinusitis after sinus surgery: Clinical outcome and effects on mucociliary parameters and nasal nitric oxide. Otolaryngology Head Neck Surgery 126: 481-489. [Crossref]
- Zeng M, Long X B, Cui Y H, Liu Z (2011) Comparison of efficacy of mometasonefuroate versus clarithromycin in the treatment of chronic rhinosinusitis without nasal polyps in Chinese adults. Am J Rhinol Allergy 25: 203-207. [Crossref]
- Psaltis AJ, Ha KR, Beule AG, Tan LW, Wormald PJ (2007) Confocal Scanning Laser Microscopy Evidence of Biofilms in Patients with Chronic Rhinosinusitis. Laryngoscope 117: 1302-1306. [Crossref]
- Healy DY, Leid JG, Sanderson AR, Hunsaker DH (2008) Biofilms with fungi in chronic rhinosinusitis. Otolaryngol Head Neck Surg 138: 641-647. [Crossref]
- Psaltis AJ, Weitzel EK, Ha KR, Wormald PJ (2008) The effect of bacterial biofilms on post-sinus surgical outcomes. Am J Rhinol 22: 1-6. [Crossref]
- Sanderson AR, Leid JG, Hunsaker D (2006) Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis. Laryngoscope 116: 1121-1126. [Crossref]
- Zhang Z, Adappa ND, Chiu AG, Doghramji LJ, Cohen NA, et al. (2015) Biofilm-forming bacteria and quality of life improvement after sinus surgery. Int Forum Allergy Rhinol 5: 643-649. [Crossref]
- Harvey RJ, Lund V (2007) Biofilms and chronic rhinosinusitis: systemic review of evidence current concepts and directions for research. Rhinology 45: 3-13. [Crossref]
- Takeda H, Oogaki N, Kikuchi N, Kobayashi H, Akashi T (1992) A study to clarify the mechanism of the usefulness of the macrolides--the influence of clarithromycin to biofilm with P. aeruginosa. Kansenshogaku Zasshi 66: 1454-1461. [Crossref]
- Wagner MB, Waite DW, Hoggard M, Douglas RG, Taylor MW, et al. (2017) Bacterial community collapse: a meta-analysis of the sinonasal microbiota in chronic rhinosinusitis. Environ Microbiol 19: 381-392. [Crossref]
Editorial Information
Editor-in-Chief
Tomoko Hasunuma
Kitasato University School of Medicine
Article Type
Research Article
Publication history
Received: March 09, 2021
Accepted: March 19, 2021
Published: March 30, 2021
Copyright
©2021 Han C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Han C, Bing Z, Qian H, Cheng L, Yubin W, et al. (2021) Efficacy and Safety of long-term low-dose clarithromycin in patients with refractory chronic sinusitis after endoscopic sinus surgery: A prospective clinical trial. J Clin Invest Stud. 4. DOI: 10.15761/JCIS.1000126

Figure 1. VAS scores box chart.

Figure 2. VAS scores 3D variation trend chart.

Figure 3. Endoscopic scores box chart.

Figure 4. Endoscopic scores 3D variation trend chart.

Figure 5. Comparison of endoscopy before and after treatment a: Before treatment, the mucosa in the surgical cavity showed obvious rhinedema, vesiculation, with a large volume of viscous secretions; b: In the 4th week of medication, rhinedema of mucosa in the surgical cavity was obviously mitigated, with small volume of viscous secretions; c: In the 12th week of medication, the mucosal epithelialization in the surgical cavity was good, the sinus ostia were unobstructed and no obvious secretion was observed.

Figure 6. Comparison of endoscopy before and after treatment a: Before treatment, the mucosa in the surgical cavity showed obvious rhinedema, vesicle and granulation, with a large volume of viscous secretions; b: In the 4th week of treatment, the mucosa in the surgical cavity was still edematous with a large volume of viscous secretions; c: In the 12th week of treatment, the mucosal epithelialization in the surgical cavity was good, and the sinus ostia were unobstructed and no obvious secretion and neoplasm were observed.

Figure 7. 3D variation trend chart of the top 7 generas in terms of relative abundance at genera level.

Figure 8. 3D variation trend chart of the top 4 strains in terms of relative abundance at species level.

Figure 9. Diversity index variation trend chart.
Table 1. Basic characteristics of subjects.
Basic characteristics |
Result (N=17) |
Age (year) |
|
Median age |
42 |
Quartiles |
35,56 |
Male/Female |
12/5 |
EOS% |
2.62%±1.55% |
Smoker (%) |
6 (35.3%) |
Concomitant asthma |
0 |
Average operations |
2 |
Interval between last surgery and the first medication of Clarithromycin (Month) |
2.45 |









