Abstract
Recent advances in understanding the molecular pathology which underlies age associated loss of substantia nigra dopamine neurons in Parkinson's disease (PD), indicates impaired energy metabolism, oxidative/nitrosative stress, protein misfolding, impaired gene transcription, circadian rhythm dysfunction, inflammation and calcium stress resulting in complex dysregulation of physiology, and producing the motor and non-motor dysfunction seen in the clinic [1,2].
A wealth of preclinical studies show that progression of these events is potentially sensitive to the activity of some key physiological agents [2,3,4,5,6].
This brief report summarises observations in a small group of 5 individual case studies in which conventional treatments were in each case supplemented with 5 nutritional agents; R-lipoic acid, acetyl-L-carnitine, coenzyme Q10/ubiquinol, melatonin (circadin), and vitamin D3, each targeting relevant factors of the underlying disease process, specifically in the age-related form 'sporadic' PD (sPD), as outlined previously [2,3].
Volunteers from the local PD society (age 59-71) with a history of not more than 5 years of symptoms, were followed for 2 years and their symptoms and signs rated with the UPDRS (II and III) protocol. A simple subjective self-rating scale also rated subjects' personal overall assessment of progress as better (B) or worse (W) relative to state at baseline. In addition to continuation of conventional therapy, all subjects were instructed in suitable dose and timing for the 5 agents listed. Special attention was given to protocol details for optimal dose and timing of an intermittent schedule of nocturnal melatonin intake. (Regional regulatory variations concerning melatonin may be met by employing melatonin receptor agonists e.g. ramelteon (USA), agomelatine (EU), circadin(UK) in future trials). Informed consent was given for all supplements subject to agreement from the neurologist in charge (Figure 1).
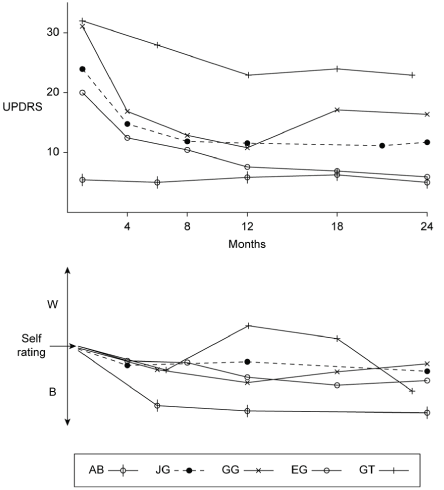
Figure 1. All supplements subject to agreement from the neurologist in charge
UPDRS scores at each interview and self ratings are indicated below. Total UPDRS at baseline for all 5 subjects combined was 112 as compared to a score of 74 at the final interview after 24 months of supplemental intake, suggesting an improved group outcome. Self rating of subjective impressions for the group as a whole followed this trend towards improvement.
Although group trend results pointed towards supplement efficacy, there was a range of outcomes in individual cases.
Subjects who clearly showed core sPD clinical features; bradykinesia, resting tremor, rigidity, response to L-dopa ( GG;JG;EG ) also showed an age related correlation with scores at both baseline and outcomes at 24 months ( 70;65;63 respectively ); a finding in line with statistical age of onset dependence [1]. These 3 subjects responded well to the 5 agents directed against age dependent dysfunctions [2,3]. This suggests that supplements could be deployed early in sPD with the aim of perhaps delaying and reducing requirements for L-dopa therapy.
With respect to age, however, one subject (GT) showed atypical results for sPD, with the highest UPDRS score and youngest age (59). Furthermore, although initially given a diagnosis of PD, there was a poor response to conventional therapy, an atypical tremor, postural instability, freezing and prominent autonomic symptoms. After further tests, diagnosis was reassigned as multiple system atrophy (MSA; with both parkinsonism and cerebellar features). In this case a series of falling episodes occurred as noted in the subjective records, but these were successfully controlled with a combination of high dose CoQ10/ubiquinol (600-900mg/d) and choline (250-500mg/d). These interventions followed evidence that MSA is associated with CoQ10 deficiency in plasma and cerebellum [7,8,9] and a report that motor disability scores in MSA correlate with loss of marker for cholinergic terminals of ponto-thalamic neurons of the CH5-6 cell group [10]. Significantly, CH5 neurons make direct excitatory synaptic contact with dopaminergic neurons of nigrostriatal pathway [11] and in cases of PD about 50% of CH5 neurons are lost [12]. Taken together, this data suggests a loss of cholinergic function in both PD and MSA from CH5.
In case GT, the marked effect of the extra interventions was described as “vast improvements”. These persisted and were associated with better sleep reports.
The remaining atypical case AB (age 71 at baseline) reported a minimal total score changing little after 24 months and with symptoms suggesting mild parkinsonism rather than sPD [1].
Taken together the evidence suggests that agents known to be effective in controlling age related dysfunctions [2,3] may be beneficial in restraining progression in early sPD, while in MSA additional choline and CoQ10 supplementation appears effective.
For future study, a greater focus on sleep functions in sPD is indicated as sleep disturbances are reported to precede the onset of motor symptoms [13]. Moreover in MSA, loss of brainstem cholinergic neurons CH5-6 appears more severe than that found in PD [14,12], and in MSA this correlates with severity of obstructive sleep apnea [15].
CDPcholine use in sPD has been found effective in moderating L-dopa dose requirements [16]. This form of choline (cytidine 5’-diphosphocholine; ‘citicoline’) may more likely incorporate into membrane phospholipids, whilst unconjugated choline, when taken up by the choline transporter at presynaptic cholinergic terminals, is a direct precursor to acetylcholine synthesis, and may thus instead help to restore losses in cholinergic neurotransmission in both MSA and sPD.
References
- Hindle JV (2010) Ageing, neurodegeneration and Parkinson’s disease. Age Ageing 39: 156-161. [Crossref]
- Phillipson OT (2017) Alpha-synuclein, epigenetics, mitochondria, metabolism, calcium traffic and circadian dysfunction in Parkinson’s disease. An integrated strategy for management. Ageing Research Reviews 40: 149-167. [Crossref]
- Phillipson OT (2013) Management of the aging risk factor for Parkinson’s disease. Neurobiology of Aging 35: 847-857. [Crossref]
- Mack JM, Schamne MG, Sampaio TB, Pertile RA, Fernandes PA, et al. (2016) Melatoninergic system in Parkinson’s disease: from neuroprotection to the management of motor and non-motor symptoms. Oxidative Medicine and Cell Longevity 3472032 (Epub 2016 Oct 18).
- Curry DW, Stutz B, Andrews ZB, Elsworth JD (2018) Targeting AMPK signaling as a neuroprotective strategy in Parkinson’s disease. Journal of Parkinson’s Disease 8: 161-181. [Crossref]
- Norwitz NG, Hu MT, Clarke K (2019) The mechanisms by which the ketone body D-beta-hydroxybutyrate may improve the multiple cellular pathologies of Parkinson’s disease. Frontiers in Nutrition 6: 1-8. [Crossref]
- Barca E, Kleiner G, Tang G, Ziozi M, Tadesse S, et al. (2016) Decreased coenzyme Q10 levels in multiple system atrophy cerebellum. Journal of Neuropathology and Experimental Neurology 75: 663-672. [Crossref]
- Mitsui J, Matsukawa T, Yasuda T, Ishiura H, Tsuji S, et al. (2016) Plasma coenzyme Q10 levels in patients with multiple system atrophy. JAMA Neurology 73: 977-980. [Crossref]
- Kasai T, Tokuda T, Ohmichi T, Ushii R, Tatebe H, et al. (2016) Serum levels of coenzyme Q10 in patients with multiple system atrophy. PloS One 11: e0147574.
- Mazere J, Meissner WG, Sibon I, Lamare F, Tison F, et al. (2013) [(123)I]-IBVM SPECT imaging of cholinergic systems in multiple system atrophy: A specific alteration of the ponto-thalamic cholinergic pathways (Ch5-Ch6). Neuroimage Clinical 3: 212-217. [Crossref]
- Bolam JP, Francis CM, Henderson Z (1991) Cholinergic input to dopaminergic neurons in the substantia nigra: a double immunocytochemical study. Neuroscience 41: 483-494.
- Pahapill PA, Lozano AM (2000) The pedunculopontine nucleus and Parkinson’s disease. Brain 123: 1767-1783. [Crossref]
- Srinivasan V, Cardinali DP, Srinivasan US, Kaur C, et al. (2011) Therapeutic potential of melatonin and it’s analogs in Parkinson’s disease: focus on sleep and neuroprotection. Therapeutic Advances in Neurological Disorders 4: 297-317. [Crossref]
- Schmeichel AM, Buchhalter LC, Low PA, Parisi JE, Boeve BW, et al. (2008) Mesopontine cholinergic neuron involvement in Lewy body dementia and multiple system atrophy. Neurology 70: 368-373.
- Gilman S, Chervin RD, Koeppe RA, Consens FB, Little R, et al. (2003) Obstructive sleep apnea is related to a thalamic cholinergic deficit in MSA. Neurology 61: 35-39. [Crossref]
- Que D-LS, Jamora RDG (2021) Citicoline as adjuvant therapy in Parkinson’s disease: a systematic review. Journal of Clinical Therapeutics 43: e19-e31. [Crossref]

