Housing of laboratory animals has changed dramatically in the last 20 years; however, the effect of modern rodent housing on behavior has not been extensively evaluated and published findings are conflicting. In the present study, aged (19 mo) Sprague-Dawley rats were single-housed in either suspended wire-mesh cages or suspended plastic cages for two months. Thereafter, rats completed a battery of balance, coordination, and strength tests, including walking on a horizontal rod, planks of varying widths, and rotarod as well as clinging to an inclined screen and horizontal wire. Rats also completed a working memory task in the water maze. Following introduction to the rodent housing, plastic cage-housed rats initially lost body weight but returned to baseline when water was made available through an additional route. Although no pathologies were observed in the rats’ extremities, analysis of behavioral data showed that plastic-housed rats balanced significantly longer on medium and wide planks and were able to cling longer to an inclined screen. No housing effects were observed in results from the water maze. These findings support concerns that modernization of rodent housing may impact measures of both motor and ingestive behavior.
rodent housing, wire cage, behavior, rat
For researchers employing rodent models, the type of housing used in their experiments is often dictated by their animal facility and is thus given little thought. Only a decade ago, studies involving rats frequently employed suspended wire-mesh cages (wire) [1]. Over the past ten years, rodent housing has transitioned from wire caging to solid-bottom caging (plastic) to individual ventilated caging (IVC) systems. However, the effects of housing on rodent behavior have infrequently been reported and are poorly characterized. The wide variety of housing systems currently in use presents a challenge to the interpretation of reported findings.
The Guide for the Care and Use of Laboratory Animals noted that “Rodents are often housed on wire flooring [2]. However, some evidence suggests that solid-bottom caging, with bedding, is preferred by rodents. Solid-bottom caging, with bedding, is therefore recommended for rodents” while the Guide for the Care and Use of Laboratory Animals considers most traditional caging but comments that: “animals should be provided with adequate bedding substrate and/or structures for resting and sleeping” [3]. While these recommendations have provided the impetus for a shift, not only from wire-mesh cages to plastic caging but also for the need for a substrate to enable resting and thermoregulation, there exists few objective reports regarding the superiority of these accommodations to caging over wire-mesh predecessors.
A few studies have examined rats’ preference for housing conditions by allowing access to both housing types; however, the results are inconsistent. In one study, rats spent most of their time (88%) in solid bottom plastic caging during periods of inactivity, but only slightly more time during periods of activity (55%), when both solid-bottom and wire housing were available [4]. Furthermore, when the two caging areas were separated by a weighted guillotine gate, rats were willing to work to obtain access to plastic caging on which to rest, irrespective of their previous housing condition [5]. However, in another study, rats preferred wire cages when allowed ad libitum access to both environments [6].
While rats display varied preferences when given their choice of housing, stress may result when one housing type is imposed. Among rats reared in wire cages, plasma corticosterone levels were increased relative to rats reared on bedding [7]. Eriksson and colleagues also reported signs suggestive of stress (e.g. decreased weight gain and increased defecation frequency) when rats housed in plastic caging were switched to wire metabolic cages, although no changes in fecal corticosterone levels were observed [8]. In a more recent study, rats that were housed in wire cages had similar baseline levels of corticosterone to those housed in solid plastic cages [9]. However, increased corticosterone levels were observed in wire-housed rats, relative to plastic-housed controls, after 1 h of restraint stress. Furthermore, these increased levels were still present 48 h after rats were returned to their home cages. While not observed in all studies, these findings suggest that choice of housing condition can increase baseline levels of stress and potentiate stress-responses in rats [10].
There exist concerns regarding the potential for injury to rats when housed on wire caging, specifically lesioning of the hind paws and/or negative impact on the peripheral nervous system [3,11,12]. When rats were housed in either wire cages or plastic caging for four weeks, no lesions or other pathological differences were observed [13]. However, Peace et al. found that rats, housed for two years in wire cages, were more likely to develop hind-paw plantar abnormalities after one year of age – particularly among heavier animals [14]. Notably, neither cage type was found to impact technicians’ ability to identify signs of ill health among rats [15].
Ultimately, housing conditions can affect rodent behavior. When telemetric sensors were used to monitor vital signs, rats housed in wire cages for 16 h displayed behavioral and physiological changes consistent with stress, including decreased locomotor activity, increased heart rate during dark phase, and decreased body weights [16]. Rock et al. found that rats housed in wire cages showed higher levels of food intake, as well as heightened levels of dark-cycle activity, relative to those housed in plastic caging [17]. Rats housed in plastic caging less frequently displayed successive negative contrast effects while consuming sucrose water [18]. However, no differences were observed in the rate of acquisition of an operant task among young (4 mo) rats housed in either wire cages or plastic caging [9]. These findings demonstrate how housing conditions may independently impact different aspects of behavior, particularly activity and ingestive behavior.
To further explore the effect of housing type on rodent behavior, rats in the present study were housed in either wire cages or plastic cages for eight weeks. Aged rats were used because they may be the most sensitive to negative effects of housing condition and the wire-housed rats formed a negative control group for a separate, ongoing study. Following exposure to either wire or plastic housing conditions, rats were tested on a variety of behavioral tasks including both motor and cognitive components. It was hypothesized that housing condition would mediate motor and cognitive behavior.
Animals
Thirty male Fischer-344 rats (19 mo) were obtained from the National Institute on Aging (NIA; Harlan Laboratories, Barrier 217, Indianapolis, IN) where they were group housed (3/cage) in solid plastic caging with Tek-Fresh bedding (Harlan #T.7099) with ad lib access to NIH-31/NIA diet (NIA). Rats underwent an 18 d habituation period, spent in solid plastic cage housing, upon arrival at the testing facility. Rats were then weight-matched and randomly assigned to one of two housing conditions (n=15): wire cage housing or solid plastic cage housing. Wire cages were changed every other week, but liners were changed 3/week while plastic cages and their bedding were changed 2/week; however, experimenters handled rats extensively prior to behavioral testing. Rats were fed a modified NIH-31 diet (Harlen/Teklad, Madison, WI), maintained on a 12-hour light/dark cycle, weighed at multiple time points, and health monitored daily [19]. Five rats had age-related pathologies and were removed from the study; sample size was twelve (wire-housed) and thirteen (plastic-housed) at the time of testing (8th-10th weeks). Rats were euthanized at the beginning of the 11th week following group assignment. All procedures, including an exemption from the Guide for housing conditions, were approved by the Institutional Animal Care and Use Committee at the Human Nutrition Research Center on Aging (HNRCA) at Tufts University.
Housing
Rats were housed in either wire or solid plastic caging. Rats maintained in the wire cages were individually housed in suspended stainless-steel cages (18 cm H x 18 cm W x 25 cm D) (Figure 1A). The cages’ front and floor were constructed from welded stainless-steel wire mesh (1.1 cm spacing) while the sides and back floor were constructed from 22-guage type 304 stainless steel plates. Rats received water via automatic watering provided by a drinking sipper that extended 2 cm into the cage at a height of 9 cm from the cage floor. Rats had ad libitum access to food pellets in an external hopper located 1 cm from the cage floor. Wire caging was changed every other week and cage liners were changed 3/week.

Figure 1. Rodent Housing Images of (A) wire-mesh cage and (B) plastic tub housing used in the present study Also shown are the racks used to house and hydrate the rodent housing
Rats in the solid bottom plastic caging were individually housed in suspended solid bottom clear polycarbonate cages (20 cm H x 17.75 cm W x 23.5 cm D) (Figure 1B). Plastic cages were filled with TEK-Fresh laboratory animal bedding (Harlan Teklad, Madison, WI) to a height of 3 cm. Rats received water via a drinking sipper, 1 cm outside the cage, which rats accessed via a 2 cm aperture in the rear of the cage and located at a height of 9 cm from the cage floor. Rats had ad libitum access to food pellets in an internal hopper located 5 cm from the cage floor. Plastic caging was changed 2/week.
Motor tasks
Rats underwent a battery of age-sensitive psychomotor tasks at the end of seven weeks following group assignment, described in detail [20]. All motor tests were conducted during the light portion of the rats’ light/dark cycle. To test balance and coordination, rats were placed on horizontal planks of varying widths (13 mm, 25 mm, and 38 mm), as well as an 26 mm diameter horizontal rod, located 23 cm above a thick foam-core pad; latency to fall was recorded (max 60s). To assess forelimb strength, rats were allowed to grasp a suspended wire 55 cm above a thick foam-core pad; latency to fall was recorded. To assess overall limb strength and stamina, rats were placed on an inclined (60°) metal screen and latency to fall was recorded (max 600s). To assess fine motor coordination and stamina, rats were tested on an accelerating rotarod (Ugo Basile, Collegeville, PA), consisting of a slowly accelerating (+2 rpm / 30s; 20 rpm max), rotating 7 cm diameter dowel, and latency to fall was recorded (max 300s).
Cognitive tasks
Cognitive testing was conducted during the 9th and 10th weeks following group assignment, during the light portion of the rats’ light/dark cycle. Rats were tested in the Morris Water Maze using a working memory protocol in which the escape platform is relocated each session [20]. In short, rats completed two acquisition trials, separated by a ten minute intertrial interval, twice daily for each of four consecutive days (8 pairs of trials in total). During each trial, rats were placed into one of four, quasi-random, start locations. Rats then searched the pool until they either found the escape platform or swam for 120 seconds and were guided to the escape platform. Once on the platform, rats were allowed 15 seconds to observe their location before being dried with a towel and returned to their home cage. All trials were recorded and swim paths and latencies were analyzed using image tracking software (HVS Image, Hampton, UK).
Statistical analysis was conducted using SYSTAT (SPSS, Inc., Chicago, IL, USA). For each behavioral measure, data was subjected to a between-groups analysis of variance (ANOVA). Where appropriate, within-group factors were added to the statistical model. Significance was assessed at the 0.05 alpha level for all analyses.
After introduction to the housing environments, plastic-housed rats showed an initial decrease in body weight (Figure 2A). Analysis of variance showed a main effect of time point [F (6,138)=2.533, p=0.023] and a housing condition x time interaction [F(6,138)=2.361, p<0.033] despite statistically similar food intakes (21.04 g/d vs 20.84 g/d for wire-bottom and plastic, respectively). Some plastic-housed rats experienced weight loss during the first three weeks of the study; thereafter, water dishes were added to their cages and body weights slowly returned to parity. Daily health monitoring by vivarium staff revealed no lesions or other pathology related to rodents’ paws in either housing condition.
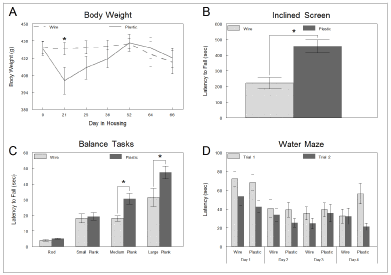
Figure 2. (A) Rats housed in wire cages-maintained body weight during the course of the study. Rats housed in plastic cages lost weight initially but returned to parity when in-cage water dishes were supplied. (B) Rats housed in plastic cages clung to an inclined screen significantly longer, and (C) balanced significantly longer on medium and large planks than those housed in wire cages. (D) On average, rats located a hidden platform more quickly during the second trial in each session when tested in a working memory version of the water maze, regardless of housing condition. Error bars represent standard error of the mean; the alpha level was set at 0.05 for all analyses (* indicates p<0.05)
Motor tasks
Fall latencies were assessed individually for all strength tests. When rats clung to an inclined screen, plastic-housed rats clung significantly longer [F(1,23)=17.668, p<0.001] relative to rats housed in wire cages (Figure 2B). No significant differences in forelimb strength were observed during wire suspension.
Fall latencies were assessed individually for all coordination and balance tests. When rats balanced on the medium or large planks, plastic-housed rats displayed significantly longer fall latencies [F(1,23)=9.871, p=0.005 and F(1,23)=5.244, p=0.032 respectively], relative to rats housed in wire cages (Figure 2C). However, no significant differences were observed when rats balanced on the accelerating rotarod, fixed horizontal rod, or small plank.
Cognition
Latency to locate a hidden platform in the MWM was averaged separately for the first and second trials across days. A 2 group (wire vs plastic) by 2 trial (1st trials vs 2nd trials) by 4 day (1-4) mixed model ANOVA revealed a main effect of day [F(3,69)=8.745, p<0.001], indicating that rats improved performance from day to day. It also revealed a main effect of trial [F(1,23)=18.644, p<0.001], indicating that all rats located the hidden platform faster during a subsequent trial (trial 2) at the same platform location (Figure 2D). Analysis of trial latencies failed to reveal a main effect of group or any significant interactions. Of note, in a separate analysis of day 4, a 2 time (morning vs afternoon) by 2 trial (1 vs 2) by two group (wire vs plastic) mixed model ANOVA revealed a main effect of time [F(1,23)=7.923, p=0.010] in which rats found the platform sooner in the morning, and a main effect of trial [F(1,23)=5.933, p<0.023], in which rats located the platform sooner during a subsequent trial (trial 2) at the same platform location. However, there was also a significant trial by group interaction [F(1,23)=5.861, p<0.024] in which plastic-housed rats took significantly longer to locate the hidden platform during the first trial (trial 1) during both time points (Figure 2D) than did wire-housed rats.
Distance travelled to locate the platform was also analyzed and, likewise, the ANOVA revealed a main effect of day [F(3,69)=4.183, p=0.009] and trial [F(1,23)=26.341, p<0.001] indicating that all rats improved performance from day to day and that they traveled a shorter distance to locate the platform during the second trial irrespective of housing condition (data not shown). Analysis of swim distances failed to reveal a main effect of group or any significant interactions. Just as with the latency measure, in a separate analysis of day 4, a 2 time (morning vs afternoon) by 2 trial (1 vs 2) by two group (wire vs plastic) mixed model ANOVA revealed a main effect of time [F(1,23)=8.716, p=0.007] in which rats travelled a shorter distance to reach the platform in the morning, and a main effect of trial [F(1,23)=8.094, p<0.009], in which rats travelled a shorter distance to reach the platform during a subsequent trial (trial 2) at the same platform location. However, there was also a significant trial by group interaction [F(1,23)=4.399, p<0.047] in which plastic-housed rats travelled farther to reach the platform during the first trial (trial 1) during both time points (data not shown) than did wire-housed rats.
This study reports three main effects of housing condition on laboratory rodents: 1) temporary fluctuations in body weight, following housing transition, which may have resulted from changes to water intake, 2) rats housed in plastic cages displayed increased cling-time on an inclined screen and 3) rats housed in plastic cages displayed increased balance-time on medium and large plank tests.
In the present study, rats housed in plastic caging showed initial decreases in body weight, likely due to reduced fluid intake. Both groups were provided with water supplied via rack-mounted automatic watering systems; however, the water sippers in the solid bottom cages do not protrude directly into the plastic cages and rats may have initially been less likely to notice or less able to reach them. Although water intake was not quantified during the study, the temporary decline in body weight was reversed when in-cage water dishes were supplied and, at the time of behavioral testing, no differences in body weight were observed. Housing has previously been reported to alter food intakes of rats but it seems likely that reduced water consumption was the cause of the initial weight loss observed in the present study [17].
Strength was assessed by allowing rats to cling to an inclined screen in which rats use all four limbs. Rats housed in plastic cages fell significantly later than those housed in wire cages, indicating that plastic cage-housed rats had increased skeletal muscle strength relative to wire-housed rats. While this result would usually be attributed to differences in overall skeletal muscle and/or grip strength, no differences were observed during the subsequent wire suspension task, suggesting that the forelimb muscle strength was not affected by housing condition but perhaps hind leg strength was. Although wire-housed rats’ hind paws could be more or less sensitized to walking on the wire-mesh, this result was still somewhat counterintuitive because rats housed in a wire cages could be expected to be more familiar with gripping and moving about on wire-mesh surfaces (i.e. the inclined screen).
Balance was assessed by placing rats on a variety of narrow balance surfaces. Rats housed in plastic cages fell significantly later than those housed in wire cages, when placed on the medium- or large-width planks, indicating increased ability to maintain balance. However, no differences in fall latency were observed on the rod and narrow-width plank. All rats displayed difficulty standing astride the narrower surfaces. As discussed previously, housing-induced alteration of the hind-limbs could lead to impairments in balance; however, this impairment may only be evident on tasks where the hind limbs maintain contact with the balance apparatus.
One variable differentiating the two housing conditions of the current study was the necessary inclusion of paper pulp bedding in the plastic caging condition. The inclusion of nesting or bedding materials to rodent housing may constitute a form of environmental enrichment by providing an aspect of their environment that is malleable while increasing the tactile complexity of the environment [21]. Both motor and cognitive ability can be improved through environmental enrichment, even when initiated late in life [22]. Rats reared in complex environments display enhanced spatial learning and memory in the water maze [23]. In the present study, both groups demonstrated comparable learning and plastic cage-housed rats performed no better in the water maze than those on the wire cages. Therefore, the inclusion of bedding materials, while marginally enriching the environment, was insufficient to produce measurable cognitive enhancement in our study.
The rats in the present study also served as controls for a larger study on the effects of dietary raspberry on mobility and cognition of aged rats. Rats in that study underwent the same procedures described here but were fed a diet that also included raspberry. Despite the enhanced motor performance of plastic cage-housed rats presented here, motor ability was significantly improved by raspberry only among rats housed in wire cages (data not shown). This additional observation highlights a concern of many researchers faced with replacing their existing rodent housing systems – that the behavior of their control animals may be altered, thus obscuring experimental effects or hindering replication of prior findings [24]. Given the modulatory effect of housing on ingestive behavior shown here, changes in rodent housing should be considered when planning experiments which manipulate nutritional variables. Also, given the potentially deleterious effect of housing on hind-limb mobility, rodent housing should be carefully considered in aging research where this effect may be maximized by long-term exposure to housing surfaces during studies employing longitudinal and lifespan paradigms.
Because rats show an inconsistent preference for both wire and solid bottom housing conditions, a compromise adopted by some institutional animal care and use committees (IACUCs) is the addition of a piece of solid flooring or a raised platform to wire mesh cages [24-26]. The addition of a platform provides a solid surface, on which animals can rest, laying atop or elevated above the wire-mesh floor of the cage. While it has been reported that rats will move off of a solid platform to urinate, we have observed that Plexiglas platforms retain liquids and standing atop a wet platform increases the incidence of hind paw lesions [24]. The additional flooring surface is utilized by rats but, to date, no studies have been conducted investigating their ability to mitigate the effects of long-term exposure to the flooring in the remainder of the cage.
In conclusions, the effects of laboratory housing on rodents are often overlooked or ignored in behavioral research. Following the recommendations published in the Guide for the Care and Use of Laboratory Animals, many research facilities have abandoned wire cages in favor of the use of plastic, solid bottom caging [2,3]. Pressure to update laboratory housing has met with resistance from some researchers who cite the unforeseen impact on the consistency of their data. Given the present findings, additional scrutiny should be given to the effect of rodents housing conditions utilized in behavioral studies.
This research was funded by the United States Department of Agriculture and the Washington Red Raspberry Commission. The authors declare no competing financial interests.
- Stark DM (2001) Wire-bottom versus solid-bottom rodent caging issues important to scientists and laboratory animal science specialists. Contemp Top Lab Anim Sci 40: 11-14. [Crossref]
- ILAR (1996) Guide for the care and use of laboratory animals: National Academies.
- ILAR (2010) Guide for the care and use of laboratory animals (8 ed.): National Academies. [Crossref]
- Manser C, Morris T, Broom D (1995) An investigation into the effects of solid or grid cage flooring on the welfare of laboratory rats. Lab Anim 29: 353-363.
- Manser C, Elliott H, Morris T, Broom D (1996) The use of a novel operant test to determine the strength of preference for flooring in laboratory rats. Lab Anim 30: 1-6. [Crossref]
- Sherer A, Rigel D, Iversan W (2001) Exploratory study to determine the preference of the Sprague-Dawley rat for a solid or a wire cage floor. Paper presented at the Abstract. ACLAM Forum, Mobile, Ala.
- Heidbreder C, Weiss I, Domeney A, Pryce C, Homberg J, et al. (2000) Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 100: 749-768. [Crossref]
- Eriksson E, Royo F, Lyberg K, Carlsson HE, Hau J (2004) Effect of metabolic cage housing on immunoglobulin A and corticosterone excretion in faeces and urine of young male rats. Exp Physiol 89: 427-433. [Crossref]
- Freed C, Martinez , Sarter M, DeVries C, Bergdall V (2008) Operant task performance and corticosterone concentrations in rats housed directly on bedding and on wire. J Am Assoc Lab Anim Sci 47: 18-22.[Crossref]
- Holson R, Scallet A, Ali S, Turner B (1991) “Isolation stress” revisited: Isolation-rearing effects depend on animal care methods. Physiol Behav 49: 1107-1118.
- Grover-Johnson N, Spencer PS (1981) Peripheral nerve abnormalities in aging rats. J Neuropathol Experi Neurol 40: 155-165.
- Ortman JA, Sahenk Z, Mendell JR (1983) The experimental production of Renaut bodies. J Neurol Sci 62: 233-241.
- Sauer MB, Dulac H, Clark S, Moffitt KM, Price J, et al. (2006) Clinical pathology laboratory values of rats housed in wire-bottom cages compared with those of rats housed in solid-bottom cages. J Am Assoc Lab Anim Sci 45: 30-35. [Crossref]
- Peace TA, Singer AW, Niemuth NA, Shaw ME (2001) Effects of caging type and animal source on the development of foot lesions in Sprague Dawley rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 40: 17-21. [Crossref]
- Van Vleet TR, Rhodes JW, Waites CR, Schilling BE, Nelson DR, et al. (2008) Comparison of technicians' ability to detect clinical signs in rats housed in wire-bottom versus solid-bottom cages with bedding. J Am Assoc Lab Anim Sci 47: 71-75. [Crossref]
- Giral M, García-Olmo DC, Kramer K (2011) Effects of wire-bottom caging on heart rate, activity and body temperature in telemetry-implanted rats. Lab Anim 45: 247-253. [Crossref]
- Rock FM, Landi MS, Hughes HC, Gagnon RC (1997) Effects of caging type and group size on selected physiologic variables in rats. J Am Assoc Lab Anim Sci 36: 69-72. [Crossref]
- Wood M, Daniel AM, Daniels E, Papini MR (2006) Effects of housing on consummatory successive negative contrast in rats: Wire-bottom cages versus polycarbonate tubs. Lab animal 35: 34-38. [Crossref]
- Youdim K, Shukitt-Hale B, Martin A, Wang H, Denisova N, et al. (2000) Short-term dietary supplementation of blueberry polyphenolics: Beneficial effects on aging brain performance and peripheral tissue function. Nutr Neurosci 3: 383-397.
- Shukitt-Hale B, Mouzakis G, Joseph JA (1998) Psychomotor and spatial memory performance in aging male Fischer 344 rats. Experi Geron 33: 615-624.
- Gonder JC, Laber K (2007) A renewed look at laboratory rodent housing and management. Ilar J 48: 29-36. [Crossref]
- Fernandez C, Collazo J, Bauza Y, Castellanos M, Lopez O (2004) Environmental enrichment‐behavior‐oxidative stress interactions in the aged rat: Issues for a therapeutic approach in human aging. Ann N Y Acad Sci 1019: 53-57. [Crossref]
- Tees RC, Buhrmann K, Hanley J (1990) The effect of early experience on water maze spatial learning and memory in rats. Dev Psychob 23: 427-439. [Crossref]
- Anonymous (2000) Wire-Bottom caging for rodents: Striking a balance between animal well-being and sound science. Connec Summer 1: 6-9.
- Benevenga N, Kaiser M, Clagett-Dame M (2005) Development of the rat loft. Tech Talk 10: 17-21.
- www.nia.nih.gov/research/dab/aged-rodent-colonies-handbook/barrier-environmental-information.


