Abstract
Objective: The use of essential oil of lavender has demonstrated positive results in different clinical areas, suggesting efficacy in reducing anxiety, stress and the entire emotional complex linked to compulsions and motivations. This research has the main objective to evaluate the acute effects of the inhaled administration of lavender in adult smokers with the withdrawal syndrome.
Methods: Sixty smokers who voluntarily assigned to Tobacco Treatment Program of Campina Grande-PB were assigned into three groups of twenty participants: Lavender Group, Nicotine Group and Placebo Group. Lavender Group held inhalation of lavender essential oil once through a surgical mask for a period of five minutes. The same procedure was adopted for the Placebo Group; however, it was used distilled water. The Nicotine Group received conventional treatment of transdermal nicotine replacement.
Results: The lavender essential oil showed significant reduction in anxiety, craving for tobacco, systolic blood pressure and heart rate when compared to placebo. Experimental substances showed similar efficacy to the conventional treatment of the tobacco referred to the control of anxiety and craving.
Conclusion: These findings suggest that lavender, for its anxiolytic effects, could be an alternative to treat craving.
key words
anxiety disorder generalized, community mental health, group therapy, tests/interviews, psychometric, psychoactive substance use disorder
Introduction
The craving for tobacco has been defined as an intense or compulsive desire for the consumption of cigarettes [1]. It is a relevant concept in the area of addictions because it is the main characteristic of dependence and is generally responsible for the processes of drug search and relapse [2]. In a study carried out by Zeni and Araújo [3], it was evidenced that there is a positive correlation of moderate intensity between the symptoms of anxiety and craving of tobacco, so that the increase in anxiety symptoms is associated with the increase in craving and vice-versa. In addition, the studies conducted by Cox et al. [4] suggest that the high level of craving and anxiety symptoms is the factor that most hinders the process of smoking abstinence. Thus, it is important to study strategies to control these variables.
The pharmacological treatment of smoking is used to reduce the discomfort of the symptoms of the abstinence. The first-line pharmacotherapy is usually Nicotine Replacement Therapy or by Bupropion, followed by nicotinic receptor blockers and biochemical mediator reuptake inhibitors [5]. However, the pharmacological therapy of smoking has the disadvantages of being expensive, presenting high rates of adverse effects, while not proving successful for all its users [6]. Lavender essential oil has been considered an alternative tool for the treatment of anxiety, stress and the whole emotional complex linked to compulsions and motivations. This compound possesses powerful calming and sedative effects, converting them into popular aromatherapy practices as a complementary therapy for various diseases [7]. For instance, the study conducted by Karaman et al. [8] evaluated the effects of lavender essential oil (1%), by inhalation in patients during a surgical procedure. The Analog Visual Scale was used to assess anxiety, and the results indicated that there was a reduction of anxiety in patients treated with lavender essential oil compared with the control group that was submitted to the same procedure with a placebo substance. Moreover, the study carried out by Braden et al. [9] showed anxiolytic activity of lavender, evaluated by self-report questionnaires, by inhaling a drop of pure oil (undiluted) on a cotton ball, in 150 patients who underwent surgery. However, Perry et al. [10]. evaluated clinical trials conducted with the administration of lavender in different ways (oral, inhaler, massage, etc.) and different concentrations and found no consensus about the effects of lavender on the reduction of anxiety and stress, so the authors assume that the lack of stringency in conducting the trials and the differences in concentration of the essential oil could have been the cause of these discrepancies.
Despite the diversity of research carried out with the essential oil of lavender described in the scientific literature, there is evidence of a lack of standardization of the administration methods. This fact showed by Lee et al. [11] the reason that compromise the effectiveness of the adopted approaches, therefore it is necessary to investigate more about the mechanisms of lavender actions and the adequate conditions of administration of this substance, using more rigorous methodological approaches. So, this randomized controlled experimental design aims to evaluate the acute effects of the inhaled administration of lavender in adult smokers with the withdrawal syndrome.
Experimental procedures
Setting and sample
A prospective, randomized, single-blind, controlled experimental pretest-posttest design was performed in smokers. A convenience sample of 42 women and 18 men with a mean age of 48 years (± 11.26) who voluntarily seek for the Tobacco Treatment Program of Campina Grande-PB, Brazil was selected. The inclusion criteria were: a) be a participant of a smoking treatment group of the Basic Units of Familiar Health (BUFH's) of Campina Grande; b) be over 18 years old; c) attend the all meetings of the Treatment Group; d) Do not use depressants drugs of the central nervous system; Exclusion criteria were: a) be haemophiliac or make use of anticoagulant substances; b) be hypotensive; c) have problems in the upper airway. The information about the inclusion and exclusion criteria was obtained by analysing the files of each patient available in the BUFH. Patients were randomly assigned to one of three groups: 20 tobacco users with the withdrawal syndrome who used the essential oil of lavender through inhalation (surgical mask), therefore, called Lavender Group (LG); 20 tobacco users with the withdrawal syndrome that used a placebo substance by inhalation, called Placebo Group (PG); 20 tobacco users, who used the conventional treatment of transdermal nicotine, thus Nicotine Group (NG). All participants provided written informed consent prior to be included in the study.
Instruments
The degree of nicotine dependence was assessed using the Fagerstrom Test for Nicotine Dependence – FTND [12]. To assess the level of craving generated by cigarettes, we administered the Brazilian version of the Questionnaire of Smoking Urges-Brief (QSU-B) originally proposed by Cox et al. [4] and translated into Portuguese by Araújo et al. [13].
To assess the level of anxiety, the State-Trait Anxiety Inventory (STAI) instrument developed by Spielbergert et al. [14] was used. This is a test consisting of two self-assessment questionnaires: the STAI-Trait (STAI-T), which defines the individual's trait anxiety, contrasting the tendency to react to situations identified as threatening, and the STAI-State (STAI -S), which identifies the state of anxiety to an anxious or anguish situation.
To further assess the level of anxiety was applied the Visual Analogue Mood Scale (VAMS), a self-assessment scale, originally proposed by Norris [15] which provides a more specific analysis of the subject mood, accounting the emotional feelings as a whole.
Measurement of the physiological parameters
The physiological parameters can reflect the clinical signs characteristic of the anxiety, which mainly include tachycardia, increase in blood pressure, hyperventilation, sweating and reduction on the temperature of body extremities [16]. Thus, the measurement of some parameters in combination with the psychometric instruments will corroborate the change in state of anxiety presented by subjects in this study. Respiratory Rate (RR), Heart Rate (HR), Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP) and Oxygen Blood Saturation (OBS) were measured. For such procedures, a portable pulse oximeter and a digital tensiometer was used.
The blood pressure measurements were recorded with the Pulse Automatic Digital Blood Pressure Device of TechLine Model Z-43. The measurement of heart rate and oxygen saturation in the blood was recorded with the Finger Pulse Oximeter Fingertip MBO-24 of Medical Rossmax Innortek Corp. The respiratory rate was obtained by counting the amount of hyperventilation during a 1-minute period, so that the patients were not aware of the recording in order not to induce the correct value.
Procedures
The experiment was developed in individual and group sessions. Brief explanations about the objectives of the study and what would be done (group section) were carried out before starting the experiment itself. Subsequently, the screening interview, the STAI-T, and the FTND were administered and the Free Informed Consent of the participants (individual section) was obtained.
The Smoking Treatment held in BUFHs was based upon that recommended by the Guidelines for Tobacco Cessation of the Brazilian Thoracic Society [17], which consists, initially, of 4 weekly informative group meetings for the approach of the contents of 4 specific guidebooks (understanding the approach on nicotine dependence) and then a group meeting every two weeks for follow-up, development of analysis and discussion of relevant strategies to smoking cessation.
For experimental and placebo groups, the pharmacological trial occurred in the second week of the informative meetings (mentioned above), which is the critical period of the symptoms of nicotine the withdrawal [1]. The participants were requested to answer STAI-S, VAMS and QSU-b questionnaires, together with the measurement of physiometric parameters (SBP, DBP, HR RR, and OBS) before and after the trial (except the Nicotine Group). Lavender Group made inhalation of 1 drop undiluted Lavandula angustifolia essential oil, while the Placebo Group did the inhalation of a placebo substance (distilled water), both only once, for a period of 5 minutes in a disposable surgery mask. The participants were informed about the trial as follows: “You will be submitted to the inhalation of a substance for a period of 5 minutes through a surgical mask, and then it will be necessary to answer some questionnaires and allow me to take some physiological measures.”
Participants of Nicotine Group only answered the psychometric instruments STAI-S, VAMS and QSU-b during the last informative meeting (4th guidebook), when the physiometric measures were also recorded to obtain pretest parameters. From this day on, the administration of 21 mg transdermal nicotine patch began. After four days, they returned to BUFH to collect the values of the STAI-S, VAMS, QSU-b and physiological parameters (SBP, DBP, HR, RR, and OBS), thereby constituting the posttest procedure for the aforementioned group. Four days of use of the transdermal nicotine patch were required before collecting the psychometric and physiological measures because this route of administration reaches stable levels only after a long period of time [18].
Statistical analyses
Statistical analyses were performed with GraphPad Prism (version 6.01, GraphPad Software Inc., San Diego, CA, USA). Hypothesis tests were defined according to whether the data are normally distributed or not, so the non-parametric methods were chosen for the instruments STAI-T, STAI-S, VAMS, FTND and QSU-b, while parametric tests were selected for the physiological parameters. It was accepted a 95% confidence level throughout the study.
The statistical coefficient of Cronbach's alpha for reliability of the data was for the STAI-T α = 0.816 at pretest, and for the other scales were: α = 0.852 and α = 0.887 for the VAMS; α = 0.847 and α = 0.887 for the STAI-S; α = 0.935 and α = 0.964 for the QSU-b, for pretest and posttest times respectively. These values represent high reliability, because all of them are higher than 0.8 and, thereby the scales are the reliable and items accurately and adequately measure the constructs [19].
The ANOVA test was adopted to compare the means of physiological parameters SBP, DBP, HR, RR, OBS between the groups at pretest and posttest, while for intragroup comparisons the Student test was used.
To compare the differences at pre and posttest between groups (Lavender Group, Placebo Group and Nicotine Group) in psychological parameters QSU-b, VAMS and STAI-S was used ANOVA of Kruskal-Wallis test. For the multiple comparison tests used the method of Dunn's multiple comparison test taking as a basis the Nicotine Group medians. For comparison intra groups at both times the paired t test of Wilcoxon was applied. To analyse the significant differences between Nicotine Group and Lavender Group the t test of Mann-Whitney was used.
Results
Sample sociodemographics and history of smoking characteristics
The average age was 48 (± 11.26) years old, the majority of participants were female (70%, n = 42) and more than half of the participants studied elementary school (65%, n = 39). The smoking background of research participants indicates that 81% (n = 49) consume less than 20 cigarettes per day, 63% (n = 38) started smoking before 15 years old and 72% (n = 44) of them have been smokers for the period between 21 and 50 years old. Regarding attempts to quit smoking, 38% (n = 23) have never tried to quit smoking and 48% (n = 29) were not successful when trying to quit smoking. Among the participants, 33% (n = 20) are searching treatment at least for the second time, while 40% (n = 24) of the sample have unsuccessfully used medications to quit smoking.
Dependence level assessment
The level of dependence for each of the groups was obtained using the Fagerstrom Test for Nicotine Dependence – FTND [20] (Table 1). At the beginning, ANOVA of Kruskal-Wallis was performed, between the groups, to check the sample homogeneity for the level of the dependence parameter, and it was observed that there were not significant differences between Lavender Group, Nicotine Group and Placebo Group (H = 4.712, p = 0.0948). As part of this, the multiple comparison tests based on the medians of Nicotine Group showed no differences. So, it can be concluded that the sample is homogeneous in the level of nicotine dependence.
Table 1. Rank by groups according to nicotine dependence obtained by FTND
|
Level of nicotine dependence |
|
Very low |
Low |
Moderate |
High |
Very high |
Total |
Group |
Lavender |
2 (10%) |
5 (25%) |
2 (10%) |
6 (30%) |
5 (25%) |
20 |
Placebo |
5 (25%) |
5 (25%) |
4 (20%) |
3 (15%) |
3 (15%) |
20 |
Nicotine |
4 (20%) |
7 (35%) |
2 (10%) |
7 (35%) |
0 (0%) |
20 |
Total |
11 (18%) |
17 (29%) |
8 (13%) |
16 (27%) |
8 (13%) |
60 |
Anxiety-trait assessment
Table 2 shows the levels of anxiety-trait obtained through the STAI-T for each of the groups. The classification was established according to Biaggio and Natalício [21] The means of the scores were for Lavender Group 49.5 (± 10.77), for Nicotine Group 48.2 (± 10.56) and for Placebo Group 48.4 (± 10.99). It can be observed similar distributions and moderate anxiety rating (according the rating of the mentioned author) for each group. This was confirmed by the Kruskal-Wallis ANOVA test, which demonstrated there were not significant differences between Lavender Group, Nicotine Group, Placebo Group (H = 0.7587, p = 0.8598), also, in the multiple comparison tests based on the medians of the Nicotine Group we observed homogeneity in the level of anxiety- trait.
Table 2. Rank by groups according to the anxiety score obtained by STAI-T
|
Anxiety Level |
|
Low |
Medium |
High |
Total |
Group |
Lavender |
6 (30%) |
12 (60%) |
2 (10%) |
20 |
Placebo |
6 (30%) |
12 (60%) |
2 (10%) |
20 |
Nicotine |
4 (20%) |
13 (65%) |
3 (15%) |
20 |
Total |
16 (27%) |
37 (62%) |
7 (11%) |
60 |
Physiological parameters assessment
In the intra-group comparisons at the pretest and posttest time, it was proved that there were only significant changes in the following parameters and groups: Systolic Blood Pressure, and Heart Rate (W = -133.00, p = 0.0007; W = -119.00, p = 0.0071 respectively) for Lavender Group, while the Nicotine Group was modified on Heart Rate (W = 105.00, p = 0.0329) and Respiratory Rate (W = -1.00, p = 0.0457) after the intervention. The Placebo group did not show statistically significant changes in any of these parameters (Table 3). Thus, when the intragroup values obtained before and after the intervention were compared, it was observed that the values of Systolic Blood Pressure and Heart Rate reduced for Lavender Group and, on the other hand, for the Nicotine Group, the Heart Rate parameter apparently increased and Respiratory Rate showed a reduction.
Table 3. Intergroup comparison of the means of physiological parameters using Student t test
|
|
Pretest |
Posttest |
p-value |
Parameters |
NG |
PG |
LG |
NG |
PG |
LG |
NG x NG |
PG x PG |
LG x LG |
SBP |
110.5
(±18.77) |
118 (±25.87) |
118 (±16.42) |
112.5 (±16.82) |
113.5 (±22.54) |
106.5 (±17.85) |
0.5190 |
0.1429 |
0.0002* |
DBP |
71 (±10.71) |
76.5 (±16.31) |
73 (±12.61) |
71.5 (±11.82) |
76 (±15.69) |
68.5 (±11.82) |
0.7715 |
0.6663 |
0.0351* |
HR |
72.4 (±9.90) |
78.55 (±11.64) |
85.7 (±5.99) |
76.8 (±10.13) |
76.95 (±10.21) |
82.1 (±8.18) |
0.0275* |
0.1803 |
0.0288* |
RR |
22.9 (±2.36) |
23.85 (±3.50) |
25.45 (±3.47) |
22.1 (±1.71) |
23.25 (±3.42) |
25.25 (±4.45) |
0.0457* |
0.1806 |
0.7498 |
OBS |
98.95 (±0.22) |
97.9 (±3.58) |
98.1 (±1.52) |
98.9 (±0.31) |
97.9 (±3.59) |
97.95 (±1.47) |
0.3299 |
> 0 .999 |
0.0828 |
*p < 0,05. n = 60. NG: Nicotine Group, PG: Placebo Group, LG: Lavender Group, RR: Respiratory Rate, HR: Heart Rate, SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure, OBS: Oxygen Blood Saturation.
Craving and anxiety assessment
The evidence carried out by the intra-group comparison tests at pretest and posttest moments showed that for the QSU-b instrument there were significant differences between the medians of the Nicotine Group and the Lavender Group (W = -193.00, p = < 0.0001; W = -144.00, p = 0.0007 respectively); after the intervention in both cases, the values suggested the craving was reduced, while the Placebo Group did not have any statistically significant change (Figure 1). The median values (25 percentile -75 percentile), at the pretest and posttest time, for the Nicotine Group ranged from 26.5 (16.50 – 36.00) to 17.0 (11.00 – 22.75), and according to the level craving classification proposed by Araújo et al. [13] These parameters has been modified from moderate to low level. Similarly, the Lavender Group showed median values of 38.5 (22.50 – 62.25) in the pretest and 20.0 (13.75 – 44.00) in the posttest, the craving was reduced from moderate to low level. The Placebo Group, as was expected, presented values of median statistically non-significant (from 28 to 27 points). After these results, the Mann Whitney t test proved that there was no significant variation (U = 147.5, p = 0.1580) between the experimental substance (lavender essential oil) and the substance used in the positive control group (nicotine).
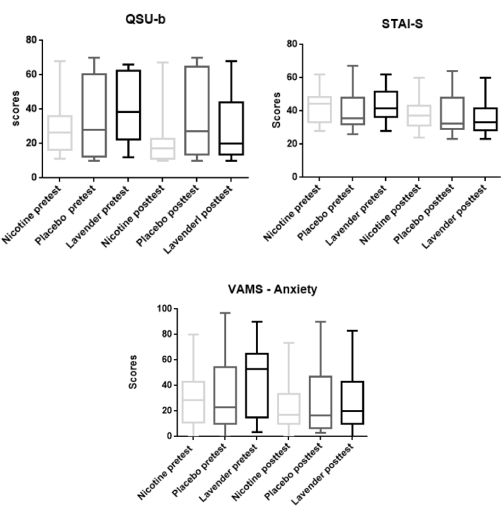
Figure 1. Intergroup scores comparisons of nicotine, placebo and lavender essential oil effect in the anxiety and craving
The intra-group tests performed with the STAI-S instrument before and after the interventions show significant differences between the medians of Nicotine Group and Lavender Group (W = -118.00, p = 0.0258; W = -134.00, p = 0.0103 respectively), and on the other hand, the Placebo Group did not show significant statistical variations (Figure 1). The median values of Nicotine Group vary from 44.5 (33.00 to 48.50) to 37.0 (31.25 to 43.00), showing, therefore, changes in the level of anxiety (from medium anxiety to lower anxiety) according to the classification proposed by Biaggio and Natalício [21]. Similarly, the Lavender Group shows a median value of 41.5 (36.25 to 51.75) in the pretest and 33.0 (28.25 to 41.50) in posttest, thus changing the rate anxiety from medium to low. The Placebo Group, as expected, has a median variation not statistically significant. After these findings, a Mann Whitney t test was held to prove that there is no significant difference between the Lavender Group and the substance used in the Nicotine Group (U = 165.5, p = 0, 3576).
The intra-group statistical tests performed for the anxiety factor of the VAMS show a significant decrease (W = -160.00, p = 0.0017) of that feeling after the approach, only for Lavender, whose median is 28.28 (11.01 to 42.92) in the pretest and 22.86 (9.98 to 54.48) in posttest (Figure 1). The other factors (cognitive impairment, sedation and discomfort) did not change significantly after the use of lavender essential oil to any group. The Mann Whitney t test made comparing the efficacy of the substance used in the Nicotine Group and Lavender Group did not show statistical significance (U = 181.5, p = 0.6251).
Discussion
A randomized, single-blind, controlled experimental design was performed to evaluate the acute effect of essential oil of lavender, administered by inhalation, on adult smokers with the withdrawal syndrome. There was a significant influence of the essential oil of Lavandula angustifolia in reducing anxiety, craving, Systolic Blood Pressure and Heart Rate when compared to the Placebo Group. When the Nicotine Group was compared with placebo, there was a significant reduction of anxiety, craving and Respiratory Rate, while Heart Rate showed a significant increase after the intervention. The experimental group experienced a decreasing level of anxiety from mild to low based on the VAMS (anxiety factor) and STAI-S scores. Besides that, both the Nicotine and Lavender group experienced reduced levels of craving from moderate to low according to QSU-b scores. Statistical analyses showed that there was no significant difference between the results of the use of essential oil and nicotine in controlling anxiety and craving parameters, so it is possible to say that they have similar characteristics in these aspects.
It was evidenced substantially similar results for all participants based on each substance administered. However, in Lavender Group, two of them did not present changes in the levels of anxiety evaluated through the self-report instrument, even though they have shown alterations in the physiological parameters in favour of the reduction of anxiety. The most logical explanation for this event would be to mention the biological differences of each individual, but since there is discrepancy between the analysis of the measures of the physiological parameters and the self-report questionnaires, it could be admitted that one of these two components was not measured correctly. Studies generally assess anxiety through self-report instruments, however, due to the complexity of this phenomenon, as others of multidimensional subjective nature (such as craving), it is important to consider the use of other instruments altogether that corroborate the content validity of the variables that are intended to be analysed [23]. For this reason, the use of self-report instruments was adopted in association with the measurement of physiological parameters in the sense of strengthening the internal validity of the study. Both anxiety and craving can be demonstrated by characteristic clinical signs, mediated mainly by the stimulation of sympathetic activity, which constitute neurovegetative responses such as tachycardia, increased breathing, muscle tension, trembling, etc. [22]
Another problem found in the literature is that there is no consensus about which the ideal placebo substance for the accomplishment of the clinical trials of aromatherapy would be. Usually distilled water, jojoba essential oil, grape seed essential oil or no component are used [9]. The pertinence of the use of distilled water as a placebo substance could be questioned, once the characteristic of essential oils is the release of strong aromatic substance-related odours. Although the results provided by the present study with the use of water as placebo appear to be consistent, once during the administration of the experimental and placebo substances, at no time were the participants informed about the type of the substance to which they would be submitted. Due to differences in methodologies adopted in the clinical trials described in the scientific literature, it would be difficult to predict the best ways of administration, adequate number of sessions and a placebo substance to obtain a better performance in the use of the lavender essential oil. However, the methodological rigor and the manner in which the variables were measured allow to assume that the present work shows consistent results in relation to what was initially proposed.
This study corroborate2021 Copyright OAT. All rights reserval. [8] that studied the effects of lavender essential oil in preoperative patients by similar administration methodology. They submitted 150 patients randomly divided into 3 groups: control (standard care), experimental (lavender essential oil inhalation) and placebo (jojoba essential oil inhalation) and observed anxiolytic activity for experimental substances by inhalation of 1 drop of pure oil (undiluted) on a cotton ball. Lee et al. [10] whose systematic review study, between 1990 and 2010, showed 4 clinical trials with a significant reduction in anxiety evaluated by self-report instruments, through inhalatory administration of lavender essential oil. Bikmoradi et al. [23] studied the effects of inhalation of lavender essential oil in reducing mental stress and improving vital signs in patients undergoing coronary artery bypass surgery and observed that Systolic Blood Pressure showed significant reduction after 5 and 30 minutes, three days after surgery, compared to placebo. The Diastolic Blood Pressure exhibited the same change after 5 minutes of aromatherapeutic approach. These findings are close to those obtained in this study, since systolic blood pressure, diastolic blood pressure and heart rate decreased after essential oil administration. The increase in heart rate and decrease in respiratory rate presented in nicotine group are probably related to the use of transdermal nicotine as pharmacotherapy and smoking cessation, because despite the complexity of substance acting scope, genetic and environmental factors, the main adverse reactions have increased blood pressure, peripheral vasoconstriction, tachycardia, etc [24].
The results obtained through aromatherapy in reducing anxiety, stress and all the emotional complexes linked to compulsions and motivations are disclosed, however, still there have not been fully elucidated the understanding by which aromatic substances exert this role when neuroanatomical are neurophysiological aspects are correlated [25]. It is known that the olfactory system receives stimuli of essential oils occupying specific sites in the respiratory epithelium of the vomeronasal organ and triggers numerous chemical reactions that generate nerve impulses that are intended to cortical and subcortical areas of the central nervous system, mainly those connections related to amygdala which is responsible for affective, emotional and motivational hue of potentially dangerous situations that expose humans to anxiogenic experiences [26]. Chioca et al. [27] and Woronuk et al. [28] differ in the mechanism of action by which lavender essential oils promote their pharmacological effects, however, they assume anxiolytic properties comparable to standard pharmacotherapy, such as benzodiazepines (diazepam, lorazepam, etc). The first author suggested mechanism of action predominantly in serotonergic neurotransmission, while the second proposes neurobiochemical change in the transmission of gamma-aminobutyric acid (GABA). On the other hand, Nikfarjam et al. [29] has demonstrated antidepressant properties of the essential oil involved, compared to antidepressant selective serotonin reuptake inhibitors (citalopram, escitalopram, etc).
Although the findings from the current study are encouraging, there are some limitations that deserve mention. This study was a single-blind trial, and despite the fact that a nurse was responsible for collecting the physiological measures and one of the authors had applied the anxiety and craving questionnaires, both were aware of the purpose of the study and the type of active the substance that was used. A better alternative would have been to dispose of a person responsible for such procedures who was not aware of either the study objectives or the properties of the substance used. Another limitation is the lack of nicotine biomarkers used for confirmation of smoking the withdrawal symptoms. It does not use an expired carbon monoxide test to confirm smoking status or other tests to confirm that the patients present the withdrawal symptoms, but that self-inform was considered. Finally, it is difficult to propose a strict comparison between two substances of different pharmacokinetics, that is, lavender essential oil was administered by the inhalation route, while nicotine was used by transdermal application. The absorption of nicotine from all nicotine replacement therapies is slower and the increase in nicotine blood levels is more gradual than from other substances administered by inhalation [30].
This study was important in the sense that, of my knowledge, so far, no scientific controlled trials were elaborated using lavender essential oil for the control of craving and anxiety in smokers. So, it provides relevant scientific evidence that the essential oil of Lavandula angustifolia has characteristics important to reduce anxiety and craving. Beyond that, based on the anxiolytic properties of lavender essential oil, coupled with the premise that anxiety and craving for tobacco share a positive correlation, the use of lavender essential oil may represent a natural, viable and low-cost alternative as a complementary treatment for smoking. Future studies using different experimental models, as a long-term double-blinded clinical essay with greater control regarding to the manifestation of the withdrawal syndrome symptoms should be made to expand the evaluation of the clinical efficacy of the essential oil of Lavandula angustifolia in controlling anxiety and craving.
References
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders. (5th Edn). Arlington: American Psychiatric Publishing.
- Koopmann A, Lippmann K, Schuster R, Reinhard I, Bach P, et al. (2017) Drinking water to reduce alcohol craving? A randomized controlled study on the impact of ghrelin in mediating the effects of forced water intake in alcohol addiction.Psychoneuroendocrinology85: 56-62. [Crossref]
- Zeni TC, Araújo RB (2011) [Relationship between craving for tobacco and craving for crack in patients hospitalized for detoxification]. J Bras Psiq 60: 28-33.
- Cox LS, TIffany ST, Christen AG (2001) Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 3: 7-16. [Crossref]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz N, et al. (2008) Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville, MD: U. S. Department of Health and Human Services, Public Health Service.
- Alba LH, Murillo R, Becerra N, Páez N, Cañas A, et al. (2013) [Recommendations for smoking cessation in Colombia].Biomedica33: 186-204. [Crossref]
- Dias P, Sousa MJ, Pereira OR (2014) Uso da Aromaterapia no Controlo de Estresse e Ansiedade. X Colóquio de Farmácia. Escola Superior de Tecnologia da Saúde do Porto. Porto, Portugal.
- Karaman T, Karaman S, Dogru S, Tapar H, Sahin A, et al. (2016) Evaluating the efficacy of lavender aromatherapy on peripheral venous cannulation pain and anxiety: A prospective, randomized study. Complement Ther Clin Pract 23: 64-68. [Crossref]
- Braden R, Reichow S, Halm MA (2009) The use of the essential oil lavandin to reduce preoperative anxiety in surgical patients.J Perianesth Nurs24: 348-355. [Crossref]
- Perry R, Terry R, Watson LK, Ernst E (2012) Is lavender an anxiolytic drug? A systematic review of randomised clinical trials.Phytomedicine19: 825-835. [Crossref]
- Lee YL, Wu Y, Tsang HW, Leung AY, Cheung WM (2011) A Systematic Review on the Anxiolytic Effects of Aromatherapy in People with Anxiety Symptoms. J Altern Complement Med 17: 101-108. [Crossref]
- Fagerstrom KO, Schneider NG (1989) Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med 12: 159-182. [Crossref]
- Araújo RB, Oliveira MS, Moraes JF, Pedroso RS, Porto F, et al. (2007) Validation of the Brazilian version of Questionnaire of Smoking Urges-Brief. Rev. psiquiatr. clín 34: 166-175.
- Spielbergert C, Gorsuch R, Lushene R (1970) Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press.
- Norris H (1971) The action of sedatives on brain stem oculomotor systems in man.Neuropharmacology10: 181-191. [Crossref]
- Almeida AA (2009) Alterações psicofisiológicas e vocais em indivíduos submetidos ao teste de simulação de falar em público [thesis]. São Paulo: Universidade Federal de São Paulo.
- Sociedade Brasileira de Pneumologia e Tisiologia (2008) Diretrizes para Cessassão do Tabagismo. J Bras Pneumol 34: 845-80.
- Laranjeira R, Giglioti A (2000) Tratamento da dependência de nicotina. Psiquiatr Prat Med 33: 9-18.
- Hernández S, Fernández C, Baptista L (2010) Metodología de la investigación. Cd. de México: McGraw-Hill.
- Fagerström KO (1978) Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment.Addict Behav3: 235-241. [Crossref]
- Biaggio AM, Natalício L (1979) Manual para o Inventário de Ansiedade Traço-Estado (IDATE). Rio de Janeiro: Centro Editor de Psicologia Aplicada (CEPA).
- Prous MJC, Salvanés FR, Ortells LC (2009) Validación de cuestionarios. Reum Clin 4: 1-7.
- Bikmoradi A, Seifi Z, Poorolajal J, Araghchian M, Safiaryan R, et al. (2015) Effect of inhalation aromatherapy withlavender essential oil on stress and vitalsigns in patients undergoing coronary arterybypass surgery: A single-blindedrandomized clinical trial. Complement Ther Med 23: 331-338. [Crossref]
- Ramos WP, Ramos AO (2006) Receptores de Acetilcolina. In: Silva P, Farmacologia. Rio de Janeiro: Guanabara Koogan; p. 208-9.
- Hur MH, Song JA, Lee J, Lee MS (2014) Aromatherapy for stress reduction in healthy adults: a systematic review and meta-analysis of randomized clinical trials. Maturitas 79: 362-369. [Crossref]
- Guyton A, Hall J (2010) Textbook of Medical Physiology (12th ed). Philadelphia: Saunders Elsevier.
- Chioca LR, Ferro MM, Baretta IP, Oliveira SM, Silva CR, et al. (2013) Anxiolytic-like effect of lavender essential oil inhalation in mice: participation of serotonergic but not GABAA/benzodiazepine neurotransmission.J Ethnopharmacol147: 412-418. [Crossref]
- Woronuk G, Demissie Z, Rheault M, Mahmoud S (2011) Biosynthesis and Therapeutic Properties of Lavandula Essential Oil Constituents. Planta Med 77: 7-15. [Crossref]
- Nikfarjam M, Parvin N, Assarzadegan N, Asghari S (2013) The Effects of Lavandula Angustifolia Mill Infusion on Depression in Patients Using Citalopram: A comparison Study. Iran Red Crescent Med J 15: 734-739. [Crossref]
- Rasmussen S, Horkan KH, Kotler M (2018) Pharmacokinetic Evaluation of Two Nicotine Patches in Smokers. Clin Pharmacol Drug Dev 7: 506-512. [Crossref]

