Effects of nano-sized particles on sensitive population have not been well elucidated. Furthermore, their size dependency is still understudied. We have previously examined the effects of CBNP on lung inflammation related to lipopolysaccharide (LPS) or allergen in mice. This study examined the effects of pulmonary exposure to carbon black nanoparticles (95 nm: CBNP95) on these lung inflammation. In protocol 1, ICR male mice were divided into 4 experimental groups that intratracheally received a single exposure to vehicle, CBNP95 (250 μg/animal), or LPS plus CBNP95. In protocol 2, ICR male mice were divided into 4 experimental groups that intratracheally received repeated exposure to vehicle, CBNP95 (100 μg/animal), allergen (ovalbumin: OVA; 1 μg/animal), or allergen plus CBNP95. In protocol 1, CBNP95exacerbated lung edema elicited by LPS, showing an overall trend of amplified lung expressions of proinflammatory cytokines. Furthermore, LPS plus CBNP95 significantly elevated circulatory levels of fibrinogen, macrophage chemoattractant protein-1, and keratinocyte-derived chemoattractant as compared with LPS alone. In protocol 2, CBNP95 significantly enhanced the pathophysiology of allergic asthma, characterized by eosinophilic lung inflammation. These results suggest that CBNP with size of 95nm exacerbated two types of (innate and adaptive immunity-dominant) lung inflammation. This study illustrates the need of further accumulation of evidence about dose-dependency of nanoparticles on sensitive lung diseases.
nanomaterials, lung inflammation, lipopolysaccharide, coagulatory disturbance, systemic inflammation, allergic asthma, neutrophil
Environmental particulate matters (PM) such as diesel exhaust particles (DEP), are well recognized to be toxic for health, including respiratory systems [1]. In 21st century, nanoparticles, particles less than 0.1 μm in mass median aerodynamic diameter, have been focused to affect cardiopulmonary systems [2-4]. Nanoparticles are theoretically able to penetrate deeply into the respiratory tract and have a larger surface area than the particles with larger size, thus resulting in a greater inflammatory response [5,6]. Indeed, two in vivo studies have demonstrated that nanoparticles have marked pulmonary toxicity compared with larger particles [7,8]. Previously, we have demonstrated that carbon nanoparticles can aggravate both LPS- and antigen-related airway inflammation [9,10]. The enhancing effects are more prominent with 14nm nanoparticles than with larger particles (56nm) in overall trend [9,10]. However, effects of nanoparticles, in particular their size effects, on pulmonary inflammatory conditions related to bacterial endotoxin are not fully investigated.
Therefore, in order to delineate the size dependency of toxicity regarding CBNP, the present study was designed to elucidate the effects of carbon black nanoparticles (95 nm) on lung inflammation induced by intratracheal administration of bacterial endotoxin and allergen to delineate toxic dose-dependency of nanoparticles. Furthermore, we added the comparison study of CBNP.
Animals: Male ICR mice 6 wk of age and weighing 29 to 33 g (Japan Clea Co., Tokyo, Japan) were used in all experiments. They were fed a commercial diet (Japan Clea Co.) and given water ad libitum. The mice were housed in an animal facility that was maintained at 24 to 26° with 55 to 75% humidity and a 12-h light/dark cycle.
CBNP: Carbon black nanoparticles at a size of 95 nm (PrinteX 90) was purchased from Degussa (Dusseldorf, Germany) suspended in the same vehicle every week for 6 wk.They were used as suspension in the vehicle (phosphate-buffered saline [PBS] at pH 7.4 [Invitrogen Co., Carlsbad, CA] containing 0.05% Tween 80 [Nacalai Tesque, Kyoto, Japan]) by sonication for 3 min using an Ultrasonic disrupter (UD-201; Tomy Seiko, Tokyo, Japan).
Study protocol: In protocol 1, mice were divided into four experimental groups. The vehicle group received above mentioned vehicle. The LPS group received 75 μg of LPS dissolved in PBS. The CBNP95 group received 250 μg of CBNP95 suspension in the vehicle. The LPS + CBNP group received the combined treatment using the same protocols as the LPS and the CBNP95 groups (n = 20-21 in each group; n = 8 for BAL with ELISA for lung homogenates and plasma fibrinogen, and n = 12-13 for lung water contents [n = 8], lung histology [n = 4-5], and ELISA for serum proinflammatory cytokines [n = 10]). Vehicle, CBNP95, LPS, or LPS + CBNP95 were suspended in 0.1-ml aliquots, and inoculated once intratracheally through a polyethylene tube under anesthesia with 4% halothane (Takeda Chemical Industries, Tokyo, Japan). The animals were sacrificed and studied 24 h after the intratracheal administration with BAL, pulmonary vascular permeability of water and protein, protein levels of cytokines and chemokines in the lung tissue supernatants and the sera, and fibrinogen. In protocol 2, mice were divided into four experimental groups. The vehicle group received PBS containing 0.05% Tween 80 once a week for 6 wk. The OVA group received 1 μg of OVA (Grade IV: Sigma Chemical, St. Louis, MO) dissolved in the same vehicle every 2 wk for 6 wk (Figure 1). The CBNP95 group received 100 μg of above mentioned three sizes of CBNP95 suspended in the same vehicle every week for 6 wk. The OVA + CBNP95 group received the combined treatment in the same protocol as the OVA and CBNP95 group (n = 12-13 in each group; n = 8 for BAL with ELISA for lung homogenates, n = 4-5 for lung histology, and n = 12-13 for ELISA for serum Ig titers in experiments using 100 μg of CBNP95). Vehicle, CBNP95, OVA, or OVA + latex nanomaterials were suspended in 0.1-ml aliquots, and inoculated once by the intratracheal route through a polyethylene tube under anesthesia with 4% halothane (Takeda Chemical Industries). The animals were sacrificed and studied 24 h after the final intratracheal administration, with BAL, lung histology, protein levels of cytokines and chemokines in the lung tissue supernatants and Ig production. The studies adhered to the National Institutes of Health guidelines for the experimental use of animals according to Institutional Animal Care and Use Committee (IACUC: www.iacuc.org). All animal studies were approved by the Institutional Review Board.
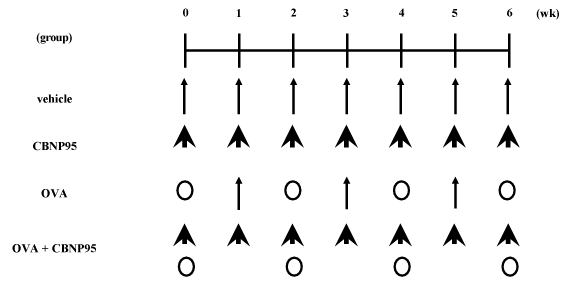
Figure 1. Study design for protocol 2
BAL process: BAL and cell counts were conducted as previously described (n = 8 in each group at each protocol; [9-12]). After the BAL procedure, supernatants were analyzed for protein assay (n = 8 in each group), and the lungs were removed, snap-frozen in liquid nitrogen, and stored at -80° until assay of cytokines and chemokines.
Pulmonary vascular permeability: In protocol 1, in a separate series of experiments, the lungs were weighed and dried. The wet lung weight – the dry lung weight/body weight was calculated (ref [9,12]: n = 8 in each group). In another experiment, protein concentrations in BAL fluid were determined using the commercially available Bradford protein assay (Bio-Rad Laboratory Inc., Hercules, CA: n = 8 in each group) with bovine serum albumin as the standard.
Lung histology: In another experiment, the lungs were fixed and stained with hematoxylin and eosin as previously described [9-12]. For semi-quantitative assessment in protocol 1, neutrophil infiltration was assessed by averaging the number of neutrophils enumerated in 30 randomly selected high power fields (HPFs; ×600) in each slide. Histologic sections were evaluated in a blind fashion (n = 4-5 in each group at each protocol).
Quantitation of cytokine and chemokine protein levels in the lung tissue supernatants: The frozen lungs after BAL were homogenized in each protocol as described previously [9-12]. ELISA for interleukin (IL)-1β(R&D Systems, Minneapolis, MN), IL-5 (Endogen, Cambridge, MA), IL-13 (R&D Systems), IL-18 (MBL, Nagoya, Japan), macrophage chemoattractant protein (MCP)-1 (R&D Systems), and keratinocyte-derived chemoattractant (KC: R&D Systems), eotaxin (R&D systems), and macrophage inflammatory protein (MIP)-1α (R&D Systems) in the lung tissue homogenates were conducted according to the manufacturer’s instructions and the values were expressed as pg/total lung supernatants (n = 8 in each group at each protocol).
Assays for circulatory levels of fibrinogen: In protocol 1, citrate plasma level of fibrinogen (n = 8 in each group) was determined using commercial kits (Diagnostica Stago, Roche, Tokyo, Japan) on STA Compact (Diagnostica Stago, Roche) as described previously [13].
Allergen-specific Ig determination: In protocol 2, blood was retrieved by cardiac puncture. Serum was prepared and frozen at – 80° until assayed for allergen-specific IgG1 and IgE. Allergen-specific IgG1 antibody was measured by ELISA with solid-phase allergen as previously described (10, 11: n = 12-13 in each group). Allergen-specific IgE antibody was measured by commercial ELISA kit (Dainippon Sumitomo Pharmaceutical Co. Osaka, Japan) according to the manufacturer’s instructions (n = 12-13 in each group).
RNA preparation and quantitative RT-PCR analysis: Lung tissue was homogenized in ISOGEN (Nippon Gene, Tokyo, Japan), and total RNA was extracted according to the manufacturer's instructions. The real-time qRT-PCR was performed using the Applied Biosystems 7900HT Real-Time PCR System and the Taqman Gene Expression Assay (Applied Biosystems, Austin, TX). The quantification of mRNA levels was performed using an ABI 7300 real-time PCR system (Applied Biosystems) and commercial specific primers and TaqMan probes, synthesized by Applied Biosystems according to the protocol provided by Applied Biosystems. Negative controls were run in parallel, and the relative values for each gene were normalized using beta-actin as the reference gene and analyzed with Taqman Analysis Software SDS2.4 (Applied Biosystems).
Statistical analysis: Data are reported as means ± SE. Differences between groups were determined using analysis of variance (ANOVA: Stat view version 4.0; Abacus Concepts, Inc., Berkeley, CA). If differences between groups were significant (P < 0.05) using one-way ANOVA, the Bonferroni correction was used for multiple comparison.
Effects of CBNP95 on LPS-related lung inflammation and pulmonary vascular permeability: We examined the cellular profile of BAL fluid and lung water content 24 h after the intratracheal instillation in protocol 1 (Table 1). CBNP95 alone increased the number of neutrophils as compared with vehicle (P < 0.05). LPS exposure also significantly increased the number as compared with vehicle exposure (P < 0.01). The number in the LPS + CBNP95 group was greater than those in the LPS group without statistical significance, but greater than those in the corresponding CBNP95 group (P < 0.01). Pulmonary exposure to CBNP95 elevated lung water content (P < 0.05) as compared with exposure to vehicle. The value was significantly greater in the LPS group than in the vehicle group (P < 0.01). These values were further greater in the LPS + latex nanomaterial groups than in the LPS (P < 0.05) or the corresponding CBNP95 (P < 0.01) group.
Table 1. The number of total cells and neutrophils and protein concentration in bronchoalveolar lavage (BAL) fluid and lung water content after intratracheal challenge (protocol 1)
Group |
Neutrophils |
Lung Water Content |
IL-1β |
IL-6 |
KC |
MIP-1α |
MIP-2 |
MCP-1 |
(×104/BALF) |
(wet weight-dry weight/body weight) |
(ng/total lung supernatants) |
(pg/total lung supernatants) |
vehicle |
16.5 ± 10.4 |
4.1 ± 0.1 |
0.9 ± 0.6 |
68.2 ± 10.2 |
49.2 ± 33.6 |
28.1 ± 2.6 |
44.2 ± 3.4 |
69.6 ± 26.3 |
CBNP95 |
113.4 ± 14.7 * |
5.6 ± 0.2 * |
1.8 ± 0.4 |
91.0 ± 12.4 |
172.1 ± 75.7 |
159.3 ± 2.7 |
105.5 ± 11.8 |
221.8 ± 64.3 * |
LPS |
711.6 ± 46.6 * |
7.1 ± 0.1 * |
36.5 ± 4.1 ** |
919.0± 131.2 * |
3568.1 ± 176.4 ** |
1911.9 ± 2.8 |
1689.8 ± 11.9 |
2080.6 ± 222.6 ** |
LPS + CBNP95 |
765.8 ± 143.0 * $ |
7.4 ± 0.2 * # $ |
66.7 ± 9.6 ** # $$ |
1817.2 ± 475.2 ** # $$ |
4567.8 ± 323.4 ** # $$ |
3660.9 ± 2.9 |
2800.0 ± 11.10 |
3763.8 ± 522.9 ** # $$ |
Results are expressed as mean ± SE (n = 8 in each group for neutrophil number and protein concentration in BAL and lung water content, n = 4-5 in each group for neutrophil number in lung parenchyma). * P < 0.05 versus the vehicle group, ** P < 0.01 versus the vehicle group, # P < 0.05 versus the LPS group, ## P < 0.01 versus the LPS group, $ P < 0.01 versus the CBNP group.
Effects of CBNP95 on LPS- related histological changes in the lung: We evaluated lung specimens stained with hematoxylin and eosin 24 h after the intratracheal instillation in protocol 1. No pathological change was seen in the lung obtained from the vehicle group. Infiltration of neutrophils was moderately seen in the lungs from the CBNP95 group and moderately seen in those from the LPS group. Combined treatment with LPS and CBNP95 enhanced leukocyte (mainly neutrophil) sequestration into the lung parenchyma as compared with LPS treatment alone (supplementary Figure1).
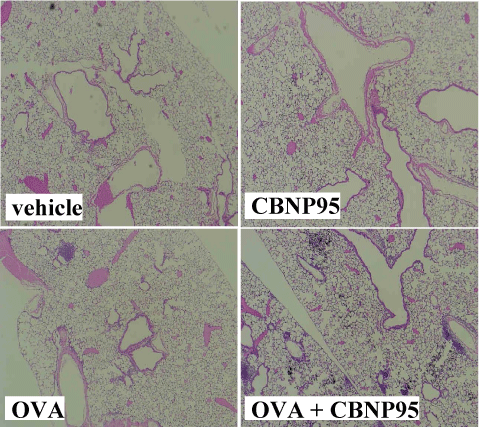
Supplementary Figure. Effects of CBNP95 on LPS- related histological changes in the lung
Effects of CBNP95 on the expression of LPS-related proinflammatory cytokines and chemokines in the lung: We next measured the protein levels of IL-1β, KC, MIP-1αMIP-2, and MCP-1 in the lung tissue supernatants 24 h after the intratracheal instillation in protocol 1 (Table 1). Pulmonary exposure to CBNP95 alone elevated the levels of these molecules (P < 0.05 for 25 or 50 nm latex nanomaterial) as compared to that to vehicle. LPS challenge significantly elevated the levels of all of these proteins as compared with vehicle challenge (P < 0.01). The levels were further greater in the LPS + CBNP95 group than in the LPS (P < 0.05 for the IL-1β level; P < 0.01 for the MCP-1 or the KC) or the corresponding CBNP95 (P < 0.01) group.
Effects of CBNP95 on a LPS-related coagulatory parameter: We next analyzed serum fibrinogen 24 h after the intratracheal challenge in protocol 1 (Table 2). CBNP95 or LPS challenge significantly elevated the fibrinogen level as compared to vehicle challenge (P < 0.01). The level was further greater in the LPS + CBNP95 group than in the LPS group (P < 0.05) or the corresponding CBNP95 group (P < 0.01).
Table 2. Gene levels (mRNA expression) of HO-1, iNOS, TLR-2 ans -4, and ICAM-1 in the lung tissue supernatants after intratracheal challenge (protocol 1)
Group |
HO-1 |
iNOS |
TLR-2 |
TLR-4 |
ICAM-1 |
(relative mRNA expression) |
vehicle |
2.60 ± 1.93 |
0.31 ± 0.18 |
0.38 ± 0.2 |
4.02 ± 2.76 |
0.4 ± 0.18 |
CBNP95 |
3.28 ± 1.06 |
0.43 ± 0.14 |
0.49 ± 0.11 |
7.12 ± 2.18 |
0.68 ± 0.12 |
LPS |
2..80 ± 0.31 |
0.79 ± 0.15 |
2.38 ± 0.42 ** |
3.5 ± 0.5 |
2.59 ± 0.33 ** |
LPS + CBNP95 |
8.60 ± 2.19 * # $ |
5.50 ± 1.87 * # $ |
5.33 ± 1.17 ** # $$ |
6.9 ± 2.14 # |
4.26 ± 1.07 ** # $$ |
Results are expressed as mean ± SE (n = 8 in each group). * P < 0.05 versus the vehicle group, ** P < 0.01 versus the vehicle group, # P < 0.05 versus the LPS group, ## P < 0.01 versus the LPS group, $ P < 0.01 versus the CBNP group.
Effects of CBNP95 on gene levels of LPS-related oxidative stress, TLRs, and ICAM-1: We measured the transcript levels of HO-1, iNOS, TLR-2 and -4 and ICAM-1 in the lung 4 h after the intratracheal instillation in protocol 1 (Table 2). CNBP95 exposure elevated all the levels as compared to vehicle exposure. LPS challenge significantly elevated the level of TLRs and ICAM-1 as compared with vehicle challenge (P < 0.05). The level was greater in the LPS + CBNP95 group than in the LPS (P < 0.05) or the corresponding CBNP95 group (P < 0.01).
Effects of CBNP95 on allergic airway inflammation: We investigated the cellular profile of BAL fluid in protocol 2 (Table 3). The numbers of total cell (data not shown) and eosinophil were significantly greater in the OVA or the OVA + CBNP95 group than in the vehicle group (P < 0.05 for OVA, P < 0.01 for OVA + CBNP95). The number was greater in the OVA + CBNP95 group than in the OVA or corresponding CBNP95 group (P < 0.05). The number of neutrophils was greater in the CBNP95 group than in the vehicle group (P < 0.01). The number was greater in the OVA + CBNP95 group than in the OVA or the corresponding CBNP95 group (P < 0.01).
Table 3. Protein levels of cytokines in the lung tissue supernatants after the final intratracheal challenge (protocol 2)
Group |
Eosinophils |
IL-2 |
IL-4 |
IL-5 |
IL-6 |
IL-13 |
IFN-γ |
(×104/BALF) |
(pg/total lung supernatants) |
vehicle |
0 ± 0 |
47.2 ± 4.2 |
233.1 ± 17.5 |
7.97 ± 1.2 |
107.2 ± 6.0 |
2.3 ± 0.8 |
4332.7 ± 218.7 |
CBNP95 |
0.09 ± 0.01 |
40.9 ± 3.7 |
224.7 ± 14.7 |
12.0 ± 1.8 |
134.1 ± 7.9 |
0.6 ± 0.2 |
4333.2 ± 242.1 |
OVA |
0.74 ± 0.11 * |
54.5 ± 7.6 |
254.8 ± 15.1 |
20.1 ± 17.1 |
130.6 ± 7.6 * |
15.7 ± 9.4 * |
4986.8 ± 239.4 |
OVA + CBNP95 |
7.94 ± 2.05 ** ## |
83.5 ± 12.1 * # |
267.5 ± 21.7 |
89.2 ± 31.6 * # |
225.7 ± 28.9 * # |
53.7 ± 15.0 ** ## |
4803.2 ± 135.3 |
Results are expressed as mean ± SE (n = 8 in each group). * P < 0.05 versus the vehicle group, ** P < 0.01 versus the vehicle group, # P < 0.05 versus the LPS group, ## P < 0.01 versus the LPS group, $ P < 0.01 versus the CBNP group.
Effects of CBNP95 on allergen - related histological changes in the lung: We evaluated lung specimens stained with hematoxylin and eosin 24 h after the final intratracheal instillation in protocol 2 (data not shown). No pathological change was seen in the lung obtained from the vehicle group. Infiltration of neutrophils was slightly seen in the lungs from the CBNP95 group. On the other hand, infiltration of eosinophils was moderately observed in the lung from the OVA group. Combined treatment with OVA and CBNP95 apparently worsened polymorphonuclear leukocyte (mainly neutrophil) sequestration into the lung parenchyma as compared with OVA treatment alone (Figure 2).

Figure 2. Representative histological findings of the Hematoxylin & Eosin-stained lung obtained from the vehicle, CBNP95, LPS, or LPS + CBNP95 24 h after the intratracheal administration. Animals received intratracheal instillation of vehicle, CBNP95, LPS, or LPS + CBNP95. Lungs were removed and fixed 24 h after the intratracheal administration (n = 4-5 in each group). Original magnification ×25.
Effects of CBNP95 on local expression of cytokines and chemokines in the presence of allergen: We quantitated protein levels of IL-2, IL-4, IL-5, IL-6, IL-13, and IFN-γ in the lung tissue supernatants (Table 3). OVA challenge increased these level of as compared with vehicle challenge (P < 0.01). All of the level were significantly greater in the OVA + CBNP95 group than in the vehicle, CBNP95, or OVA group (P < 0.01). On the other hand, CBNP95 exposure elevated all of the chemokines examined (MIP-1α, MCP-1, eotaxin, RANTES, and TARC) as compared to vehicle exposure. OVA exposure significantly elevated the level of eotaxin as compared to vehicle exposure. All of these chemokine levels were significantly greater in the OVA + CBNP95 group than in the vehicle, OVA, or CBNP95 group.
Effects of CBNP95 on allergen-specific production of IgG1 and IgE: Finally, we measured allergen-specific IgG1 and IgG2a (Table 4). The allergen-specific IgG1 was significantly greater in the OVA + CBNP95 group than in the vehicle, OVA, or CBNP95 group (P < 0.01). On the other hand, the allergen-specific IgG2a was significantly lower in the OVA + CBNP95 group than in the vehicle, OVA, or CBNP95 group (P < 0.05) (Table 5).
Table 4. Protein levels of chemokines in the lung tissue supernatants after the final intratracheal challenge (protocol 2)
Group |
MIP-1α |
MCP-1 |
Eotaxin |
RANTES |
TARC |
(pg/total lung supernatants) |
vehicle |
15.3 ± 0.6 |
66.6 ± 5.0 |
64.7 ± 3.7 |
182.0 ± 24.2 |
22.8 ± 2.7 |
CBNP95 |
185.7 ± 24.8 ** |
80.4 ± 8.8 |
90.6 ± 11.9 |
213.7 ± 22.3 |
92.0 ± 5.7 * |
OVA |
23.6 ± 6.9 |
50.5 ± 11.2 |
131.6 ± 28.7 * |
153.0 ± 28.3 |
54.1 ± 13.6 |
OVA + CBNP95 |
180.7 ± 29.7 ** ## |
163.0 ± 14.7 * # |
867.3 ± 125.4 * # |
299.4 ± 43.6 * # |
170.1 ± 19.0 * # |
Results are expressed as mean ± SE (n = 8 in each group). * P < 0.05 versus the vehicle group, ** P < 0.01 versus the vehicle group, # P < 0.05 versus the OVA group, ## P < 0.01 versus the OVA group, $ P < 0.05 versus the CBNP group, $$ P < 0.01 versus the CBNP group.
Table 5. Levels of allergen-specific IgG (protocol 2)
Group |
IgG1 |
IgG2a |
(titer) |
vehicle |
1148.4 ± 280.0 |
20.3 ± 9.6 |
CBNP95 |
3716.4 ± 1997.9 |
13.7 ± 7.7 |
OVA |
2048.0 ± 455.8 |
17.0 ± 9.2 |
OVA + CBNP95 |
17835.5 ± 9253.0 * # |
6.6 ± 2.9 * |
Results are expressed as mean ± SE (n = 8 in each group). * P < 0.05 versus the vehicle group, ** P < 0.01 versus the vehicle group, # P < 0.05 versus the OVA group, ## P < 0.01 versus the OVA group, $ P < 0.05 versus the CBNP group, $$ P < 0.01 versus the CBNP group.
The present study has demonstrated that single pulmonary exposure to carbon black nanoparticle with average size of 95 nm enhances neutrophilic lung inflammation with pulmonary vascular permeability related to LPS resulted from activated innate immunity. The enhancement is concomitant with the increased local (lung) expression of proinflammatory cytokine such as IL-1β, and chemokines such as MCP-1 and KC. In addition, combined challenge with LPS and CBNP95 further increases circulatory levels of fibrinogen as compared with challenge with LPS alone. Furthermore, repetitive exposure to CBNP95 also significantly exacerbated the hallmarks of allergic airway inflammation characterized by eosinophilic lung inflammation with Ig productions predominant consequence of activated adaptive immunity.
Although there are many studies concerning the effects of exposure to nanomaterials, their size dependent effects have been considerably less explored. Liu et al. have shown that smaller ZnO2 NPs induced more significant toxic response in human neuroblastoma cells [14]. On the other hand, Avalos et al. have demonstrated that AgNPs-induced cytotoxicity was size-dependent (smaller > larger), whereas AuNPs was not [15]. Also, it was reported that silver NPs with smaller size have significant toxicity as compared to that with larger size in vitro [16,17]. Interestingly, Kong et al. applied three sizes (14 nm, 51 nm, 95 nm) of CBNP and showed that their cytotoxicity including oxidative and nuclear damages) was inversely size-dependent (95 nm > 51 nm > 14 nm) in mouse macrophage RAW264.7 cells [18], although the larger nanoparticles the authors used was not ours (provided from Flammruss 101). Nonetheless, previously, we have proved/reported that 14 nm CBNP theatrically augmented the same pathological condition in the lungs on the same experimental protocol as the current study, whereas 50 nm CBNP did not, especially, LPS-provoked lung inflammation. In the present study, however, 95 nm CBNP significantly exacerbated both types of lung inflammation. Thus; our consecutive studies seem to be not consistent with the study by Kong et al. It is possible that not only particle size but also characteristics of these particles contribute to adverse effects of nanoparticles. Future accumulation of studies focusing size dependent efficacy may clarify the issue.
Alternatively, sensitivity to nanoparticles may depend on pathological types of lung inflammation. Recordati et al. showed that AgNPs (10 nm, 40 nm, 100 nm) intravenously administered exhibited size-dependent (10 nm > 40 nm, 100 nm) hepatotoxicity in healthy mice [19]. Combined with our previous studies (both 14 and 56 nm CBNPs exacerbated the allergic airway inflammation model; ref [10]) and the current one, particularly, CBNP can exacerbate allergic lung inflammation in mice, regardless of size less than 100 nm. On the other hand, our previous study has shown that latex nanoparticle (25 nm ~ 100 nm) did not influence on this type of lung inflammation in the same protocol as the current study [20]. Recently, particulate matters such as alum have been reported to serve as adjuvant in allergic response through inflammasome dependent or non-dependent pathway [21]. However, series of our experiments complicated the issue. On the other hand, our previous and current studies have shown that CBNP did not exhibit size-dependent adverse effects on LPS-related lung inflammation (14 nm > 95 nm > 56 nm?), whereas TiO2 [22] and latex [20] NPs did with overall trend. Further studies concerning the effects of not only nanoparticles/materials on preexisting lung diseases, but their size-dependent efficacy is needed in the future.
In conclusion, this study has highlightened that 95 nm CBNP enhance lung inflammation related to bacterial endotoxin. The enhancement is mediated through the increased local expression of IL-1β and chemokines via TLR-dependent pathway. 95 nm CBNP also enhance coagulatory disturbance accompanied by lung inflammation. Furthermore, this size of CBNP significantly exacerbated allergen-related airway inflammation. This study illustrates the need of further accumulation of evidence about dose-dependency of nanoparticles on sensitive lung diseases. Alternate, this study adds the hazardous effects of nanoparticles nearly 100 nm, even relatively large ones as nanoparticles, on sensitive pulmonary diseases.
This study was supported by Grants-in-Aid for Scientific Research (S) 18390188 (to H. Takano) from Japan Society for the Promotion of Science.
- Dockery DW, Pope CA (1994) Acute respiratory effects of particulate air pollution. Annu Rev Public Health 15: 107-132. [Crossref]
- Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622-627. [Crossref]
- Peters A, Wichmann HE, Tuch T, Heinrich J, Heyder J (1997) Respiratory effects are associated with the number of ultrafine particles. Am J Respir Crit Care Med 155:1376-1383. [Crossref]
- Utell MJ, Frampton MW (2000) Acute health effects of ambient air pollution: the ultrafine particle hypothesis. J Aerosol Med 13:355-359. [Crossref]
- MacNee W, Donaldson K (2000) How can ultrafine particles be responsible for increased mortality? Monaldi Arch Chest Dis 55:135-139. [Crossref]
- Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, et al. (2001) Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med 164, 1665-1668. [Crossref]
- Ferin J, Oberdorster G, Penney DP (1992) Pulmonary retention of ultrafine and fine particles in rats. Am J Respir Cell Mol Biol 6: 535-542. [Crossref]
- Li XY, Brown D, Smith S, MacNee W, Donaldson K (1999) Short-term inflammatory responses following intratracheal instillation of fine and ultrafine carbon black in rats. Inhal Toxicol 11:709-731. [Crossref]
- Inoue K, Takano H, Yanagisawa R, Hirano S, Sakurai M, et al. (2006). Effects of airway exposure to nanoparticles on lung inflammation induced by bacterial endotoxin in mice. Environ Health Perspect 114: 1325-1330. [Crossref]
- Inoue K, Takano H, Yanagisawa R, Sakurai M, Ichinose T, et al. (2005) Effects of nano particles on antigen-related airway inflammation in mice. Respir Res 6:106. [Crossref]
- Takano H, Yoshikawa T, Ichinose T, Miyabara Y, Imaoka K, et al. (1997) Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. Am J Respir Crit Care Med 156: 36-42. [Crossref]
- Takano H, Yanagisawa R, Ichinose T, Sadakane K, Yoshino S, et al. (2002) Diesel exhaust particles enhance lung injury related to bacterial endotoxin through expression of proinflammatory cytokines, chemokines, and intercellular adhesion molecule 1. Am J Respir Crit Care Med 165:1329-1335. [Crossref]
- Inoue K, Takano H, Yanagisawa R, Sakurai M, Shimada A, et al. (2004) Protective role of interleukin-6 in coagulatory and hemostatic disturbance induced by lipopolysaccharide in mice. Thromb Haemost 91: 1194-1201. [Crossref]
- Liu J, Kang, Y, Yin S, Song B, Wei L, et al. (2017) Zinc oxide nanoparticles induce toxic responses in human neuroblastoma SHSY5Y cells in a size-dependent manner. Int J Nanomedicine 12: 8085-8099. [Crossref]
- Ávalos A, Haza AI, Mateo D, Paloma Morales P (2015) Effects of silver and gold nanoparticles of different sizes in human pulmonary fibroblasts. Toxicol Mech Methods 25: 287-295. [Crossref]
- Soares T, Ribeiro D, Proença C, Chisté RC, Fernandes E, et al. (2016) Size-dependent cytotoxicity of silver nanoparticles in human neutrophils assessed by multiple analytical approaches. Life Sci 145: 247-254. [Crossref]
- Park MVDZ, Neigh AM, Vermeulen JP, de la Fonteyne LJJ, Verharen HW, et al. (2011) The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 32: 9810-9817. [Crossref]
- Kong H, Zhang Y, Li Y, Cui Z, Xia K, et al. (2013) Size-dependent cytotoxicity of nanocarbon blacks. Int J Mol Sci 14: 22529-22543. [Crossref]
- Recordati C, De Maglie M, Bianchessi S, Argentiere S, Cella C, et al. (2016) Tissue distribution and acute toxicity of silver after single intravenous administration in mice: nano-specific and size-dependent effects. Part Fibre Toxicol 13:12. [Crossref]
- Inoue K, Takano H, Yanagisawa R, Koike E, Shimada A (2009) Size effects of latex nanomaterials on lung inflammation in mice. Toxicol Appl Pharmacol 234: 68-76. [Crossref]
- He P, Zou Y, Hu Z (2015) Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccin Immunother 11: 477-88. [Crossref]
- Inoue K, Takano H, Ohnuki M, Yanagisawa R, Sakurai M, et al. (2008) Size effects of nanomaterials on lung inflammation and coagulatory disturbance. Int J Immunopathol Pharmacol 21: 197-206. [Crossref]
Editorial Information
Editor-in-Chief
Salim Surani
A&M University of Texas
Article Type
Research Article
Publication history
Received: January 04, 2021
Accepted: January 13, 2021
Published: January 18, 2021
Copyright
©2021 Inoue KI. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Inoue KI, Yamada K, Suzuki W, Takano H, Shimada A (2021) Effects of 95nm carbon black nanoparticles on inflammatory conditions in the murine lung - Controversy to size dependent efficacy. J Respir Dis Med 3. DOI: 10.15761/JRDM.1000127.

Figure 1. Study design for protocol 2

Figure 2. Representative histological findings of the Hematoxylin & Eosin-stained lung obtained from the vehicle, CBNP95, LPS, or LPS + CBNP95 24 h after the intratracheal administration. Animals received intratracheal instillation of vehicle, CBNP95, LPS, or LPS + CBNP95. Lungs were removed and fixed 24 h after the intratracheal administration (n = 4-5 in each group). Original magnification ×25.

Supplementary Figure. Effects of CBNP95 on LPS- related histological changes in the lung
Table 1. The number of total cells and neutrophils and protein concentration in bronchoalveolar lavage (BAL) fluid and lung water content after intratracheal challenge (protocol 1)
Group |
Neutrophils |
Lung Water Content |
IL-1β |
IL-6 |
KC |
MIP-1α |
MIP-2 |
MCP-1 |
(×104/BALF) |
(wet weight-dry weight/body weight) |
(ng/total lung supernatants) |
(pg/total lung supernatants) |
vehicle |
16.5 ± 10.4 |
4.1 ± 0.1 |
0.9 ± 0.6 |
68.2 ± 10.2 |
49.2 ± 33.6 |
28.1 ± 2.6 |
44.2 ± 3.4 |
69.6 ± 26.3 |
CBNP95 |
113.4 ± 14.7 * |
5.6 ± 0.2 * |
1.8 ± 0.4 |
91.0 ± 12.4 |
172.1 ± 75.7 |
159.3 ± 2.7 |
105.5 ± 11.8 |
221.8 ± 64.3 * |
LPS |
711.6 ± 46.6 * |
7.1 ± 0.1 * |
36.5 ± 4.1 ** |
919.0± 131.2 * |
3568.1 ± 176.4 ** |
1911.9 ± 2.8 |
1689.8 ± 11.9 |
2080.6 ± 222.6 ** |
LPS + CBNP95 |
765.8 ± 143.0 * $ |
7.4 ± 0.2 * # $ |
66.7 ± 9.6 ** # $$ |
1817.2 ± 475.2 ** # $$ |
4567.8 ± 323.4 ** # $$ |
3660.9 ± 2.9 |
2800.0 ± 11.10 |
3763.8 ± 522.9 ** # $$ |
Results are expressed as mean ± SE (n = 8 in each group for neutrophil number and protein concentration in BAL and lung water content, n = 4-5 in each group for neutrophil number in lung parenchyma). * P < 0.05 versus the vehicle group, ** P < 0.01 versus the vehicle group, # P < 0.05 versus the LPS group, ## P < 0.01 versus the LPS group, $ P < 0.01 versus the CBNP group.
Table 2. Gene levels (mRNA expression) of HO-1, iNOS, TLR-2 ans -4, and ICAM-1 in the lung tissue supernatants after intratracheal challenge (protocol 1)
Group |
HO-1 |
iNOS |
TLR-2 |
TLR-4 |
ICAM-1 |
(relative mRNA expression) |
vehicle |
2.60 ± 1.93 |
0.31 ± 0.18 |
0.38 ± 0.2 |
4.02 ± 2.76 |
0.4 ± 0.18 |
CBNP95 |
3.28 ± 1.06 |
0.43 ± 0.14 |
0.49 ± 0.11 |
7.12 ± 2.18 |
0.68 ± 0.12 |
LPS |
2..80 ± 0.31 |
0.79 ± 0.15 |
2.38 ± 0.42 ** |
3.5 ± 0.5 |
2.59 ± 0.33 ** |
LPS + CBNP95 |
8.60 ± 2.19 * # $ |
5.50 ± 1.87 * # $ |
5.33 ± 1.17 ** # $$ |
6.9 ± 2.14 # |
4.26 ± 1.07 ** # $$ |
Results are expressed as mean ± SE (n = 8 in each group). * P < 0.05 versus the vehicle group, ** P < 0.01 versus the vehicle group, # P < 0.05 versus the LPS group, ## P < 0.01 versus the LPS group, $ P < 0.01 versus the CBNP group.
Table 3. Protein levels of cytokines in the lung tissue supernatants after the final intratracheal challenge (protocol 2)
Group |
Eosinophils |
IL-2 |
IL-4 |
IL-5 |
IL-6 |
IL-13 |
IFN-γ |
(×104/BALF) |
(pg/total lung supernatants) |
vehicle |
0 ± 0 |
47.2 ± 4.2 |
233.1 ± 17.5 |
7.97 ± 1.2 |
107.2 ± 6.0 |
2.3 ± 0.8 |
4332.7 ± 218.7 |
CBNP95 |
0.09 ± 0.01 |
40.9 ± 3.7 |
224.7 ± 14.7 |
12.0 ± 1.8 |
134.1 ± 7.9 |
0.6 ± 0.2 |
4333.2 ± 242.1 |
OVA |
0.74 ± 0.11 * |
54.5 ± 7.6 |
254.8 ± 15.1 |
20.1 ± 17.1 |
130.6 ± 7.6 * |
15.7 ± 9.4 * |
4986.8 ± 239.4 |
OVA + CBNP95 |
7.94 ± 2.05 ** ## |
83.5 ± 12.1 * # |
267.5 ± 21.7 |
89.2 ± 31.6 * # |
225.7 ± 28.9 * # |
53.7 ± 15.0 ** ## |
4803.2 ± 135.3 |
Results are expressed as mean ± SE (n = 8 in each group). * P < 0.05 versus the vehicle group, ** P < 0.01 versus the vehicle group, # P < 0.05 versus the LPS group, ## P < 0.01 versus the LPS group, $ P < 0.01 versus the CBNP group.
Table 4. Protein levels of chemokines in the lung tissue supernatants after the final intratracheal challenge (protocol 2)
Group |
MIP-1α |
MCP-1 |
Eotaxin |
RANTES |
TARC |
(pg/total lung supernatants) |
vehicle |
15.3 ± 0.6 |
66.6 ± 5.0 |
64.7 ± 3.7 |
182.0 ± 24.2 |
22.8 ± 2.7 |
CBNP95 |
185.7 ± 24.8 ** |
80.4 ± 8.8 |
90.6 ± 11.9 |
213.7 ± 22.3 |
92.0 ± 5.7 * |
OVA |
23.6 ± 6.9 |
50.5 ± 11.2 |
131.6 ± 28.7 * |
153.0 ± 28.3 |
54.1 ± 13.6 |
OVA + CBNP95 |
180.7 ± 29.7 ** ## |
163.0 ± 14.7 * # |
867.3 ± 125.4 * # |
299.4 ± 43.6 * # |
170.1 ± 19.0 * # |
Results are expressed as mean ± SE (n = 8 in each group). * P < 0.05 versus the vehicle group, ** P < 0.01 versus the vehicle group, # P < 0.05 versus the OVA group, ## P < 0.01 versus the OVA group, $ P < 0.05 versus the CBNP group, $$ P < 0.01 versus the CBNP group.
Table 5. Levels of allergen-specific IgG (protocol 2)
Group |
IgG1 |
IgG2a |
(titer) |
vehicle |
1148.4 ± 280.0 |
20.3 ± 9.6 |
CBNP95 |
3716.4 ± 1997.9 |
13.7 ± 7.7 |
OVA |
2048.0 ± 455.8 |
17.0 ± 9.2 |
OVA + CBNP95 |
17835.5 ± 9253.0 * # |
6.6 ± 2.9 * |
Results are expressed as mean ± SE (n = 8 in each group). * P < 0.05 versus the vehicle group, ** P < 0.01 versus the vehicle group, # P < 0.05 versus the OVA group, ## P < 0.01 versus the OVA group, $ P < 0.05 versus the CBNP group, $$ P < 0.01 versus the CBNP group.



