Abstract
The first study evaluated the effect of repeated use of single cervical tuberculin test (SCTT) and gamma-interferon (IFN-γ) test at different time intervals in infected cattle. The hypothesis was that the IFN-γ test could be repeatedly used in desensitized cattle for bovine tuberculosis diagnosis without affecting its sensitivity. 32 dairy cattle positive to cervical comparative tuberculin test (CCTT) were selected from an infected herd and randomly divided into 4 groups (n=8). All animals were tested with the in-vitro test on day 0, then a second tuberculin test and IFN-γ test was performed at different time intervals according to the group, at 15, 30, 45 and 60 days post-CCTT. They all were submitted to post-mortem inspection, histopathological analysis and Mycobacterium bovis culture. In comparison with the first tuberculin test, the mean skin thickness decreased significantly when the SCTT was performed at 15 and 30 days, with presentation of false negative results even after 45 days. The sensitivity of the IFN-γ test applied repeatedly in these desensitized animals was not affected. The second investigation evaluated the specificity of the caudal fold test (CFT) and SCTT used repeatedly in non-infected cattle. The hypothesis was that this repeated used generate a sensitization to tuberculin, with the presentation of false positive results. 100 beef cattle were classified into two groups (n=50). One group received 6 rounds of CFT and the other 6 of SCTT, with 60-day intervals between tests. The IFN-γ test was performed in all the animals on day 0 obtaining a specificity of 97%. Regarding the SCTT, a significant increase (0.48mm, p=0.0034) in the mean skin thickness was observed after six rounds of the test compared to the first test, but no positive animal was observed. All the animals remained negative to CFT during the course of this experiment, without affecting its specificity.
Key words
bovine, desensitized, diagnosis, Mycobacterium bovis, sensitization, tuberculosis
Introduction
The single intradermal tuberculin test is the screening method used in the control and eradication programs of Bovine Tuberculosis (BTB) in many countries, as it is in Uruguay. Recognized by the World Organization for Animal Health (OIE) as a reference technique for the international trade of animals. However, repeated and short-term use of these in-vivo tests directly influences their sensitivity. After using the intradermal tuberculin test on an infected animal with Mycobacterium bovis, the ability to respond to another inoculation of the purified protein derivative (PPD) decreases substantially, because the animal goes through a stage of "desensitization" to the PPD [1]. This effect has been demonstrated at 4 and 7 days post-inoculation of PPD in experimentally infected cattle [2] and naturally infected cattle [3] and lasts up to 60 days. Some authors suggest that this period may last more time [4-6]. This desensitization demonstrated after performing the caudal fold test (CFT) and comparative cervical tuberculin test (CCTT), can result in failures on the detection of infected animals, compromising the success of health programs. No scientific studies have been found that evaluate this desensitization effect using the single cervical tuberculin test (SCTT), considered by some authors as the most sensitive tuberculin test [7,8].
It is generally accepted that tuberculin tests, individually used, are not effective in eradicating BTB [9], due to a lack of sensitivity and specificity reported, with evidence of cross-reactions in the presence of other environmental mycobacteria [9,10,11]. Given the need of an alternative diagnostic test, [12] developed in the late 1980s, in Australia, the gamma-interferon test (IFN-γ test). Currently, it is an ancillary test approved by the OIE for use in ante-mortem diagnosis [13], incorporated in the health programs of countries such as Australia [14], USA [15] and European countries [16]. This in-vitro test has some advantages, such as the greater specificity compared to the single tuberculin tests [13,16]. Also, the use of specific antigens, like ESAT-6, CFP-10 [17-19], and more recently Mb2845c [20], are capable of reduce the presentation of cross-reactions. On the other hand, the phenomenon of desensitization does not occur because it is an in-vitro test, what allows repetitions of the test without the need of waiting any period [12,17,21]. Moreover, this test can be used in combination with tuberculin tests and applying some interpretation strategies allows improving the detection of the infection [6,11,19,22-26].
In Uruguay, the BTB is present for more than 100 years, first diagnosed and documented in 1888 [27]. With the aim of reducing the prevalence of the disease until its eradication, there is a national health program with joint efforts of the public and private sectors. The CFT used during field epidemiological surveillance detect infected animal and the CCTT performed by official veterinarians confirm de infection. Occasionally, this strategy has not been able to lead to a quick sanitation of some infected dairy herds, resulting in the need to study the incorporation of new ancillary techniques to advance towards the eradication of the disease in the country. One of the aims of the present study was to observe how the sensitivity of the SCTT and the IFN-γ test are affected when they are used repeatedly with short time intervals.
Another situation that caught the attention of the health authorities was that the proportion of positive animals to tuberculin tests with visible lesions, during post-mortem inspection, has oscillated between 40 and 60%. Moreover, in some farms that have repeatedly applied the SCTT, the number decreased to values below 10%. The sensitivity of the detection of macroscopic lesions in slaughter is very low [13,28], and the time elapsed since a bovine becomes infected until it is detected in slaughter, is very long [15]. Considering this, one possible explanation is that some animals were identified and eliminated in early stages of the disease, without developing lesions. If this were the situation, it would have a positive impact on sanitation and an important drop in the prevalence of reactors, which has not happened in all farms with infected animals. Another hypothesis, which was considered in the present experiment, is that the repeated use of tuberculin tests in non-infected animals has determined the presentation of false positive results, with the consequent drop in the specificity of the diagnosis. Little is known about the repeated use of these tests on cattle that are BTB-free. Is possible that the constant exposure of the animal's immune system to the PPD stimulates the differentiation of T lymphocytes to Th1 lymphocytes with antigen-specific memory, determining a state of "sensitization" of the animals to the PPD with the consequent presentation of nonspecific reactions.
The elimination of dairy cattle of high production originates an important cost for the farmers, due to the productive and genetic losses. Also due to their contributions to the indemnity funds, as well as to the public funds for the operating costs of the health campaign. Therefore, the other aim of the present investigation was to evaluate the effect of the repeated use of the single tuberculin tests (CFT and SCTT) over its specificity.
Materials and methods
Animal selection
Effect on the sensitivity of SCTT and IFN-γ test used repeatedly in infected cattle: We started from a population of BTB-positive cattle (positive for CFT and CCTT, with a serial interpretation), identified through routine procedures of epidemiological surveillance in dairy farms, according to official diagnostic protocols of the Ministry of Livestock, Agriculture and Fisheries of Uruguay (MGAP). These animals came from quarantined dairy farms with isolation of M. bovis from the departments of Rocha and Florida, Uruguay.
32 adult female cattle (older than 2 years), Holando and Jersey breed, were randomly selected. In order not to affect the results of the evaluated tests, we selected non-pregnant females, with no record of recent birth and without treatment with corticosteroids. This selection criterion, also applied for the non-infected cattle, was because in the literature is mentioned that these factors are able to affect the intradermal reaction to PPD, increasing the probability of presenting false negatives results [1,19,28,29].
The animals were randomly classified into 4 groups of 8 members each. During the months of February and September of 2015, the SCTT and IFN-γ test were performed repeatedly at different time intervals after confirmation with CCTT, as shown in figure 1.

Figure 1. Protocol scheme applied in field tests to evaluate the sensitivity of SCTT and IFN-γ test in bovine tuberculin desensitized cattle.
Protocol scheme applied in field tests to evaluate the sensitivity of SCTT and IFN-γ test in bovine tuberculin desensitized cattle.
Day 0 was the PPD inoculation day of the CCTT. To carry out the IFN-γ test, blood samples were collected from all animals within 2 days after reading the CCTT (3 to 5 days after PPD inoculation).
The animals of group 1 received the SCTT and de IFN-γ test 15 days after PPD inoculation, group 2 after 30 days, group 3 after 45 days and those of group 4 after 60 days. The collection of the blood samples was the same day of the SCTT, prior to PPD inoculation.
All the animals in this part of the study were slaughtered and subjected to a post-mortem inspection, looking for macroscopic lesions compatible with BTB. Lymph node samples were taken for histopathological diagnosis and M. bovis culture.
Effect on the specificity of CFT and SCTT used repeatedly in non-infected cattle: 100 adult female cattle (older than 2 years), without history of BTB, were selected from an experimental farm in the department of Tacuarembó, property of the Ministry of Livestock, Agriculture and Fisheries (MGAP). The animals were randomly classified into two groups of 50 animals. Between December 2015 and November 2016, the CFT and SCTT were performed repeatedly with intervals of 60 days. Each animal received six bovine PPD (PPDb) inoculations as shown in figure 2.
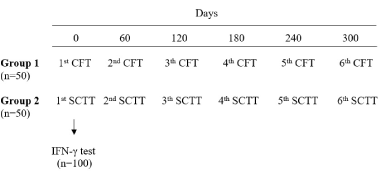
Figure 2. Protocol scheme applied in field tests to evaluate the specificity of caudal fold test and single cervical tuberculin test used repeatedly in non-infected cattle.
Protocol scheme applied in field tests to evaluate the specificity of caudal fold test and single cervical tuberculin test used repeatedly in non-infected cattle.
On day 0, prior to the inoculation of PPDb, blood samples were taken from all the animals to perform the IFN-γ test at the official laboratory of the Division of Veterinary Laboratories (DILAVE, Miguel C. Rubino – MGAP).
Caudal Fold Test (CFT)
This test was carried out following the national guidelines and the recommendations of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals [30]. Cattle of group 1 (n=50) were skin tested by intradermal injection of 0.1 ml of PPDb in the caudal fold, approximately 6cm from the base of the tail. Prior to PPD inoculation the area was cleaned and disinfected with alcohol, verifying that this area has no wounds or scars on the skin that may affect the results. The PPD used in this test, as well as in the SCTT, was elaborated by the official laboratory of the DILAVE Miguel C. Rubino, from the M. bovis standard strain, AN5 of Rotterdam.
Changes in skin thickness at the injection sites were recorded 3 days later (72 ± 6 hours), examined by visual inspection and palpation. The animal that presented any change at the inoculation site was considered as positive to BTB.
Single Cervical Tuberculin Test (SCTT)
Cattle were skin tested using SCTT by intradermal injection of 0.1 ml of bovine PPD in the middle neck region. Prior to PPD inoculation, the hair of the area was shaved (5 cm diameter) and disinfected with alcohol, to then measure and record the skin thickness.
Changes in skin thickness at the injection sites were recorded 3 days later (72 ± 6 hours), examined by inspection, palpation and measurement of the skin thickness. The interpretation applied was according to the criteria described by the World Organization for Animal Health (OIE) manual, BTB-positive = increase in skin thickness greater than or equal to 4 mm.
Interferon-gamma test (IFN-γ test)
This test was carried out following the instructions of use recommended by the laboratory that developed this kit (Prionics AG – Zurich, Sweden). Blood samples were collected with anticoagulant (lithium heparin) from the coccygeal vein. The blood was kept at room temperature (22 ± 5ºC) during transport to the laboratory. The time elapsed from the collection of the sample until the arrival to the laboratory to be processed did not exceed 6 hours.
In a first stage of the test, 250μl of each blood sample was added to a 96-well culture plate. 25μl of the Nil control antigen, bovine PPD, avian PPD (PPDa) and pokeweed mitogen were added to the wells containing the blood samples. The culture plates were incubated at 37°C in a humidified environment for 16-24 hours. After this period, the plates were centrifuged to harvest the plasma of each sample.
In a second stage, an immune enzymatic assay was carried out with the commercial kit of IFN-γ EIA, complying with the manufacturer's specifications. The pokeweed mitogen allowed the validation of the results since it evaluated the viability of the T cells. The criteria applied for the results interpretation was the following: (1) Positive=PPDb Optical Density (OD) – Nil OD ≥ 0.1 and PPDb OD – PPDa OD ≥ 0.1; (2) Negative=PPDb OD – Nil OD < 0.1 or PPDb OD – PPDa OD < 0.1.
Post-mortem inspection
Post-mortem examination was carried out following the protocols established by the Decree 369/983. The tissues selected were those most affected by M. bovis according to the literature [31-36]. Lungs, liver, pleura and lymph nodes of the head (retropharyngeal, submandibular, and parotid), thorax (mediastinal and four bronchus-associated) and mesenteric lymph nodes were examined for gross lesions by visual inspection and cuts.
Lymph node samples, with and without lesions, were sent to the official laboratory of the Veterinary Laboratories Division (DILAVE Miguel C. Rubino), in an isothermal box with refrigerants to maintain the right conditions to be processed.
Histopathological diagnosis
Lymph node samples were remitted to the histopathology section of the DILAVE Miguel C. Rubino, kept in 10% buffered formalin for one week. Subsequently, they were checked with special care so that the observed lesions were contemplated in them. The samples were included in paraffin and then cuts of 4 microns were made. Finally, they were stained with Hematoxylin and Eosin to be observed with microscope for evidence of BTB.
Mycobacterium bovis culture and identification
Specialized professionals at the Tuberculosis Laboratory of the DILAVE Miguel C. Rubino performed this confirmatory test. After sample decontamination with 5% oxalic acid and macerated by mechanical means, the obtained sediment was seeded in conventional selective culture media (Löwenstein-Jensen y Stonebrink), following the recommendation of the Pan American Zoonosis Center, PAHO / WHO. The sowings were incubated for eight weeks at 37ºC, performing a weekly reading for the detection of typical M. bovis colonies.
Statistical analysis
For the study of the sensitivity of SCTT and IFN-γ tests, an analysis of covariance was applied to observe how the intradermal reaction is affected by the repeated use of the tuberculin test. The skin reaction to day 0 and the groups defined according to the days for the second PPD inoculation were included as a continuous covariate. For the categorical analysis of the variables for both the tuberculin and the IFN-γ tests, an exact Fisher test was run in order to identify if there is an association between the time groups and the behavior of the diagnostic tests.
In the second study about the specificity of CFT and SCTT, to observe how the intradermal reaction was affected by the repeated use of the tuberculin tests (CFT and SCTT), an ANOVA test for repeated measures was applied in order to detect significant differences between the number of repetitions. A simple linear regression test was applied to confirm the existence of a significant relationship between the increase in repetitions of the tests and the intradermal reaction to PPDb.
Ethic approval
This study was carried out in accordance with the national guidelines of the law Nº 18.611 and was approved by the Ethics Commission for the Use of Experimental Animals, Veterinary School, University of the Republic, Uruguay.
Result
Effect on the sensitivity of SCTT and IFN-γ test used repeatedly in infected cattle
Sensitivity of SCTT performed at different intervals within 60 days after the inoculation of PPD: It was found that the repetition of the tuberculin test in short periods significantly affects the response to PPDb (p ≤ 0.01). As we move away from the first PPD inoculation day the intradermal reaction increases as shown in figure 3.
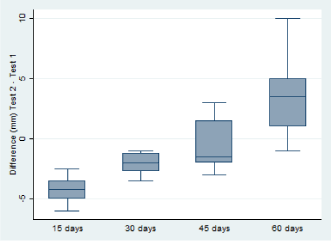
Figure 3. Influence of a second inoculation of PPDb at short intervals on the intensity of intradermal response in naturally infected cattle.
Comparison of the intensity of the intradermal reaction (mm) to the PPDb between the second tuberculin test and the first, according to the interval applied between tests. A significant reduction (p ≤ 0.01) in the mean skin thickness values was observed when tuberculin test was repeated within 15, 30 days, compared to the response obtained in the first test, with a skin thickness decrease of 4.25 mm and 2.06 mm, respectively.
Influence of a second inoculation of PPDb at short intervals on the intensity of intradermal response in naturally infected cattle.
This reduction in the intensity determined that some animals initially classified as positive, were negative in the second test. Only 12.5%, 37.5% and 87.5% were positive when the SCTT was reused at 15, 30 and 45 days, respectively. It was observed with a statistical significance (p ≤ 0.01) that the diagnosis of BTB was negatively affected by the time elapsed between the first and the second test. The shorter the time between the two tests, the lower was the ability of the test to re-detect infected animals.
Based on the results of the culture, BTB confirmed in 21 animals, it was observed that a second inoculation of PPDb at 15 days determined the appearance of 7 false negative results, at 30 days a total of 2 false negatives and at 45 days only one negative animal was truly infected.
First IFN-γ test: The cattle in this study were selected because they were CFT and CCTT-positive. Blood samples were taken after reading the CCTT (3-5 days post-inoculation of PPD) to perform the IFN-γ test. Table 1 shows the results obtained with this in-vitro technique.
Table 1. Results of the first IFN-? test and post-mortem diagnosis in positive cattle (n=32) to the comparative cervical tuberculin test.
| |
|
Culture |
Histopathology |
Lesion (slaughter) |
| |
|
(+) |
(-) |
(+) |
(-) |
(+) |
(-) |
IFN-? a |
(+) |
20 |
4 |
18 |
6 |
19 |
5 |
|
(-) |
1 |
7 |
1 |
7 |
4b |
4 |
a Cut-off point: Positive = OD PPDb - OD PPDa ≥ 0.1 and OD PPDb - OD Nil ≥ 0.1.
b This 4 IFN-? negative animals with post-mortem lesions were diagnosed by histopathology as nonspecific abscesses and were considered in the investigation as BTB-negative.
24 animals were positive for the IFN-γ test. Based on the post-mortem diagnostic tests, it was found that 9 CFT and CCTT-positive animals (28.1%) did not present visible lesions compatible with BTB.
Second IFN-γ test performed in different periods within 60 days after the tuberculin test: A second blood sample was collected from each animal for the in-vitro measurement of IFN-γ at 4 different times according to the group of animals (n = 8): 15 days; 30 days; 45 days and 60 days post-inoculation of PPD.
The release of IFN-γ in blood stimulated with PPDb and PPDa was compared between groups. A greater release of IFN-γ was registered when the test was repeated at 30 days post-CCTT (OD PPDb = 2.806 ± 1.087 and OD PPDa = 2.203 ± 1.352). However, this difference was not statistically significant. There were also no significant differences in the release of IFN-γ comparing the first and second tests. It should be noted that the mean IFN-γ in all the groups was maintained at a DO value PPDb - DO PPDa > 0.1, along the different sampling points.
2021 Copyright OAT. All rights reserv
Unlike the SCTT, the repetition of the in-vitro test in the different time intervals evaluated did not decrease its ability to detect positive animals, as shown in figure 4.
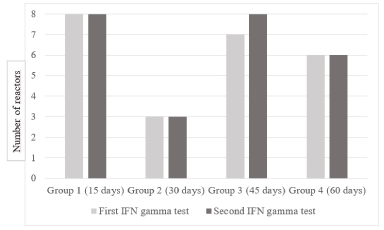
Figure 4. Comparison of the ability to detect infected animals between the first and second IFN-γ test.
The number of reactors to the second test was equal to or greater than the first, determining that the ability to recognize the infection did not decrease when the test was repeated at the different time intervals evaluated. Only one animal, truly infected, reacted differently in both tests, going from negative to positive when the test was repeated after 45 days.
(Figure 4). Comparison of the ability to detect infected animals between the first and second IFN-γ test.
Effect on the specificity of CFT and SCTT used repeatedly in non-infected cattle
IFN-γ test specificity: As recommended to assess specificity in healthy animals [36], using the most specific cut-off point (positive animal = PPDb OD – PPDa OD ≥ 0.1 and PPDb OD – Nil OD ≥ 0.1), three positive animals were detected. Considering that they were animals without history of BTB, the specificity recorded for this test was 97%.
Single Cervical Tuberculin Test specificity: Using as cut-off point an increase in skin thickness equal or greater than 4mm, all animals remained BTB-negative during the course of this experiment. With the ANOVA test for repeated measurements, significant differences were found in the mean of skin thickness between the number of repetitions of the SCTT (p<0.0005). Applying the linear regression test, a significant increase in skin thickness was detected in the animals only after receiving 6 PPD inoculations, with a positive association. Registering 0.48mm more (IC 95%, 0.2396407 a 0.7203593, p<0.0005) compared to the mean skin thickness recorded in the first round of the test, as shown in Figure 5.
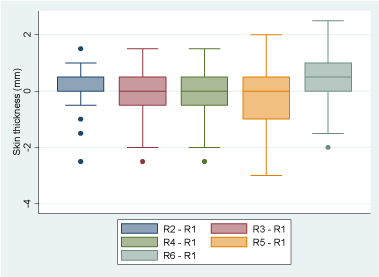
Figure 5. Influence of the repeated use of the single cervical tuberculin test, on the increase in skin thickness in non-infected cattle.
Variation of the mean of skin thickness as the number of PPD inoculations increases, compared to the reaction to one PPD (first SCTT). Skin thickness increased significantly after 6 repetitions of the SCTT.
Influence of the repeated use of the single cervical tuberculin test, on the increase in skin thickness in non-infected cattle.
Caudal Fold Test specificity: As with SCTT, the animals received 6 inoculations of PPDb in the caudal fold, with an interval of 60 days between tests. All the animals studied remained negative to the CFT during the course of the successive repetitions.
Discussion
Effect on the sensitivity of SCTT and IFN-γ test used repeatedly in infected cattle
In the present study, a scenario based on the reality of epidemiological field surveillance in Uruguay was used, starting from a population of dairy cattle positive to the presumptive test (CFT) and to the confirmatory test (CCTT). The performance of the IFN-γ test was evaluated on these animals when it was used within a period in which tuberculin tests are not recommended to detect the infection (desensitization period). Seeking in this way to verify beneficial characteristics to use this in-vitro technique as an alternative for the sanitation an infected farm in the country.
In these CFT and CCTT-positive cattle, it was found that some of them did not present visible lesions post-mortem (9/32) and, in an even greater proportion, it was not possible to isolate M. bovis (11/32). It is generally accepted that the sensitivity of detection of gross lesions in slaughter is very low, at around 28.5% [13]. This is due, on one hand, to the fact that the primary complex can often go unnoticed by the inspection if it is not detailed [34]. On the other hand, the time elapsed since a bovine becomes infected until it is detected in slaughter, is very long [15]. Such is the case that scientific studies have recorded high percentages of positive tuberculin animals with no visible lesions post-mortem, between 44.5% [33] and 75.5% [35]. The CCTT has high specificity values [16,37] and combined with CFT, with serial interpretation, it is possible to minimize the proportion of false positive animals [8]. Considering this, it is expected that in the current experiment the tuberculin tests have been able to detect the infection early, prior to the development of lesions, decreasing the probability of isolating the agent.
Regarding the repeated use of tuberculin tests in short periods, it has already been described the effect of desensitization to PPD that occurs within 60 days after a first inoculation. The first to detect this phenomenon were the researchers [2], who showed that animals experimentally infected with M. bovis and CFT-positive, developed a lower intradermal reaction compared to a second test (CCTT) performed at 4 and 7 days later. When these animals were retested after 60 days, in most of them this negative effect disappeared, returning to the original sensitivity. A decade later, [3] again demonstrate the desensitization effect, in this case on naturally infected cattle, CFT-positive. They found that the practice of repeating the CCTT with a 7-day interval decreased the response of the animals to the PPDb and PPDa compared to the second test, significantly reducing the sensitivity of the test. These findings suggest that repeated use of tuberculin tests in short periods would reduce their ability to detect effectively M. bovis infection. Although sensitivity is recovered after 2 months [2,38], some researchers suggest that this period may be somewhat longer, both for the use of the CFT [5], as for the CCTT [6].
The results of the current experiment demonstrated a significant effect of desensitization to the PPDb when the tuberculin test was repeated within 30 days, somewhat shorter than that recorded in previous studies. This may be because the period between day 10 and 60 post-inoculation of PPD has not been evaluated. On the other hand, in the present study it was used the single cervical tuberculin test, which has a higher sensitivity compared to the other tuberculin tests [7,8], that may explain why the desensitization effect lasted less.
The IFN-γ test has been widely studied by the scientific community over the years, demonstrating that it is a good tool for health programs since it has a high sensitivity, equal to or greater than the single tuberculin tests [13,16]. It also can be used in combination with tuberculin tests with a high overall performance [19,22,26]. In the present investigation, this in-vitro test was not able to recognize all the CCTT-positive cattle (24/32). The high values of IFN-γ in blood stimulated with PPDa of some negative animals could be due to a co-infection of M. bovis with other environmental mycobacteria, including the agent of Bovine Paratuberculosis (Mycobacterium avium subsp. paratuberculosis) [16], decreasing the sensitivity of the IFN-γ test. This may explain the finding where a truly infected animal was incorrectly classified as negative. Because of this, for future research it will be necessary the use of specific antigens that have already been shown to be able to differentiate animals infected with M. bovis, as in the case of ESAT-6; CFP-10 [18,39] and Mb2845c [20].
A finding of relevance was the absence of significant differences in the release of IFN-γ between repetitions of the test, what determined that the sensitivity of the test remained unaffected, or even improved, in the different time intervals evaluated. This confirm the repeatability property of the test already described [3,17,21,40] and highlights its ability as an ancillary test for BTB diagnosis.
Effect on the specificity of CFT and SCTT used repeatedly in non-infected cattle
One of the aims of this study was to obtain information on how the IFN-γ test behaves in non-infected cattle and evaluate its applicability as a complementary test to incorporate into the health program of Uruguay. [11], in an extensive investigation evaluated and compared the specificity of the CFT and the IFN-γ test in more than 6000 cattle from herds without history of BTB. Using different cut-off points, they found a specificity between 96.2 and 98.1%, with a proportion of false positive results between 1.9 and 3.8%. [23], using the same cut-off point that was applied in the current investigation found a specificity of 93%. The specificity observed in the present study (97%), despite having evaluated a smaller number of animals, was similar to the report of these researchers. However, the tuberculin tests had a higher performance in terms of this parameter. Unexpected result according to international reports that described that the IFN-γ test is more specific than the single tuberculin tests [13,16,19,22,40]. Despite of this finding, to confirm the specificity values of the current investigation it would be appropriate to carry out confirmatory tests, which were not possible due to the cost involved.
There are scientific investigations in different countries that evaluated the specificity of SCTT and CFT for the diagnosis of BTB. For the SCTT the registered specificity values are between 75 and 99%. While for the CFT, the values described are between 89.2 and 98.8% [1,8,16,37]. The current results indicate an ideal specificity using any of the tuberculin tests evaluated.
The successive inoculation of PPD implies a constantly exposure of the animal’s immune system to this antigenic molecule. This could generate a differentiation of T lymphocytes to Th1 lymphocytes with antigen-specific memory and, consequently, the presentation of false positive results with a drop in the specificity of the test. Most scientific research that evaluate the effect of repeated use of these tests have focused on infected animals [2,3,5,6]. In one investigation developed by [4] it was used the tuberculin test repeatedly on non-infected calves, without observing significant differences in the increase of the skin thickness after five inoculations of PPD.
In the present study, the significant increase in skin thickness registered after six rounds of the SCTT could indicate a possible stage of sensitization of the animals to PPD. On the other hand, because no positive results were detected, it can be concluded that the specificity of the SCTT was not affected by the repeated use of the test. However, is possible that with more repetitions of the test this parameter can decrease in consequence.
As for the CFT, is generally accepted that has a higher specificity and lower sensitivity than the SCTT [7,8,10,13]. Therefore, it was expected that the animals treated with CFT would not be sensitized to PPD.
Conclusion
The sensitivity of SCTT decreased when used in naturally infected animals between 15 and 45 days after inoculation of PPD. The repeated use of the IFN-γ test on animals desensitized to PPD did not affect his ability to detect the infection. Based on this experiment, we suggested to considerate the use of this in-vitro test as an ancillary technique in the health program for the control and eradication of bovine tuberculosis in Uruguay. A possible strategy of use can be in combination with the CCTT for de sanitation of infected herds. This would improve the ability to detect infected animals, increasing the global sensitivity of the diagnosis and possibly reducing the sanitation period. Another possible use may be during the purchase of cattle. In order to confirm the presumptive diagnosis, it could be applied prior to the entry of new animals to the herd without the need to wait for that desensitization period of 60 days.
As for the study with BTB-free animals, the CFT, traditionally used in Uruguay for the diagnosis, can be performed repeatedly in non-infected cattle with intervals of at least 60 days, without affecting the specificity of the test. Regarding the SCTT, although a decrease in its specificity was not demonstrated, the significant increase in the skin thickness detected in the last round suggests the need to study this protocol over a longer period, complementing with techniques that study the possible stimulus on the immune response, such as the IFN-γ test.
Acknowledgment
We would like to thank the Veterinary Laboratories Division, Miguel C. Rubino, of the Ministry of Livestock, Agriculture and Fisheries of Uruguay (MGAP), where the laboratory tests were performed and to the technicians who collaborated in the realization of the different diagnostic techniques. Thanks, to all the Veterinarians and employees of the Division of Animal Health (DSA-MGAP), who were involved in the field tests and blood samples collection.
References
- Monaghan ML, Doherty ML, Collins JD, Kazda JF, Quinn PJ (1994) The tuberculin test. Vet Microbiol 40: 111-124.
- Radunz B, and Lepper A (1985) Suppression of skin reactivity to bovine tuberculin in repeat tests. Aust Vet J 62: 191-194. [Crossref]
- Doherty ML, Monaghan ML, Basset HF, Quinn PJ (1995) Effect of a recent injection of purified protein derivative on diagnostic tests of tuberculosis in cattle infected with Mycobacterium bovis. Res Vet Sci 58: 211-217. [Crossref]
- Thom M, Morgan IH, Hope JC, Villarreal-Ramos B, Martin M, et al. (2004) The effect of repeated tuberculin skin testing of cattle on immune responses and disease following experimental infection with Mycobacterium bovis. Vet Immunol Immunopathol 102: 399-412. [Crossref]
- Schneider M, Magnano G, Bergamo E, Urbani C, Herrera P, et al. (2007) Repetición de la prueba de intradermorreacción tuberculínica en bovinos naturalmente infectados y modificaciones del pliegue anocaudal. Sitio Argentino de producción animal In Vet 9: 27-33.
- Coad M, Clifford D, Rhodes SG, Hewinson RG, Vordermeier HM, et al. (2010) Repeat tuberculin skin testing leads to desensitisation in naturally infected tuberculous cattle which is associated with elevated interleukin-10 and decreased interleukin-1 beta responses. Vet Res 41:14. [Crossref]
- Errico F, de Kantor IN, Baltar J, Silva M, Millán A (1989) Comparación de la especificidad de las pruebas tuberculínicas cervical y caudal en bovinos de Uruguay. Rev sci tech Off int Epiz 8: 1021-1029.
- Farnham MW, Norby B, Goldsmith TJ, Wells SJ (2012) Meta-analysis of field studies on bovine tuberculosis skin tests in United States cattle herds. Prev Vet Med 103: 234-242. [Crossref]
- Schiller I, Waters WR, Vordermeier HM, Jemmi T, Welsh M, et al. (2011) Bovine tuberculosis in Europe from the perspective of an officially tuberculosis free country: Trade, surveillance and diagnostics. Vet Microbiol 151: 153-159. [Crossref]
- Francis J, Seiler RS, Wilkie IW, O’Boyle D, Lumsden MJ, et al. (1978) The sensitivity and specificity of various tuberculin tests using bovine PPD and other tuberculins. Vet Rec 103: 420-425. [Crossref]
- Wood PR, Corner LA, Rothel JS, Baldock C, Jones SL, et al. (1991) Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust Vet J 68: 286-290. [Crossref]
- Wood PR, Corner LA, Plackett P (1990) Development of a simple, rapid in vitro cellular assay for bovine tuberculosis based on the production of gamma interferon. Res Vet Sci 49: 46-49. [Crossref]
- Schiller I, Oesch B, Vordermeier HM, Palmer MV, Harris BN, et al. (2010) Bovine Tuberculosis: A Review of Current and Emerging Diagnostic Techniques in View of their Relevance for Disease Control and Eradication. Transbound Emerg Dis 57: 205-220. [Crossref]
- Wood PR, Rothel JS (1994) In vitro immunodiagnostic assay for bovine tuberculosis. Vet Microbiol 40: 125-135. [Crossref]
- Wells SJ, VanderWaal KL, Picasso C, Ribeiro Lima J, Craft M, et al. (2015) Epidemiología y sistemas de vigilancia basados en riesgo para la detección de Tuberculosis Bovina. Disertación. Paysandú, Uruguay: XLIII Jornadas Uruguayas Buiatría “Dr. Recaredo Ugarte”.
- de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, et al. (2006) Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, gamma-interferon assay and other ancillary diagnostic techniques. Res Vet Sci 81: 190-210. [Crossref]
- Wood PR, Jones SL (2001) BOVIGAMTM: an in vitro cellular diagnostic test for bovine tuberculosis. Tuberculosis 81: 147-155. [Crossref]
- Vordermeier HM, Chambers MA, Cockle PJ, Whelan AO, Simmons J, et al. (2002) Correlation of ESAT-6-Specific Gamma Interferon Production with Pathology in Cattle following Mycobacterium bovis BCG Vaccination against Experimental Bovine Tuberculosis. Infect Immun 70: 3026-3032. [Crossref]
- Gormley E, Doyle MB, Fitzsimons T, McGill K, Collins JD (2006) Diagnosis of Mycobacterium bovis infection in cattle by use of the gamma-interferon (Bovigam) assey. Vet Microbiol 25: 171-179. [Crossref]
- Eirin ME, Macias A, Magnano G, Morcella C, Mendez L, et al. (2015) Identification and evaluation of new Mycobacterium bovis antigens in the in vitro interferon gamma release assay for bovine tuberculosis diagnosis. Tuberculosis 95: 795-801. [Crossref]
- Gormley E, Doyle MB, McGill K, Costello E, Good M, et al. (2004) The effect of the tuberculin test and the consequences of a delay in blood culture on the sensitivity of a gamma-interferon assay for the detection of Mycobacterium bovis infection in cattle. Vet Immunol Immunopathol 102: 413-420. [Crossref]
- González Llamazares OR, Gutiérrez Martín CB, Alvarez Nistal D, de la Puente Redondo VA, Domínguez Rodríguez L, et al. (1999) Field evaluation of the single intradermal cervical tuberculin test and the interferon-gamma assay for detection and eradication of bovine tuberculosis in Spain. Vet Microbiol 70: 55-66. [Crossref]
- Ryan TJ, Buddle BM, de Lisle GW (2000) An evaluation of the gamma interferon test for detecting bovine tuberculosis in cattle 8 to 28 days after tuberculin skin testing. Res Vet Sci 69: 57-61. [Crossref]
- Whipple DL, Palmer MV, Slaughter RE, Jones SL (2001) Comparison of purified protein derivatives and effect of skin testing on results of a commercial gamma interferon assay for diagnosis of tuberculosis in cattle. J Vet Diagn Invest 13: 117-122. [Crossref]
- Rangen SA, Surujballi OP, Lutze-Wallace C, Wayne Lees V (2009) Is the gamma interferon assay in cattle influenced by multiple tuberculin injections? Can Vet J 50: 270-274. [Crossref]
- Álvarez J, Perez A, Marqués S, Bezos J, Grau A, et al. (2014) Risk factors associated with negative in-vivo diagnostic results in bovine tuberculosis-infected cattle in Spain. BMC Vet Res 10:14. [Crossref]
- Magallanes N (1998) Tambos y Tuberculosis bovina en Uruguay (1834-1963). Montevideo, Uruguay: Academia Nacional de Veterinaria.
- Snider DE Jr (1982) The tuberculin skin test. Am Rev Respir Dis 125: 108-118. [Crossref]
- Doherty ML, Basset HF, Quinn PJ, Davis WC, Monaghan ML (1995) Effects of dexamethasone on cell-mediated immune responses in cattle sensitised to Mycobacterium bovis. Am J Vet Res 56: 1300-1306. [Crossref]
- Organización Internacional de Sanidad Animal (OIE) (2009) Manual de las Pruebas de Diagnóstico y de las Vacunas para los Animales Terrestres.
- Neill SD, Pollock JM, Bryson DB, Hanna J (1994) Pathogenesis of Mycobacterium bovis infection in cattle. Vet Microbiol 40: 41-52. [Crossref]
- Whipple DL, Bolin CA, Miller JM (1996) Distribution of lesions in cattle infected with Mycobacterium bovis. J Vet Diagn Invest 8: 351-54. [Crossref]
- Liébana E, Johnson L, Gough J, Durr P, Jahans K, et al. (2008) Pathology of naturally occurring bovine tuberculosis in England and Wales. Vet J 176: 354-360. [Crossref]
- Canal AM (2013) Tuberculosis bovina: vigilancia epidemiológica en mataderos de la Provincia de Santa Fe (Argentina) y evaluación de la respuesta inmune en lesiones granulomatosas de animales infectados. Tesis Doctoral. Madrid, España: Faculta de Veterinaria de la Universidad Complutense de Madrid, Departamento de Medicina y Cirugía Animal.
- von Gehlen A (2015) Descripción de datos de ganado lechero enviado a faena sanitaria por Tuberculosis Bovina en el 2013 en Uruguay. Tesis de Grado. Montevideo, Uruguay: Facultad de Veterinaria. Universidad de la República.
- Casal C (2016) Diagnóstico de tuberculosis en rumiantes y camélidos: optimización de pruebas de base celular y humoral. Tesis Doctoral. Madrid, España: Facultad de Veterinaria de la Universidad Complutense de Madrid, Departamento de Sanidad Animal.
- Vordermeier HM, Whelan AO, Hewinson RG (2008) The scientific case for the gamma-interferon Bovigam assay. Gov Veterinary Journal. 19: 38-43.
- Thom ML, Hope JC, McAulay M, Villarreal-Ramos B, Coffey TJ, et al. (2006) The effect of tuberculin testing on the development of cell-mediated immune responses during Mycobacterium bovis infection. Vet Immunol Immunopathol 114: 25-36. [Crossref]
- Buddle BM, Ryan TJ, Pollock JM, Andersen P, de Lisle GW (2001) Use of ESAT-6 in the interferon-gamma test for diagnosis of bovine tuberculosis following skin testing. Vet Microbiol 80: 37-46. [Crossref]
- Wood P, Corner L, Rothel J, Ripper J, Fifis T, et al. (1992) A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet Microbiol 31: 71-79. [Crossref]





