Gastrin as the main hormone is responsible for stimulating gastric acid secretion. However, there are more and more indications that the increase in gastrin may promote the development of certain gastrointestinal cancers. The amidated gastrin peptides can act as growth factors in the tumor process, and their proliferative effect is at least partially dependent on COX-2 and prostaglandins. By affecting the proliferation and inhibition of apoptosis, they contribute to carcinogenesis.
The role of intermediates in the synthesis of gastrin was not known for a long time. They are therefore attributed the role of inactive peptides. Recent research has shown that non-amidated gastrin peptides do not belong to inactive precursors, suggesting that further investigation and further evaluation of the role of these particles is needed.
An attempt was made to evaluate whether or not amidated gastrin peptides could be targets for interaction between PPARγ and β-catenin nuclear receptors.
β-catenin, PPARγ-peroxisome proliferator-activated receptors, Gastrin non-amidated gastrin peptides
Gastrin (G-17) is secreted by gastric G cells and is responsible for the stimulation of gastric acid secretion [1]. In addition, many evidence suggests that amidated gastrin peptides can act as a growth factor in the tumor process. It has been proven that an increase in gastrin may significantly promote the development of certain gastrointestinal cancers [2]. Although expression of the gastrin-induced amine receptor (CCKBR) in human gastrointestinal cancer cells is controversial, there are publications indicating its temporal expression. The effects on
the CCKBR receptor are exerted only by the amidated forms of gastrin [3,4,5].
The gastrin precursor molecule is preprogastrin, which converts into progastrin. Progastrin undergoes further processing enzyme under the action of endo-and carboxypeptidases resulting in a synthesized gastrin by the glycine extended (G-Gly) [1]. Non-amidated forms of gastrin are active molecules represented by progastrin and
Gly-G. These molecules do not work through the CCKBR receptor, but through other receptors not according knowledge is not clear. Gly-G appears to be associated with ANX II (annexin II) it is not known whether directly involved in this interaction [6,7]. Increased expression of Gly-G was observed in numerous tumor biopsies and tumor cell lines. Research suggests that the differences in the levels of G-Gly may affect the proliferation and apoptosis [8,9]. This suggest that Gly-G can act as a growth factor for many cancer cells even - including pancreatic cancer [10].
Effect on proliferation, cell differentiation by regulation of gene transcription have also nuclear receptors PPAR-γ (Peroxisome proliferator-activated receptors) [11,12]. They can promote cell differentiation, have antiproliferative effects, and inhibit angiogenesis. All these processes lead to slow tumor progression [13,14]. There are also reports indicating that PPAR-γ activation is conducive to the development of colon cancer in APC in mice [9,15,16]. These results are in contrast to studies showing the activity antitumor of PPAR-y receptors. Moreover, the authors demonstrate that overexpression of β- catenin as a transcription co-factor increases PPAR-γ activity in colon cancer cells [17,18]. These results are consistent with other studies that show the relationship between β-catenin and nuclear receptor signaling [19,20].
The β-catenin protein is involved in the classic path of the WNT signaling pathway. WNT signal pathway participates in regulation of processes such as embryogenesis, differentiation, survival, cell proliferation and metabolism. So far the best known is the role of WNT proteins in the formation and development of cancer [21]. Changes in the activity of the WNT pathway and related disorders in the expression of genes regulated by this signalling pathway lead to many pathologies [22]. Mutation in the gene encoding β-catenin prevents its
proteasome degradation, leading to the accumulation of this protein in the cells. This in turn activates the transcription of genes responsible for proliferation and inhibits the expression of proapoptotic genes in cancer cells, contributing to the progression of the disease [23,24].
A more in-depth understanding of the mechanisms responsible for regulation of the WNT pathway creates new therapies for modulating the activity of the proteins of this signaling pathway.
Tissue culture
The study used a cell line PANC-1 (human pancreatic adenocarcinoma epithelial cell line) obtained from the European Collection of Animal Cell Cultures and OE-33 (human caucasian oesophageal carcinoma cell line) (Sigma Aldrich, Poland). Cell line PANC-1 were routinely in Roswell Park Memorial Institute (RPMI) 1640 culture medium (Sigma Aldrich, Poland) containing 10% heat-inactivated fetal bovine serum (FBS; Sigma, Poole, UK) and antibiotics (100 mg/l penicillin, 100 mg/l streptomycin) at 37ºC in 95% air, 5% CO2 and humidified atmosphere.
The OE-33 cell line was cultured in Dulbecco′s Modified Eagle′s Medium (DMEM) containing 4500 mg/l glucose, L-glutamine, antibiotics (100 mg/l penicillin, 100 mg/l streptomycin) and supplemented with 10% fetal bovine serum (FBS) (Sigma Aldrich, Poland) at 37ºC in 95% air, 5% CO2 and humidified atmosphere.
Reagents
The studies used the peptides human amidated gastrin-17 (G-17) (Sigma-Aldrich, USA), glycine-extended gastrin-17 (Gly-G17) (products produced by NeoMPS Strasbourg, France).
Tetrazolium-based growth assay (MTT)
Cell suspension (PANC-1, OE-33) was plated with 100 μl (1 x 104 cells per well) into 96 well tissue culture plates. Then incubated for 24 hours at 37°C and 5% CO2.
After overnight incubation at 37°C, 5% CO2 in all wells was replaced with serum free medium and incubated for 24 hours with 50 μl 5 x (10-8 to 10-5 M) gastrin (G17, heptadecapeptide, Sigma Aldrich, USA) and glycine-extended gastrin-17 (Gly-G17) (produced by NeoMPS Strasbourg, France). After 72 hours of incubation to each of the test wells, 50 μl of methyl thiazoyl tetrazolium (MTT) solution was added and the plate was incubated for 4 hours. The media was then removed and MTT was dissolved in 75 ul of DMSO per well. The optical density was measured at 550 nM.
Western Blot analysis
Protein extraction was performed using a columnar protein isolation kit - NucleoSpin® RNA/Protein (Macherey-Nagel, Germany). Protein concentration in the supernatant was measured using the spectrophotometer NanoDrop 2000 (Thermo Scientific). Total cellular protein (20-30 μg) was separated by electophoresis on a 12,5% sodium dodecyl sulfate-polyacrylamide gel and transferred onto Immobilon membrane (Millipore, Bedford, MA). After blocking, the membranes was incubated with the antibodies indicated as follows: PPAR-γ monoclonal antibody (Thermo Scientific) and rabbit anti-beta-catenin antibody (Sigma). Next membranes washed and were then probed with secondary antibody conjugated horseradish peroxidase (Sigma) for 30 minutes. In the same way actin controls were performed. Chemiluminescent visualisation and protein detection were carried out using the Amersham enhanced chemiluminescence kit (Amersham, Little Chalfont, United Kingdom). Relative protein quantification was carried out using the Chemi Genius2 Biolmaging System (Syngene, Cambridge, UK) in conjunction with the GeneTools image analysis software (Syngene).
Statistics
For evaluation of in vitro data non-parametric Mann-Whitney U test was used.
Growth effects of gastrin-17 and glycine-extended gastrin-17 on PANC-1 and OE-33 cells
Growth effects of G17 on PANC-1 and OE-33 cells were measured by MTT assay method. Results are mean±S.E.M and represent three experiments with four replicates. Exogenous gastrin-17 (G-17) administration increased the number of both PANC-1 and OE-33 cells in a dose dependent manner reaching maximum at a gastrin concentration of 10-6 to 10-5 [M] (p<0,01, non-parametric Mann-Whitney U test, significant indicated by) (Fig. 1 A, B). These results are in agreement with previous observations conducted by other investigators [1]. On the other hand, treatment of glycine-extended gastrin-17 (Gly-G17) cells increased the number of both cell types at a maximum concentration level of 10-5 [M] (p<0,01, nonparametric Mann-Whitney U test, significant indicated by) (Fig. 2 A, B). The study confirms the fact that gastrin and gastrin precursors are powerful trophic peptides that act pro-oncogenically and stimulate the growth of pancreatic cancer cells and esophageal cancer.
Investigation of the effect of gastrin and gastrin precursors on PPAR-γ receptors and β-catenin protein in PANC-1 and OE-33 cells
Western blot analysis revealed a 60 kDa protein corresponding to PPAR-γ. Under the influence of gastrin and Gly-gastrin, expression of PPAR-γ was observed to be significant only in the case of the synergistic action of both compounds. The increase in receptor expression clearly depended on the time of stimulation (Fig.1, C, E). The highest expression (p <0.001) was observed after 60 minutes of synergistic action of both compounds compared
to untreated control. Conducted studies have shown that the interaction of gastrin and its precursors (Gly-gastrin) has an effect on PPAR-γ activity (p <0.001 after 60 min). This effect was particularly evident in the PANC-1 cell line.
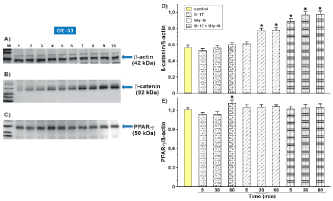
On the OE-33 cell line, however, only the positive effect of gastrin on PPAR-γ stimulation was observed. The effect of gastrin was dependent on the duration of action (p <0.001 after 60 min). No significant changes were observed in Gly-gastrin. Similar results were obtained by studying the synergistic effect of both compounds (Fig.2 C, E).
A completely different trend has been observed with β-catenin. The study demonstrated the stimulatory effect of gastrin on protein, which plays an important role in the WNT/β-catenin signaling pathway. Western blot analysis in the PANC-1 cell line showed that overexpresssion 92 kDa protein of β-catenin was observed after treatment only gastrin or Gly-gastrin. The positive effect of stimulation also appeared after the synergistic action of both compounds simultaneously. Reinforcement of the expression of the test protein was most evident after 60 minutes of stimulation (p <0.001) with gastrin alone. However, in the case of Gly-gastrin and synergism between the two compounds, the signal amplification effect was independent of stimulation time (p <0.001 after 5 min/30 min/60 min) (Fig.1 B, D).
Similar experiments carried out on the OE-33 cell line showed a clear effect of gastrin precursors and the synergistic effect of test compounds on β-catenin. Positive expression (p <0.001) was observed at 30 and 60 min. stimulation Gly-gastrin. At the same time, the effect of the compounds induced the same stimulation effect on the examined protein regardless of time (p <0.001 after 5 min/30 min/60 min) (Fig2. B, D).
Observations show an important role of gastrin and its precursors in regulation of WNT/β-catenin activity. At the same time, the synergism of the two compounds has a significant effect on the tested protein. The positive effect of stimulation on both cell lines was not time-dependent. Furthermore, based on the experience, it can be said that the increase in β-catenin expression may affect the signaling of the PPAR-γ nuclear receptor, but not necessarily in all cell lines.
In the case of OE-33 line, it was observed that the induction of β-catenin protein inactivates the activity of the PPAR-γ receptor. Glycine-extended gastrin action and synergism of gastrin and Gly-gastrin action induced up β-catenin. At the same time, no PPAR-γ expression was observed (Figure.2). In the PANC-1 cell line only the synergism of gastrin and glycine-extended gastrin activity induced upward β-catenin with the simultaneous increase in PPAR-γ expression. This effect was observed during 30 and 60 minutes of cell stimulation (Figure.1). The obtained results confirm the thesis that in most tumors, but not in all PPAR-γ is reduced, while the WNT/β-catenin pathway is regulated upwards.
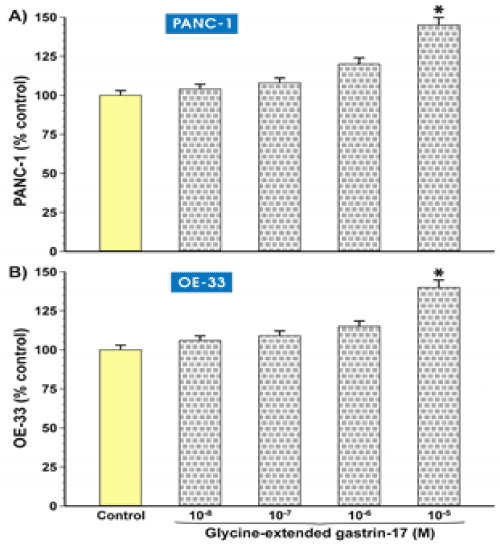
Figure 1. Effect of gastrin and gastrin precursors on PPAR-γ receptors and β-catenin protein in PANC-1 cell.
Results of the study on effects of gastrin G-17, Gly-G and synergism of these compounds (G-17 + Gly-G) obtained by Western-Blot method for PPAR- γ (C) and β-catenin proteins (B). Negative control - β-actin protein (A).
Results of expression level for PPAR-γ (E) receptor. In the investigated cell lines, expression enhancement was seen for the synergistic action of gastrin (G-17 + Gly-G) and increased at a specific incubation time. Significant expression of the test protein occurred after 60 min. (p <0.01 t = 60 min).
Results of the expression level for β-catenin protein (D). In the investigated cell lines, expression increased at a specific incubation time (p <0.01 t = 60 min) with gastrin. Gly-G stimulation and simultaneous action of both proteins (G-17 + Gly-G) induced signal amplification independent of stimulation time (p <0.001 after 5 min / 30 min / 60 min).
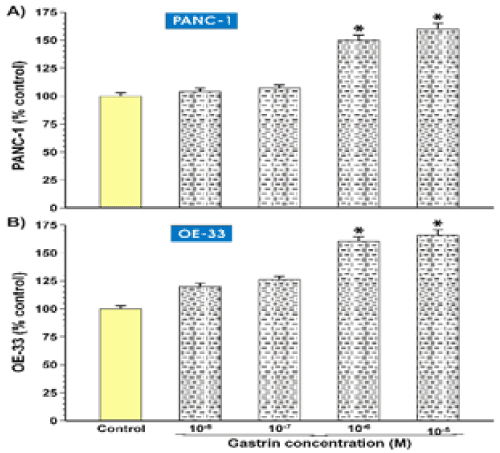
Figure 2. Effect of gastrin and gastrin precursors on PPAR- γ receptors and β-catenin protein in OE-33 cell.
Results of the study on effects of gastrin G-17, Gly-G and synergism of these compounds (G-17 + Gly-G) obtained by Western-Blot method for PPAR- γ (C) and β-catenin proteins (B). Negative control - β-actin protein (A).
Results of expression level for PPAR- γ (E) receptor. In the investigated cell lines, expression increased at a specific incubation time (p <0.01 t = 60 min) only after G-17 gastrin.
Results of the expression level for β-catenin protein (D). In the studied cell lines, expression increased at a specific incubation time (p <0.01 t = 30 min, t = 60 min) with Gly-G. Simultaneous stimulation of both proteins (G-17 + Gly-G) (p <0.01, t = 5 min, t = 30 min, t = 60 min) took place at different times.
Conducted studies confirmed the role of gastrin and its precursors in promoting tumor progression, through strong trophic effects on selected tumor cell lines [10,2]. Literature derived from in vitro studies and animal models undoubtedly suggests gastrin carcinogenicity. It is not clear at the present time whether high levels of gastrin have the same effects in humans. It is suggested that intermediate forms of gastrin have an even stronger carcinogenic
effect [8]. Therefore, further research is needed to assess the potential role of these molecules in the carcinogenic process.
It has been proven that some types of cancer produce their own gastrin, which promotes tumor growth, although the results of this study are contradictory. Certain types of cancer, such as colorectal cancer produce a high level of gastrin intermediates. These compounds have been isolated in colorectal cancer cells as well as in adenomatous polyps, and have not been identified in healthy colon-pleural membranes [3,25]. A similar tendency has been observed in Barrett's esophagus and esophagus, where high expression of gastrin and its receptor was evident in comparison to that seen in healthy epithelial cells [3,26]. The chronic form of esophageal reflux disease contributes to inflammatory conditions of the epithelium, which over time can be a premature cause of adenocarcinoma of the esophagus. Over the past several decades, the incidence rates for this type of cancer have increased significantly. Gly-gastrin levels are also significantly elevated in gastric mucosa in patients with gastrinoma [27]. In addition to the gastrointestinal tract, only a few cases of lung cancer showed a relationship of Gly-gastrin expression, which was related to the survival rate [28].
According to the study, small differences in Gly-gastrin levels may affect proliferation and apoptosis. It has been observed that this form of gastrin works trophic with many cultured cells, which also confirmed our results. Gly-gastrin also has an effect on the reduction of apoptosis by correlating with β-catenin, influencing the increase in expression of this protein in the examined tumor cell lines. The presented results also show a possible association
between β-catenin expression and PPAR-γ nuclear receptor signaling, as confirmed by other literature reports [29,30].
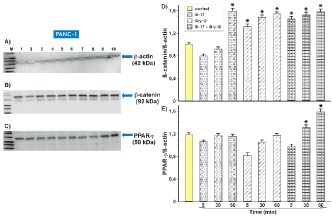
Figure 3. Growth effects after treatment of glycine extended gastrin in PANC-1 and OE-33 (A-B) cell lines. After Gly-gastrin treatment at 10-5, cell growth was observed in PANC-1 (A) and OE-33 (B) cells. Results were monitored using the MTT test (p <0.01 non-parametric Mann-Whitney U test significant indicates by).
2021 Copyright OAT. All rights reserv
Figure 4. Growth effects after treatment of glycine extended gastrin in PANC-1 and OE-33 (A-B) cell lines. After Gly-gastrin treatment at 10-5, cell growth was observed in PANC-1 (A) and OE-33 (B) cells. Results were monitored using the MTT test (p <0.01 non-parametric Mann-Whitney U test significant indicates by).
In many mammalian cells we observe the opposite effect of WNT/β-catenin and PPAR-γ. Often, the amplification of PPAR-γ induces inhibition of the β-catenin pathway, whereas activation of the WNT/β-catenin pathway results in the inactivation of PPAR-γ [18]. In most tumors, but not all, PPAR-γ is down-regulated, and the WNT/β -catenin pathway is upregulated. It has also been observed that inhibition of the WNT/β -catenin pathway induces
PPAR-γ [30].
In tumor cells, increased regulation of WNT/β-catenin signaling causes dramatic changes, which in consequence promotes cell proliferation and growth. Several studies have documented the protective role of PPAR-γ against carcinogenesis [31]. In PPAR-γ, colorectal cancer reduces oncogenic beta-catenin and inhibits cell proliferation [29]. Contrary to other studies related to PPAR-γ which show the involvement of receptors in promoting the development of cancer [32]. Therefore, the biological significance of PPAR-γ in cancer induction remains controversial and requires further research. Just like the interactions of receptors with β-catenin.
- Abdalla SI, Lao-Sirieix P, Novelli MR, Lovat LB, Sanderson IR, et al. (2004) Gastrininduced cyclooxygenase-2 expression in Barrett’s carcinogenesis. Clin Cancer Res 10: 4784-4792. [Crossref]
- Anastas JN, Moon RT (2013) WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13: 11-26. [Crossref]
- Azuma T, Magami Y, Habu Y, Kawai K, Taggart RT, et al. (1990) Carboxyl terminal glycine extended progastrin (gastrin-G) in gastric antral mucosa of patients with gastric or duodenal ulcer and in gastrinomas. J Gastroenterol Hepatol 5: 525-529. [Crossref]
- Barker N, Clevers H (2006) Mining the WNT pathway for cancer therapeutics. Nat Rev Drug Discov 5: 997-1014. [Crossref]
- Botrugno OA, Fayard E, Annicotte JS, Haby C, Brennan T, et al. (2004) Synergy between LRH-1 and β- Catenin induces G1 cyclin-mediated cell proliferation. Mol Cell 15: 499-509. [Crossref]
- Chueca E, Lanas A, Piazuelo E (2012) Role of gastrin-peptides in Barrett's and colorectal carcinogenesis. W J Gastroenterol 18: 6560-6570. [Crossref]
- Clevers H (2006) Wnt/ β-Catenin signaling in development and disease. Cell 127: 469-80. [Crossref]
- Colucci R, Blandizzi C, Tanini M, Vassalle C, Breschi MC, et al. (2005) Gastrin promotes human colon cancer cell growth via CCK-2 receptor-mediated cyclooxygenase- 2 induction and prostaglandin E2 production. Br J Pharmacol 144: 338-348. [Crossref]
- Fujisawa T, Nakajima A, Fujisawa N, Takahashi H, Ikeda I, et al. (2008) Peroxisome Proliferator-Activated Receptor γ (PPARγ) Suppresses Colonic Epithelial Cell Turnover and Colon Carcinogenesis Through Inhibition of the β-Catenin/T Cell Factor (TCF) Pathway. J Pharmacol Sci 106: 627-638. [Crossref]
- Girnun GD, Smith WM, Drori S, Sarraf P, Mueller E, et al. (2002) APC-dependent suppression of colon carcinogenesis by PPARγ. Proc Natl Acad Sci U S A 99:13771-13776. [Crossref]
- Jansson EA, Are A, Greicius G, Kuo IC, Arulampalam V, et al. (2005) The Wnt/β- catenin signalling pathway targets PPARγ activity in colon cancer cells. Proc Natl Acad Sci U S A 102:1460-1465. [Crossref]
- Johnson ML, Rajamannan N (2006) Diseases of WNT signaling. Rev Endocr Metab Disord 7: 41-49. [Crossref]
- Kersten S, Desvergne B, Wahli W (2000) Roles of PPARs in health and disease. Nature 405: 421-424. [Crossref]
- Kitamura S, Miyazaki Y, Shinomura Y, Kondo S, Kanayama S, et al. (1999) Peroxisome proliferators-acivated receptor γ induces growth arrest and differentiation markers of human colon cancer cells. Jap j Cancer Res 90: 75-80. [Crossref]
- Koh TJ, Field JK, Varro A, Liloglou T, Fielding P, et al. (2004) Glycine-extended gastrin promotes the growth of lung cancer. Cancer Res 64: 196-201. [Crossref]
- Lecarpentier Y, Claes V, Vallée A, Hébert JL (2017) Thermodynamics in cancers: opposing interactions between PPAR gamma and the canonical WNT/ β-Catenin catenin pathway. Clin Transl Med 6:14. [Crossref]
- Lefebvre AM, Chen I, Desreumaux P, Najib J, Fruchart JC, et al. (1998) Activation of the peroxisome proliferatoractivated receptor gamma promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat Med 4: 1053–1057. [Crossref]
- Liu J, Wang H, Zuo Y, Farmer SR (2006) Functional Interaction between Peroxisome Proliferator-Activated Receptor γ and β-Catenin. Mol Cell Biol 26: 5827-5837. [Crossref]
- Michalik L, Desvergne B, Wahli W (2004) Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer 4: 61–70. [Crossref]
- Murphy GJ, Holder JC (2000) PPAR-γ agonists: therapeutic role in diabetes, inflammation and cancer. Trends in Pharmacological Sciences 21: 469-474.
- Novellasdemunt L, Antas P, Li VS (2015) Targeting Wnt signaling in colorectal cancer. A Review in the Theme: Cell Signaling: Proteins, Pathways and Mechanisms. Am J Physiol Cell Physiol 309: C511–C521. [Crossref]
- Ptak-Belowska A, Pawlik MW, Krzysiek-Mączka G, Brzozowski T, Watson SA, et al. (2007) Transcriptional upregulation of gastrin in response to peroxisome proliferator-activated receptor gamma agonist triggers cell survival pathways. J Physiol Pharmacol 58: 793–801. [Crossref]
- Rengifo-Cam W, Singh P (2004) Role of progastrins and gastrins and their receptors in GI and pancreatic cancers: targets for treatment. Curr Pharm Des 10: 2345-2358. [Crossref]
- Sabatino L, Pancione M, Votino C, Colangelo T, Lupo A, et al. (2014) Emerging role of the β-catenin-PPARγ axis in the pathogenesis of colorectal cancer. World J Gastroenterol 20: 7137- 7151. [Crossref]
- Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, et al. (1998) Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat Med 4: 1058–1061. [Crossref]
- Seva C, Dickinson C, Yamada T (1994) Growth promoting effects of glycine –extended progastrin. Science 265: 410-412. [Crossref]
- Shah S, Hecht A, Pestell RG, Byers SW (2003) Transrepression of beta-catenin activity by nuclear receptors. J Biol Chem 278: 48137-48135. [Crossref]
- Siddheshwar RK, Gray JC, Kelly SB (2001) Plasma levels of progastrin but not amidated gastrin or glycine extended gastrin are elevated in patients with colorectal carcinoma. Gut 48: 47-52. [Crossref]
- Singh P, Wu H, Clark C, Owlia A (2007) Annexin II binds progastrin and gastrin-like peptides, and mediates growth factor effects of autocrine and exogenous gastrins on colon cancer and intestinal epithelial cells. Oncogene 26: 425-440. [Crossref]
- Singh P (2007) Role of Annexin-II in GI cancers: interaction with gastrins/progastrins. Cancer Lett 252: 19-35. [Crossref]
- Stepan VM, Sawada M, Todisco A, Dickinson CJ (1999) Glycineextended gastrin exerts growthpromoting effects on human colon cancer cells. Mol Med 5: 147-159. [Crossref]
- Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, et al. (1996) Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest 98: 1918- 1929. [Crossref]




 2021 Copyright OAT. All rights reserv
2021 Copyright OAT. All rights reserv