Introduction: The scientific literature shows an association between inflammatory arthritis and cardiovascular diseases (CVD) with inflammation being a shared characteristic between the two types of diseases. Among patients with arthritis, it is possible that the protective factors against inflammation, such as vitamin D, are also protective factors against the development of CVD. This effect may be different according to sex.
Objective: To evaluate the impact of serum vitamin D concentration on the association between arthritis and CVD separately among men and women (effect modification of vitamin D and sex).
Methods: Data came from a large representative sample of the US population: The National Health and Nutrition Examination Survey (NHANES) 2005-2006, which included 3406 adults aged between 20 and 69 years. Measurements of arthritis (primary independent variable) and CVD (dependent variable) were taken during face-to-face interviews, while the measurement of serum vitamin D was carried out on blood samples. Multivariate logistic regression analyses were performed in which the combined modifying effect of vitamin D (<20 ng/ml/≥20 ng/ml) and sex was tested and adjusted for several potentially confounding variables.
Results: Arthritis was statistically associated with CVD in both men and women, with <20 ng/ml or ≥20 ng/ml serum vitamin D. In men, the adjusted ratio of the odds ratios (ROR) comparing the association at <20 ng/ml vitamin D concentration to the association at ≥20 ng/ml concentration was 0.8 (95% CI 0.5 to 1.5); in women, the adjusted ROR was 0.7 (95% CI 0.3 to 1.5).
Conclusions: In this large cross-sectional study, arthritis and CVD were statistically associated, but this association was not modified by sex nor vitamin D concentrations. Vitamin D supplementation is not recommended as part of the management of patients of both sexes suffering from inflammatory arthritis to prevent CVD.
Arthritis, cardiovascular diseases, NHANES 2005-2006, sex, vitamin D.
NHANES: National Health and Nutrition Examination Survey; CVD: Cardiovascular Diseases; 25(OH)D: 25-Hydroxy Vitamin D; BMI: Body Mass Index; HIV: Human Immunodeficiency Viruses; ROR: Ratio of OR; SD: Standard Deviation; OR: Odds Ratio
In the United States of America, about 52.5 million adults aged 18 years or older have clinically diagnosed arthritis. This disease is more prevalent among women and older adults [1]. Arthritis addresses a group of diseases of the musculoskeletal system affecting the joints and surrounding tissues [1,2]. If underdiagnosed or inadequately treated, patients may suffer from joint damage, functional limitations [3] and even increased mortality compared to the general population [4,5].
An association between inflammatory arthritis and cardiovascular diseases (CVD) has been reported [6-9]. Chronic inflammation has an impact on atherosclerosis and insulin resistance, both potential cardiovascular risk factors [10]. Using NHANES dataset, we previously found a statistically significant association between arthritis and CVD [11]. It thus seemed reasonable to hypothesize that, among patients with arthritis, protective factors against inflammation could provide protection against the development of CVD. Vitamin D, an immune modulator, could be such a protective factor [10,12].
The assessment of 25(OH)D serum concentration is a relevant test diagnosing vitamin D deficiency [13-15] because 25(OH)D is more stable and has a longer half-life. Season, aging, latitude, adiposity, physical activity, smoking and diet are factors that affect the associations between vitamin D concentrations and health outcomes [16]. Vitamin D deficiency, defined as 25(OH)D serum concentration <20 ng/ml [17-20] is considered a public health concern [21].
Vitamin D deficiency is common in patients with rheumatoid arthritis [16,22] and is known to be associated with increased CVD risk [12,22,23]. It is not known, however, if this association is the same in men and women. In fact, we know that, except for children aged 1-5 years old, women have a higher prevalence of 25(OH)D deficiency than men [24], as well as a higher arthritis prevalence [1], and that sex differences do exist when it comes to inflammation [25,26] and CVD occurrence [27,28]. However, a clear effect modification of sex on this association is not yet established. The objective of this study was to assess the impact, possibly differential between men and women, of serum 25(OH) D concentrations on the association between arthritis and CVD.
Source of Data
National Health and Nutrition Examination Surveys (NHANES) are national cross-sectional surveys providing health and nutrition information on a representative sample of non-institutionalized U.S. residents of all ages that is generalizable to the entire U.S. population [29]. For the current study, we used data collected from NHANES 2005-2006 because at the time the work was done, it was the latest NHANES survey with data on arthritis, CVD, and also serum measurement of 25(OH)D.
Participants signed written informed consent. Data were collected with health questionnaires, physical examination and blood tests [29]. The questionnaires were standardized and filled out during home interviews, while the physical examination and the blood collection were done in specifically equipped mobile centers travelling when needed to the participant’s home [29].
Sample Selection
Participants included were aged between 20 and 69 years. Exclusion criteria were mainly linked to the blood samples: 1) hemophilia, 2) received chemotherapy in the last 4 weeks, 3) presence of the following on two arms: rashes, gauze dressings, casts, edema, paralysis, open wounds, missing limbs, sclerotic veins, allergies to cleansing agents, burns or scar tissues. Besides the NHANES exclusion criteria, we excluded pregnant women and participants with incomplete physical exam or missing data related to vitamin D status.
Figure 1 shows the detailed participants’ selection: 10,348 individuals participated to NHANES 2005-2006, of whom 382 were pregnant women, 5323 participants were younger than 20 years, 882 were older than 70 years, 140 (1.4%) had not completed the physical examination and 215 (2.1%) had vitamin D data missing. The final sample size available for the current study was 3406 (90.6% of those eligible).
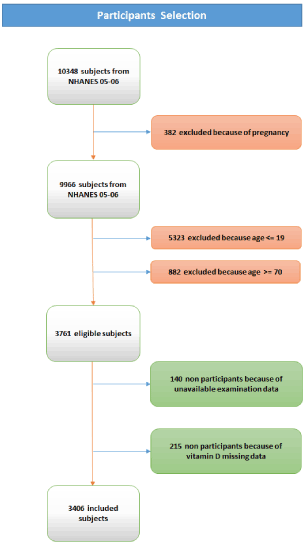
Figure 1. Participants’ selection schematization.
Main independent and dependent variables: Participants reported their health conditions through the NHANES 2005-2006 “Medical Conditions Questionnaire”. The main independent variable was the presence of arthritis; participants answered if they had been diagnosed as having arthritis, which included several diagnoses such as osteoarthritis, rheumatoid arthritis, spondylarthritis, psoriatic arthritis, etc. If the answer was “Yes” to the question: “Has a doctor or other health professional ever told you that you had arthritis?”, then we considered them as having arthritis.
The dependent variable was the diagnosis of CVD; the questions were: “Has a doctor or other health professional ever told you that you had -congestive heart failure?”; “-coronary heart disease?”; “-angina pectoris?”; “-a heart attack?”; “-a stroke?” A positive answer to any of these questions was considered as a positive CVD diagnosis. This is the approach that was used in NHANES to estimate the prevalence of CVD [30].
Other independent variables: Several variables described participants’ sociodemographic, lifestyle and physical characteristics (Table 1). Some were considered as potential confounders of the association between arthritis and CVD, based on biological plausibility and the literature [8,16,31-34]. Covariates included in analyses were age, sex, ethnicity, body mass index (BMI), total blood cholesterol concentration, 25(OH) D serum concentration and monthly dose of vitamin D supplementation. In addition, we included the administration of glucocorticoids and anticonvulsants as potential confounders. In fact, some drugs (anti-convulsant, corticosteroids) adversely affect the vitamin D absorption [35,36]. BMI was calculated as weight in kilograms divided by the square of height in meters and divided into 4 categories: underweight (BMI<18.5), normal weight (18.5<=BMI<24.9), overweight (24.9<=BMI<30), and obese (BMI >=30). Total serum cholesterol (mg/dL) was considered as a continuous variable in analyses.
Table 1. Selected characteristics of subjects1.
| |
Study sample
n (%) |
Without Arthritis n (%) |
With Arthritis n (%) |
p-value2 |
CVD Prevalence and p-value3 |
Age (years) - n [missing]
(mean ± standard deviation) |
3406 [0]
(43.0 ± 0.4) |
2680 [0]
(40.5 ± 0.4) |
726 [0]
(52.1 ± 0.6) |
<0.0001 |
<0.0001 |
20- 40 years |
1496 (43.9) |
1393 (51.5) |
103 (16.1) |
|
1.2 |
41- 60 years |
1360 (44.6) |
1000 (41.1) |
360 (57.4) |
|
6.6 |
>60 years |
550 (11.5) |
287 (7.4) |
263 (26.6) |
|
19.6 |
Ethnicity- n [missing] |
3406 [0] |
2680 [0] |
726 [0] |
<0.0001 |
0.2996 |
Mexico-American |
743 (8.4) |
654 (9.8) |
89 (3.3) |
|
4.1 |
Other Hispanic |
116 (3.7) |
100 (4.1) |
16 (2.0) |
|
3.6 |
Non-Hispanics White |
1563 (70.8) |
1167 (68.7) |
396 (78.7) |
|
5.8 |
Non-Hispanics Black |
839 (11.5) |
647 (11.8) |
192 (10.3) |
|
6.9 |
Other race (including multi-racial) |
145 (5.6) |
112 (5.6) |
33 (5.7) |
|
5.4 |
Sex- n [missing] |
3406 [0] |
2680 [0] |
726 [0] |
<0.0001 |
0.5411 |
Male |
1745 (50.1) |
1437 (52.4) |
308 (41.5) |
|
6.1 |
Female |
1661 (49.9) |
1243 (47.6) |
418 (58.5) |
|
5.3 |
Body Mass Index (BMI) - n [missing] |
3371 [35] |
2655 [25] |
716 [10] |
<0.0001 |
<0.0001 |
Underweight (BMI<18.5 kg/m2) |
36 (1.1) |
31 (1.2) |
5 (0.7) |
|
11.1 |
Normal weight (18.5-24.9 kg/m2) |
956 (31.3) |
823 (34.2) |
133 (20.4) |
|
2.4 |
Overweight (24.9-30 kg/m2) |
1129 (32.2) |
935 (33.4) |
194 (27.9) |
|
5.4 |
Obese (BMI >30 kg/m2) |
1250 (35.4) |
866 (31.1) |
384 (50.9) |
|
8.5 |
Femoral neck bone mineral density4 - n (%) |
2789 (617) |
2239 (441) |
550 (176) |
<0.0001 |
<0.0001 |
Normal |
2104 (73.1) |
1745 (75.9) |
359 (61.9) |
|
4.5 |
Osteopenia |
658 (25.9) |
477 (23.2) |
181 (36.5) |
|
7.8 |
Osteoporosis |
27 (1.0) |
17 (0.8) |
10 (1.6) |
|
16.9 |
Alcohol consumption5 - n [missing] |
3160 [246] |
2469 [211] |
691 [35] |
0.0088 |
0.0596 |
Light consumption |
2353 (71.4) |
1807 (70.2) |
546 (76.0) |
|
6.5 |
Moderate consumption |
491 (16.8) |
397 (17.2) |
94 (15.5) |
|
3.9 |
Vigorous consumption |
316 (11.8) |
265 (12.7) |
51 (8.6) |
|
3.7 |
Total cholesterol (mg/dL) - n [missing]
(mean ± standard deviation) |
3396 [10]
(199.1 ± 1.0) |
2673 [7]
(198.0 ± 1.0) |
723 [3]
(202.8 ± 1.6) |
0.0166 |
0.0205 |
Total cholesterol concentration < 200 mg/dL |
1889 (54.5) |
1501 (55.1) |
388 (52.4) |
|
6.6 |
Total cholesterol concentration >=200 mg/dL |
1507 (45.5) |
1172 (44.9) |
335 (47.6) |
|
4.4 |
Glucocorticoids intake - n [missing] |
3378 [28] |
2660 [20] |
718 [8] |
<0.0001 |
<0.0001 |
Yes |
49 (1.4) |
25 (0.9) |
24 (3.4) |
|
5.4 |
No |
3329 (98.6) |
2635 (99.1) |
694 (96.6) |
|
21.1 |
Anticonvulsants intake- n (%) |
3378 [28] |
2660 [20] |
718 [8] |
<0.0001 |
<0.0001 |
Yes |
140 (4.3) |
69 (2.7) |
71(10.1) |
|
5.1 |
No |
3238 (95.7) |
2591 (97.3) |
647 (89.9) |
|
16.7 |
Vitamin D supplementation- n (%) |
3404 [2] |
2678 [2] |
726 [0] |
0.2388 |
0.0181 |
Yes |
1129 (38.9) |
866 (38.4) |
263 (41.0) |
|
6.3 |
No |
2275 (61.1) |
1812 (61.6) |
463 (59.0) |
|
4.7 |
Monthly dose of vitamin D supplementation (IU)6 - n [missing]
(mean ± standard deviation) |
3390 [16]
(4049.0 ± 265.3) |
2667 [13]
(3794.2 ± 238.0) |
723 [3]
(4987.1 ± 731.4) |
0.12 |
0.258 |
Dose < 12000 IU |
2888 (82.2) |
2304 (83.5) |
584 (77.7) |
|
5. 5 |
Dose > = 12000 IU |
495 (17.8) |
363 (16.5) |
139 (22.3) |
|
6.4 |
Vitamin D serum concentration- n (%) |
3406 [0] |
2680 [0] |
726 [0] |
0.1146 |
0.0015 |
<20 ng/mL |
1600 (35.7) |
1251 (35.0) |
349 (38.4) |
|
7.8 |
>= 20 ng/mL |
1806 (64.3) |
1429 (65.0) |
377 (61.6) |
|
4.5 |
Cardiovascular diseases7- n (%) |
3406 [0] |
2680 [0] |
726 [0] |
<0.0001 |
NA8 |
Yes |
241 (5.7) |
114 (3.4) |
127 (14.1) |
|
NA |
No |
3165 (94.3) |
2566 (96.6) |
599 (85.9) |
|
NA |
1All values are weighted.
2Statistical significance level: 0.05 for the comparison between both groups (arthritis versus non-arthritis); chi2 test for categorical variables and Student’s t test for continuous variables.
3Prevalence of cardiovascular diseases with the p value of a chi square test that highlights the bivariate association of cardiovascular diseases with each of the participant characteristics.
4Normal when for men the femoral neck bone mineral density is >0.79 gm/cm2, and for women >0.74 gm/cm2
Osteopenia when for men the femoral neck bone mineral density is between 0,59 and 0.79 gm/cm2, and for women between 0.56 and 0.74 gm/cm2
Osteoporosis when for men the femoral neck bone mineral density is <0.59 gm/cm2 and for women <0.56 gm/cm2
5Light alcohol consumption: M: <= 3 drinks per week- F: <= 3 drinks per week
Moderate alcohol consumption: M: between 3 and 14 drinks per week- F: between 3 and 7 drinks per week
Vigorous alcohol consumption: M: more than 14 drinks per week- F: more than 7 drinks per week
6IU: International Units
7Includes congestive heart failure, coronary heart disease, angina/angina pectoris, heart attack, and stroke
8NA: Not Applicable for this table
Details concerning the administration of glucocorticoids and anticonvulsants were extracted from NHANES 2005-2006 by matching two drug files (“Dietary Supplements” and “Prescription Medications”) into a computer where an automatic match to a prescription drug database was performed [37].
Laboratory data provided measurements of serum concentrations of 25(OH)D for participants. The Diasorin 25-Hydroxyvitamin D (25(OH)D) assay, an accurate kit measuring serum concentrations of 25(OH)D [38], was performed to first extract 25(OH)D metabolites from serum, and then separate and measure them [39]. NHANES uses different procedures to control the quality of the analyses performed by the laboratories [40]. Serum concentrations of 25(OH)D were dichotomized at >20 ng/ml [17-20]. Finally, information on the participants’ vitamin D supplementation and its monthly dose were taken from several dietary data files available in the NHANES 2005-2006 database, that were merged.
Statistical Analyses
SAS software (version 9.3) was used to perform statistical analyses. Sample weights were used to produce an unbiased national estimate of the civilian noninstitutionalized U.S. population [41]. Comparisons between arthritis and non-arthritis groups were conducted using chi-squared tests for categorical variables and Student’s t test for continuous variables. To visualize bivariate associations of CVD with each of the independent variables, chi-squared tests were performed, and the prevalence of CVD was calculated for each variable category. Multiple logistic regression was used to assess the association between arthritis and CVD. Age, total cholesterol and monthly dose of vitamin D supplementation were treated as continuous variables, while other variables were left categorical. Prevalence odds ratios (OR) with 95% confidence intervals (95% CI) were calculated. Age, sex, ethnicity, BMI, total cholesterol, the administration of glucocorticoids and anticonvulsants, 25(OH)D serum concentration of vitamin D (<20 ng/ml vs. ≥20 ng/ml), and vitamin D supplementation use were considered as potential confounding factors. Confounding was defined as a change in the OR ≥ 10% brought about by taking off one variable at a time compared to the full model. Effect modification was tested on a full model that included three double interaction terms (sex*arthritis, vitamin D*arthritis and sex*vitamin D) and a triple interaction term (arthritis*sex*vitamin D). This is because the main interest in this study were possible modifying effects of sex and vitamin D serum concentrations on the association between arthritis and CVD. To measure such effects, ratios of ORs (ROR) were calculated with their 95% confidence intervals; here, a ROR of 1.00 means no effect modification.
Descriptive analyses: 3761 adults were considered eligible, while 355 were excluded. No major differences existed between study participants and eligible non-participants except for the prevalence of arthritis. Actually, the non-participant group had an arthritis prevalence of 12.4% while the prevalence in the participants’ group was 21.4%. As for the prevalence of CVD, it was 7.1% in non-participants and 5.7 % in participants.
Overall, 3406 participants comprised the study sample (Table 1), including 726 with a reported diagnosis of arthritis. Almost 90% of participants were aged between 20 and 60 years. Men and women were equally represented, with a majority of non-Hispanic Whites (70.8%). Close to a third of participants (32.2%) were overweight and 35.4% were obese. Deficient vitamin D concentrations (<20 ng/ml) were found in 35.7% of participants, while a vitamin D supplementation was taken by 38.9%.
Participants with arthritis were significantly older and more likely to be non-Hispanic Whites, female, obese and to have osteopenia than those without arthritis. They were also more likely to take glucocorticoids, anticonvulsants and higher doses of supplements of vitamin D. The prevalence of CVD was 14.1% among participants with arthritis and 3.4% among those without it (p<0.0001). CVD were significantly more prevalent with increasing age, among underweight and obese participants, those with osteopenia and osteoporosis, those with lower concentrations of total cholesterol, those not taking glucocorticoids or anticonvulsants, and those taking vitamin D supplementation. There was a significant (p=0.0015) bivariate association between serum vitamin D concentration and CVD: participants with <20 ng/ml had a prevalence of CVD of 7.8% while those with ≥20 ng/ml had a prevalence of 4.5% (Table 1).
Association of arthritis with CVD according to sex and serum vitamin D concentration:
Table 2 shows results of unadjusted and final multivariate models. In men, the unadjusted OR in participants with vitamin D <20 ng/ml was similar to the OR in participants with vitamin D ≥20 ng/ml (ROR: 1.1; 95% CI: 0.7-1.8), while there was a significant difference in women (ROR: 0.5; 95% CI: 0.3-1.0). The ROR comparing the association according to vitamin D concentration and sex was also significant (ROR: 2.3; 95% CI: 1.2-4.5). These differences disappeared however in adjusted analyses: the ROR <20 ng/ml /≥20 ng/ml among men was 0.8 (95% CI: 0.5-1.5), the ROR <20 ng/ml ≥20 ng/ml among women was 0.7 (95% CI: 0.3-1.5), and the ROR combining both sexes was 1.1 (95% CI: 0.5-3.1); all adjusted ROR were not statistically different from 1.0.
Table 2. Results of bivariate and multivariate analyses on the association between arthritis and cardiovascular diseases by sex and vitamin D concentration (n=3406)9.
| |
Men |
Women |
Ratio of OR (Men vs Women)10 |
OR or ROR (95% CI)11 |
p-value |
OR or ROR (95% CI) |
p value |
ROR (95% CI) |
p-value |
UNADJUSTED |
Vitamin D <20 ng/ml concentration |
4.8 (3.0 - 7.7) |
<0.0001 |
3.6 (2.0 - 6.6) |
<0.0001 |
_- |
_- |
Vitamin D ≥20 ng/ml concentration |
4.2 (2.9 - 6.2) |
<0.0001 |
7.3 (3.0 - 17.4) |
<0.0001 |
- |
- |
Ratio of OR (<20 ng/ml /≥20 ng/ml) [2] |
1.1 (0.7 - 1.8) |
0.5817 |
0.5 (0.3 - 1.0) |
0.0367 |
2.3 (1.2 - 4.5) |
0.0161 |
ADJUSTED12 |
|
|
|
|
|
Vitamin D <20 ng/ml concentration |
1.7 (1.0 - 3.0) |
0.0508 |
2.1 (0.9 - 5.1) |
0.1074 |
_- |
_- |
Vitamin D ≥20 ng/ml concentration |
2.1 (1.3- 3.4) |
0.0017 |
3.1 (1.1 - 9.0) |
0.0361 |
_- |
_- |
Ratio of OR (<20 ng/ml /≥20 ng/ml)13 |
0.8 (0.5 - 1.5) |
0.5008 |
0.7 (0.3 - 1.5) |
0.3457 |
1.1 (0.5 - 3.1) |
0.67914 |
9All values are weighted with the full sample 2-year interview weight and the full sample 2-year MEC exam weight.
10Combined effect modification of vitamin D and sex; interaction term (arthritis*sex*vitamin D).
11OR (95% CI): Odds Ratios with 95% confidence interval; ROR (95% CI): Ratio of OR with 95% confidence interval.
12Adjusted for age, supplementation of vitamin D and its monthly dose, body mass index, administration of anticonvulsants, total cholesterol concentration, and main effect of vitamin D serum concentration and sex.
13Effect modification by vitamin D concentration; interaction term: (arthritis*vitamin D).
14P-value for combined effect-modification
In men, the ROR<20 ng/ml /≥20 ng/ml is obtained by dividing the OR of vitamin D <20 ng/ml (1.7) by the OR of vitamin D ≥20 ng/ml (2.1). This ROR was 0.8, which reflects that the odds of CVD in men with arthritis and <20 ng/ml vitamin D concentration is 0.8 times that of men with arthritis and ≥20 ng/ml vitamin D concentration. However, it was not statistically significant (p-value: 0.50); we conclude that there was no significant difference between both ORs, and thus that no double interaction does exist in men between vitamin D concentration and arthritis.
In women, the ROR <20 ng/ml /≥20 ng/ml is obtained by dividing the OR of vitamin D <20 ng/ml concentration (2.1) by the OR of vitamin D ≥20 ng/ml concentration (3.1). This ROR is 0.7 which reflects that the odds of CVD in women with arthritis and <20 ng/ml vitamin D concentration is 0.7 times that of women with arthritis and ≥20 ng/ml of vitamin D. However, it was not statistically significant (p-value: 0.35); we conclude that there was no significant difference between both ORs, and thus that no double interaction does exist among women between vitamin D concentration and arthritis.
The ROR of 0.8 in men is not the same as that of 0.7 in women but this difference was not statistically significant. In fact, the combined ROR obtained by dividing 0.7 by 0.8 is 1.1, the confidence interval includes the null value, and its p-value is 0.68. We thus concluded that no triple interaction does exist between arthritis, vitamin D concentration and sex.
Using data from a nationwide survey, we found a statistically significant association between arthritis and CVD. However, this association was not different according to serum vitamin D concentrations nor sex in multivariate analyses.
The association between arthritis and CVD has been reported previously in several studies [7,8,31,32] as well as the confounding effect of some factors such as age and sex, obesity, vitamin D consumption and its serum concentration, total cholesterol concentration, and the administration of anticonvulsants [7-9,31].
Vitamin D serum concentration, involved in the inflammatory process [16,42], could provide better understanding of the mechanism behind the association between arthritis and CVD. The concentrations of 25(OH)D have been shown to decline during health disorders characterized by inflammation and are inversely correlated to inflammatory biomarkers such as C-reactive protein [16]. However, even if low 25(OH)D concentrations have been shown to be associated with several diseases such as arthritis and CVD, firm conclusions on vitamin D association with health disorders cannot be drawn because these associations were based on observational studies [34].
Our study was conducted on a large sample, representative of the U.S. population that provided high statistical power. The availability of serum vitamin D measures was a particularly interesting feature, providing more valid information than the usual dietary recalls.
There are some limitations that need to be discussed. The first one relates to the design of our study. Being cross-sectional, no causal relationship could be confirmed between arthritis and CVD. Another limitation is linked to the fact that 355 eligible subjects were eliminated from the study sample because of missing data related to physical examination and vitamin D status. According to NHANES, missing values are randomly distributed; however, the difference in the prevalence of arthritis between the study participants and the non-participants could reflect that arthritic subjects were more involved in the survey and blood collection than non-arthritic ones. To create an important selection bias, differences between both groups should be related not only to the exposure but also to the outcome. After comparing CVD prevalence between both groups, we did not notice a considerable difference, which brings us to conclude that the risk of selection bias is not high.
Both the dependent and the primary independent variables were reported pointing to possible misclassification. The fact of measuring simultaneously the exposure and outcome with reported measures may introduce a common method bias, generating an overestimation of the association between arthritis and CVD. Another source of non-differential misclassification exists: NHANES data provide a global measure for the arthritis disease; the term "arthritis" was not specific and included osteoarthritis, rheumatoid arthritis, spondylarthritis, psoriatic arthritis, etc. Inflammatory arthritis was not clearly defined nor measured. The disease was considered in general, and participants with inflammatory arthritis were not separated from those with non-inflammatory conditions. Also, data were derived from a survey conducted in 2005-2006 and results may be different due to advancements in arthritis treatment.
As for confounding, we have identified several potential confounders in the literature [8,16,31,32,34,43], though we were not able to fully control for all of them. The presence of osteoporosis [44] and alcohol consumption [5] were potential confounders, but we were not able to include these variables in our models because of the important number of missing values. In addition, HIV medications affect the vitamin D metabolism and blood absorption [35,45] and should have been considered as potential confounders for our analysis; however, these data were private in NHANES. Since HIV prevalence in the adults and adolescents US population was only 0.45% at the end of 2006 [46], we assume that this would not have caused big changes in our final estimates. Last, we were not able to get detailed data concerning the vitamin D daily consumption, and data related to the participant’s daily sunlight exposure was not available, so we could not include those variables as potential confounders.
In order to prevent statistical overadjustment, we removed some variables (administration of vitamin D and its monthly dose) from the regression models, to test the changes on the estimates and their precision. No major changes were noticed; the associations remained almost the same, so we kept them in our model, and we can confirm that our results were not affected by major overadjustment.
Multicollinearity between variables was tested before performing the regressions, and all correlation coefficients were neither larger than 0.9 nor smaller than -0.9; we thus conclude that multicollinearity was not important in our models.
From a clinical perspective, the CVD risk among arthritic patients should be taken more seriously and better CVD preventive interventions should be undertaken. However, since we have not found any effect modification by sex and vitamin D on the association between arthritis and CVD, we cannot conclude to the usefulness of vitamin D administration as part of the management for patients suffering from inflammatory arthritis to prevent CVD. This is in-line with some meta-analyses that have confirmed a lack of association between vitamin D deficiency and several diseases [16,34] and there are no universal recommendations related to the daily intake of vitamin D supplementation [34]. It is difficult to conclude concerning the vitamin D supplementation effect because vitamin D absorption differs among people, and optimal levels of vitamin D concentrations are not the same for all outcomes [34].
The study of a shared cause for inflammatory arthritis and CVD should continue with prospective designs and more specific measures of both arthritis and CVD. Furthermore, information on exposure to sunlight would contribute to enhance the internal validity of results.
The association between arthritis and CVD is not modified by serum vitamin D concentrations nor sex. Prospective studies with more specific definitions of inflammatory arthritis and CVD are needed to shed more light on modifiable common risk factors of arthritis and CVD.
The ethics committee of the Laval University (CERUL: https://www.cerul.ulaval.ca) validated that this type of research does not need ethical approval since it is based exclusively on secondary use of anonymous data, and information used is legally available to the public and adequately protected by the law.
All participants provided written informed consent before participating to NHANES 2005-2006.
Not applicable.
The datasets supporting the conclusions of this article are available in the NHANES 2005-2006 website (http://wwwn.cdc.gov/nchs/nhanes/search/nhanes05_06.aspx
Not applicable.
The authors declare that they have no competing interests.
Rachelle Saade participated in conception and design, acquisition of data, analysis, interpretation, and drafting the manuscript. Clermont E. Dionne participated in conception and design, interpretation, and critical revision of the manuscript. Danielle Laurin participated in interpretation and critical revision of the manuscript. All authors read and approved the final manuscript.
- Centers for Disease Control and Prevention (2019) Arthritis. https://www.cdc.gov/arthritis/basics/index.html
- Arthritis Society (2019) About arthritis. http://www.arthritis.ca/aboutarthritis
- Mease P, Garg A, Gladman D, Helliwell P (2013) Development of simple clinical criteria for the definition of inflammatory arthritis, enthesitis, dactylitis, and spondylitis: a report from the GRAPPA 2012 annual meeting. J Rheumatol 40: 1442-1445. [Crossref]
- Davenport G (2004) Rheumatology and musculoskeletal medicine. Br J Gen Pract 54: 457-464. [Crossref]
- Lahiri M, Luben RN, Morgan C, Bunn DK, Marshall T, et al. (2014) Using lifestyle factors to identify individuals at higher risk of inflammatory polyarthritis (results from the European Prospective Investigation of Cancer-Norfolk and the Norfolk Arthritis Register—the EPIC-2-NOAR Study). Ann Rheum Dis 73: 219-226. [Crossref]
- Dessein PH (2013) Vitamin D replacement therapy: a promising adjunct in cardiovascular risk management among patients with rheumatoid arthritis? J Rheumatol 40: 1463-1465. [Crossref]
- Hollan I, Meroni PL, Ahearn JM, Cohen Tervaert JW, Curran S, et al. (2013) Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev 12: 1004-1015. [Crossref]
- Soubrier M, Barber Chamoux N, Tatar Z, Couderc M, Dubost JJ, et al. (2014) Mathieu S. Cardiovascular risk in rheumatoid arthritis. Joint Bone Spine 81: 298-302.
- Meek IL, Picavet HS, Vonkeman HE, Verschuren WM, van de Laar MA (2013) Increased cardiovascular risk factors in different rheumatic diseases compared with the general population. Rheumatology (Oxford) 52: 210-216. [Crossref]
- Goshayeshi L, Saber H, Sahebari M, Rezaieyazdi Z, Rafatpanah H, et al. (2012) Association between metabolic syndrome, BMI, and serum vitamin D concentrations in rheumatoid arthritis. Clin Rheumatol 31: 1197-1203. [Crossref]
- Saade R (2016) Effets de la vitamine D et du sexe sur l'association entre l'arthrite et les maladies cardiovasculaires Quebec, Canada: Laval University.
- Baker JF, Mehta NN, Baker DG, Toedter G, Shults J, et al. (2012) Vitamin D, metabolic dyslipidemia, and metabolic syndrome in rheumatoid arthritis. Am J Med 125: 1036.e9-1036.e15. [Crossref]
- Bhan I, Tamez H, Thadhani R (2013) Impact of new vitamin D data on future studies and treatment. Curr Opin Nephrol Hypertens 22: 377-382. [Crossref]
- Abrahamsen B, Harvey NC (2013) The role of vitamin D supplementation in patients with rheumatic diseases. Nat Rev Rheumatol 9: 411-422. [Crossref]
- Greene-Finestone LS, Berger C, De Groh M, Hanley DA, Hidiroglou N, et al. (2011) 25-Hydroxyvitamin D in Canadian adults: biological, environmental, and behavioral correlates. Osteoporos Int 22: 1389-1399. [Crossref]
- Autier P, Boniol M, Pizot C, Mullie P (2014) Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol 2: 76-89. [Crossref]
- Wacker M, Holick MF (2013) Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 5: 111-148. [Crossref]
- Spedding S, Vanlint S, Morris H, Scragg R (2013) Does vitamin D sufficiency equate to a single serum 25-hydroxyvitamin D level or are different levels required for non-skeletal diseases? Nutrients 5: 5127-5139. [Crossref]
- Mertens PR, Muller R (2010) Vitamin D and cardiovascular risk. Int Urol Nephrol 42: 165-171. [Crossref]
- Cozzolino M, Ketteler M, Zehnder D (2010) The vitamin D system: a crosstalk between the heart and kidney. Eur J Heart Fail 12: 1031-1041. [Crossref]
- Khadilkar VV, Khadilkar AV (2013) Use of vitamin D in various disorders. Indian J Pediatr 80: 215-218. [Crossref]
- Haque UJ, Bathon JM, Giles JT (2012) Association of vitamin D with cardiometabolic risk factors in rheumatoid arthritis. Arthritis Care Res (Hoboken) 64: 1497-1504. [Crossref]
- Ranganathan P, Khalatbari S, Yalavarthi S, Marder W, Brook R, et al. (2013) Vitamin D deficiency, interleukin 17, and vascular function in rheumatoid arthritis. J Rheumatol 40: 1529-1534. [Crossref]
- Yetley EA (2008) Assessing the vitamin D status of the US population. Am J Clin Nutr 8: 5585-5645. [Crossref]
- Sokka T, Toloza S, Cutolo M, Kautiainen H, Makinen H, et al. (2009) Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther 11: R7. [Crossref]
- Ahlmen M, Svensson B, Albertsson K, Forslind K, Hafström I, et al. (2010) Influence of gender on assessments of disease activity and function in early rheumatoid arthritis in relation to radiographic joint damage. Ann Rheum Dis 69: 230-233. [Crossref]
- Mendis S, Puska P, Norrving B (2011) Global Atlas on Cardiovascular Disease Prevention and Control. World Health Organization Geneva.
- Regitz-Zagrosek V, Lehmkuhl E, Weickert M (2006) Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol 95: 136-147. [Crossref]
- Centers for Disease Control and Prevention (2014) National health and examination survey overview, 2013-2014. http://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013-14_overview_brochure.pdf.
- Kendrick J, Targher G, Smits G, Chonchol M (2009) 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis 205: 255-260. [Crossref]
- John H, Kitas G (2012) Inflammatory arthritis as a novel risk factor for cardiovascular disease. Eur J Intern Med 23: 575-579. [Crossref]
- Semb AG, Rollefstad S, van Riel P, Kitas GD, Matteson EL, et al. (2014) Cardiovascular disease assessment in rheumatoid arthritis: a guide to translating knowledge of cardiovascular risk into clinical practice. Ann Rheum Dis 73: 1284-1288. [Crossref]
- van Breukelen-van Der Stoep DF, Klop B, Van Zeben D, Hazes JM, Castro Cabezas M (2013) Cardiovascular risk in rheumatoid arthritis: how to lower the risk? Atherosclerosis 231: 163-172. [Crossref]
- Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP (2014) Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 348: g2035. [Crossref]
- Holick MF (2006) High Prevalence of Vitamin D Inadequacy and Implications for Health. Mayo Clin Proc 81: 353-373. [Crossref]
- Zhang R, Naughton DP (2010) Vitamin D in health and disease: current perspectives. Nutr J 9: 65. [Crossref]
- National Health and Nutrition Examination Survey (2008) Prescription Medications (RXQ_RX_D).
- Carter GD (2009) 25-Hydroxyvitamin D Assays: The Quest for Accuracy. Clin Chem 55: 1300-1302. [Crossref]
- National Health and Nutrition Examination Survey (2008) Vitamin D (VID_D).
- National Health and Nutrition Examination Survey (2017) Laboratory procedures manual.
- Mirel LB, Mohadjer LK, Dohrmann SG, Clark J, Burt VL, et al. (2013) National Health and Nutrition Examination Survey: Estimation procedures, 2007–2010. Vital Health Stat 2 159: 1-17. [Crossref]
- Hossein-nezhad A, Holick MF (2013) Vitamin D for health: a global perspective. Mayo Clin Proc 88: 720-755. [Crossref]
- Holick MF (2005) The Vitamin D Epidemic and its Health Consequences. J Nutr 135: 2739S-2748S. [Crossref]
- Holick MF (2005) Vitamin D: Important for Prevention of Osteoporosis, Cardiovascular Heart Disease, Type 1 Diabetes, Autoimmune Diseases, and Some Cancers. South Med J 9H: 1024-1027. [Crossref]
- O'Neal JD (2015) Vitamin D supplementation regimens for HIV-infected patients: a historical chart review. OHSU Digital Collections.
- Campsmith M, Rhodes P, Hall H, Green T (2008) HIV Prevalence Estimates -- United States, 2006. Div of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC.
Editorial Information
Editor-in-Chief
Andras Perl
State University of New York, USA
Article Type
Research Article
Publication history
Received: December 24, 2020
Accepted: January 04, 2021
Published: January 15, 2021
Copyright
©2020 Saade R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Saade R, Laurin D, E. Dionne C (2020) Does serum vitamin D concentration modify the association between arthritis and cardiovascular diseases in both sexes?. Rheumatol Orthop Med. 5. DOI: 10.15761/ROM.1000181

