- This work signifies a generally accepted view of contribution of chewing tobacco and gutka in oral cancer in Indian subcontinent.
- This finding shows first attempt of extraction of genotoxic components from chewing tobacco and gutka in DMSO solvents.
- This preliminary finding supports that mechanisms of action of oral carcinogenesis could be different between use of chewing tobacco and gutka, which may be attributed due to the differential ability to inflict DNA damage in vitro condition.
Background: At the global level and specifically in Indian sub-continent, the use of chewing tobacco and gutka, a type of tobacco products is reported to cause oral submucous fibrosis (OSMF), which can potentially progress to oral cancer. There is no clear view to suggest that both chewing tobacco and gutka may lead to oral carcinogenesis based on chemical ingredients such as betel quid, areca nuts, and slaked lime, sweeteners, carcinogenic compounds and their ability to act as genotoxic agents.
Methodology: In this direction, we asked to know the differential ability of chewing tobacco and gutka to show DNA damaging act as genotoxic agents that may be responsible for oral cancer. We performed a new and novel method of DMSO based extraction of genotoxic components from chewing tobacco and gutka. Then, we performed well reliable in vitro plasmid DNA damage assay and agarose electrophoresis system to determine extent of DNA damage.
Results and conclusion: In our preliminary but reproducible manner, data indicate that gutka which is a well know type of tobacco products showed very less genotoxic DNA damaging effects compared to chewing tobacco. This result appears surprising because gukta has been viewed as highly potential factor for oral carcinogenesis. Here, we suggest that in spite of lack of DNA damaging act by gutka components, these gutka components may cause oral carcinogenesis other than DNA damaging role such as epigenetic and aberration of molecular signaling in carcinogenesis.
genotoxic agents, oral cancer, epigenetic, genetic, DNA
In the developing countries of Indian sub-continent, an appreciable portion of populations are habituated to use chewing tobacco and gutka as a part of their lifestyles and to have stimulant and relaxation effects [1-6].
In recent, several clinical and basic scientists proposed views that these consumed chewing tobacco and gutka products contains several carcinogenic chemicals such as proprionitrile, nitrosamines, nicotine, sweeteners, flavoring agents [4-6]. However, there is a lack of clear evidence on the genotoxic abilities of these chewing tobacco and gutka. Besides that the molecular mechanisms are not clear to show that whether both chewing tobacco and gutka components are a major factors of oral carcinogenesis based on the extent of DNA damaging abilities of these chewing tobacco and gutka or some other factors including epigenetic modulation of oral mucosa [1-3].
Therefore, we asked to know the genotoxic abilities of these locally available chewing tobacco and gutka with simple and reliable assay. These preliminary findings are of importance to see that even though DNA damaging abilities of gutka is very less compared to the chewing tobacco, but contribution of gutka in oral carcinogenesis is much more severe than the use of chewing tobacco. Hence, this finding suggests that chewing tobacco and gutka may contribution towards oral carcinogenesis in varied molecular mechanisms like genotoxic and epigenetic pathway.
Materials
All reagents were purchased from Invitrogen India Pvt. Ltd. and Himedia India Pvt. Ltd. The chewable tobaco and gutka were purchased from the local market and their identities are not disclosed.
Preparation of genotoxic components from tobacco and gutkha
For the preparation of genotoxic components, chewable tobacco and gutka with different brands were procured from the local market. We took eppendorf tubes of capacity 2 ml and put 1 ml of sterile DMSO in each tube in the biosafety cabinet. Next, we weighed 0.15 g of chewable gutka and tobacco and added into the centrifuge tubes having DMSO as an extraction solvent. Further, added chewable gutka and tobacco mixed well and then placed for vortexing on vortex mixer for 1 h 30 min at half of its final rpm. Then after, we kept these centrifuge tubes having chewable gutka and tobacco and DMSO in microcentrifuge tube racks for sedimentation for the next 36 h.
After 36 h of sedimentation and incubation, DMSO extract of chewable gutka and tobacco were centrifuged for 30 sec at 10,000 rpm. Next, the top clear supernatant DMSO extract from both chewable tobacco and gutka were carefully removed and transferred to the fresh sterile centrifuge tube and designated as DMSO extract of chewing tobacco and gutka. Further, these DMSO extract from chewable tobacco and gutka were filter sterilized using 0.45 micron syringe filter for used in in vitro DNA damaging assay.
In vitro DNA damage assay
To assess the in vitro DNA damage ability of DMSO extract chewing tobacco and gutka, we performed DNA damage ability using routinely used plasmid pBR322 DNA. In short, in vitro reaction were performed in the presence of 1X TAE buffer, 1 µl of pBR322 plasmid DNA (0.2 mg per ml) and DMSO extract chewing tobacco and gutka. In this paper, we have set up different and separate DNA damaging reactions for DMSO extract chewing tobacco and DMSO extract gutka as plasmid control (1 µl plasmid pBR322+24 µl NFW), DMSO control (1 µl plasmid pBR322 + 1 µl sterile DMSO + 5 µl TAE 4X + 18 µl NFW), and varied concentration of DMSO extract chewing tobacco and DMSO extract gutka + 5 µl TAE+ 18.0 µl NFW). These prepared reaction mixture were incubated for 24 h at 37oC. At the end of incubation, these plasmid DNA samples with or without treatment of DMSO extract chewing tobacco and DMSO extract gutka were separated on 1% agarose gel using LifeTechnology, USA horizontal electrophoresis system. The agarose gel loaded with plasmid DNA was visualized by the help of in gel ethidium bromide staining. After observation of DNA bands, photograph was captured using BIO-RAD EZ imaging system.
It is well established that chronic use of chewing tobacco and gutka can be one of potential agent in the prevalence of early onset of oral cancers in Indian sub-continent [1-6]. In this paper, we report the clear, reliable and efficient extraction of genotoxic agents in DMSO solvent from chewing tobacco and gutka in an appreciable amount. These obtained DMSO extract from chewing tobacco and gutka was subjected to the standard in vitro DNA damage assay with slight modifications [7]. The analysis of plasmid DNA damage data revealed that DMSO extract of chewing tobacco showed extensive DNA damage up to the ten times dilution of used extract (Figure 1). On the other hand, DMSO extract obtained from gutka did not show appreciable plasmid DNA damage at varied use of DMSO extract (Figure 2). These observations were surprising as general notion that gutka is considered as more severe factor for oral carcinogenesis; at the same time extracted components in DMSO did not display genotoxic activities. In our case data is reliable and reproducible and this observation is new and first time to show that DMSO extract components from gutka is less genotoxic than the DMSO extract components from chewing tobacco. In spite of very low genotoxic effects of gutka components should be inferred that gutka may not initiate carcinogenesis, rather this observation suggests that chemical compositions within gutka may contribute towards oral carcinogenesis via epigenetic and deregulation of molecular signaling pathways. This finding may have implications in the existing views to suggest the role of genetic alterations by genotoxic agents, molecular signaling deregulations and epigenetic pathways in different type cancer development including oral cancer [8-10].
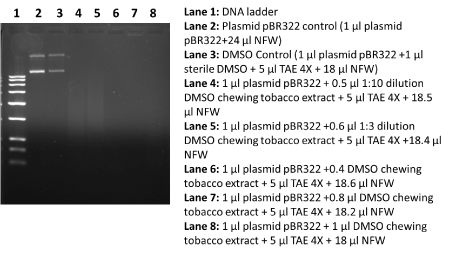
Figure 1. DNA damage effects of DMSO extract from chewing tobacco. In this figure, 1% agarose gel stained with ethidium bromide showing different lanes including DNA marker, control plasmid pBR322 and plasmid DNA treated with DMSO extract from chewing tobacco.
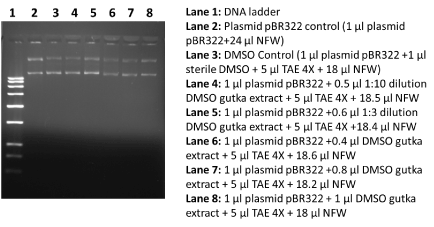
Figure 2. DNA damage effects of DMSO extract from gutka. In this figure, 1% agarose gel stained with ethidium bromide shows different lanes including DNA marker, control plasmid pBR322 and plasmid DNA treated with DMSO extract from gutka.
In our case, data support such possibility that gutka may have bigger contribution in oral carcinogenesis by modulating epigenetic landscape in normal cells that lead to the tumor initiation and progression.
In conclusion, our data suggest that genotoxic effect of extracted components from chewing tobacco is very high compared to gutka components. This finding is new and slightly surprising to the common view that gutka ingredients lead to genomic instability. We suggest that even gutka ingredients are without DNA damaging ability, these components from gutka may lead to carcinogenesis by non-genetic pathways including deregulated molecular signaling and epigenetic modulation.
The authors acknowledge financial support from DST-SERB, Government of India, New Delhi, India (SERB/LS-1028/2013) and Dr. D. Y Patil, Vidyapeeth, Pune, India (DPU/05/01/2016).
Authors declare no conflict of interest.
- Chandirasekar R, Kumar BL, Sasikala K, Jayakumar R, Suresh K, et al. (2014) Assessment of genotoxic and molecular mechanisms of cancer risk in smoking and smokeless tobacco users. Mutat Res Genet Toxicol Environ Mutagen 767: 21-27. [Crossref]
- Braakhuis BJ, Nieuwint AW, Oostra AB, Joenje H2021 Copyright OAT. All rights reserv to chromosomal breakage as risk factor in young adults with oral squamous cell carcinoma. J Oral Pathol Med 45: 189-192. [Crossref]
- Kumar A, Sarode SC, Sarode GC, Majumdar B, Patil S, et al. (2017) Beyond gene dictation in oral squamous cell carcinoma progression and its therapeutic implications. Translational Research In Oral Oncology 2: 1-14.
- Mehrtash H, Duncan K, Parascandola M, David A, Gritz ER, et al. (2017) Defining a global research and policy agenda for betel quid and areca nut. Lancet Oncol 18: e767-767e775. [Crossref]
- Niaz K, Maqbool F (2017) Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol Health 39: e2017009. [Crossref]
- Li CC, Shen Z, Bavarian R, Yang F, Bhattacharya A (2018) Oral Cancer: Genetics and the Role of Precision Medicine. Dent Clin North Am 62: 29-46. [Crossref]
- Bhatkar D, Kumar J, Purohit S, Jahagirdar D, Sharma NK (2016) ATM kinase inhibitor KU-55933 contribution in cisplatin mediated HeLa proliferation. International Journal of Pharmacology and Toxicology 4: 201-207.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646-674. [Crossref]
- Burrell RA, McGranahan N, Bartek J, Swanton C (2013) The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501: 338-345.
- Ahuja N, Sharma AR, Baylin SB (2016) Epigenetic Therapeutics: A New Weapon in the War Against Cancer. Annu Rev Med 67: 73-89.


