Abstract
Purpose: Colorectal cancer (CRC) is the third most common cancer in the United States, and prognosis is greatly influenced by stage at diagnosis. Early colorectal cancer can be subtle on CT scans showing only mild wall thickening, small polyps, or subtle lymph nodes in atypical draining location. Identifying these lesions on CT scan performed for nonspecific symptoms can help identify interval CRC and improve patient outcome. The purpose of the present study is to classify the undetected CRC on abdominal CT scan by their imaging features and whether early identification can downstage CRC patients.
Materials and methods: A retrospective analysis was conducted of patients (pts) diagnosed with CRC and receiving treatment or sought second opinion at Banner MD Anderson Cancer Center. Data collection included age, gender, ECOG, KRAS mutation status, and overall survival (OS). CT imaging was obtained from the time of diagnosis, as well as any prior abdominal imaging available. Images were reviewed for multiple CT features including appearance of mass, mesenteric infiltration, abnormal draining lymph nodes, contrast enhancement relative to adjacent mucosa, and intralesional calcifications. Staging was evaluated using available clinical note and CT scan, based on the TNM staging system for CRC.
Results: The 41 pts with 51 prediagnostic CTs from 1/1/2012 - 12/31/2015 had mean age of 68 years (range:44-90) Mean ECOG status for the population was 1.46. 41% of the prediagnostic CTs had undetected findings. 52 and 43 % of the undetected findings were in the rectosigmoid and ascending colon respectively. Of the 15 undetected masses, 9 appeared as asymmetric wall thickening, 3 as concentric wall thickening, and 3 as polyps. Of the 14 undetected lymph node groups, 2 were excluded due to stability or nonrelated condition. The remaining lymph nodes were found in the associated draining station and averaged 3±1.2 mm in size. On average, the stage at prediagnostic CT was 3A and the diagnostic CT was 3C (p=0.0015). Average time lapse between prediagnostic and diagnostic CT was 21 months (3-64 months).
Conclusion Our study demonstrated that high percentage of early-stage CRC findings are undetected on abdominal CT due to their subtle feature, with most undetected location in the rectosigmoid and ascending colon. In general, these subtle features predate the actual diagnosis by up to two years. Early detection of CRC can improve survival by lowering the stage from 3C to 3A, thus providing 36% improvement in 5-year survival. A dedicated search can be performed on the abdominal CT to improve detection by specifically looking for polyps, wall thickening, and small lymph nodes in the draining station.
Introduction
Colorectal cancer (CRC) is the third most common cancer for both males and females in the United States, responsible for 8% of the estimated cancer deaths in the United States in 2016 [1]. Survival of CRC patients depend on staging with a 5 year survival of 97% for stage I disease, 63-87% for stage II disease, 53-89% for stage III disease, and 11% for stage IV [2, 3]. Because of its slow growing biology, screening for CRC usually begins at age of 50 at a baseline frequency of once every 10 years [3]. However, there has been an increased incidence of CRC in adults aged 20-49 [4], which is concerning as this population falls outside of the routine screening age. Without a family history these patients do not undergo routine screening.
Fortunately, patients of this age group will present to medical facilities with other nonspecific complaints. In 2011, about 20% of US adults aged 20-64 visited the emergency room [5]. In patients presenting with nonspecific abdominal complaints, CT scan of the abdomen is a commonly ordered study, with an estimated 14.9 million abdominal studies done in the United States in 2006 [6]. In patients who present to the emergency department (ED) or outpatient imaging with nonspecific abdominal symptoms, identifying incidental CRC on a CT scan of the abdomen and pelvis at a preclinical stage can significantly improve their clinical outcome [3]. In evaluating abdominal disease, multiple etiologies can mimic advanced colon cancer, including diverticulitis and inflammatory bowel disease [7-9]. In advanced colon cancer, the imaging features are well established, including large obstructing mass with pericolonic infiltration and necrosis [10,11]. Once diagnosed, CT is then used to evaluate lymph node involvement and distant metastasis [12].
While there is abundant discussion of the imaging characteristics of more advanced CRC, the literature does not mention the early imaging features that are unreported. These imaging features are especially helpful in identifying incidental CRC for patients who visit the emergency department for non-cancer related symptoms. The purpose of this retrospective case series is to identify prediagnostic disease patterns associated with CRC, which may guide workup of patients presenting with nonspecific abdominal complaints and allow earlier cancer detection to dramatically improve patient outcome.
Materials and Methods
Patients
The institutional review board approved this retrospective study (Reference # 017417) and waived the requirement for informed consent regarding the acquisition of data. The study was compliant with the health insurance portability and accountability act (HIPAA). The list of patients was obtained from the tumor registry at Banner MD Anderson Cancer Center (Gilbert, AZ) from 1/1/2012 to 12/31/2015. Patient's inclusion criteria for the study were as follows: (a) histopathological diagnosis of CRC; (b) availability of imaging studies through our institution’s picture archiving and communications system (PACS, Fuji Synapse PACS) in digital imaging and communications in medicine (DICOM) format (baseline study); and (c) availability of at least one CT imaging study prior to date of diagnosis (prediagnostic study).
CT Imaging Acquisition and Analysis
Images were obtained from our institution’s PACS for all patients, including diagnostic and prediagnostic studies. Patients were anonymized and given a numerical subject ID. All available prior imaging studies were listed, in order of most remote to most recent, along with imaging from the time of diagnosis. CT images were examined by a body fellowship trained radiologist with 6 years of independent practice experience (JC). The location of the primary mass and years prior to diagnosis were recorded for each baseline study. The location of the primary mass is made known to JC when prediagnostic study is evaluated. This makes the read unblinded but does allow detection of the earliest features of CRC. The prediagnostic studies were assessed for presence of the following findings: mesenteric infiltration, presence and type of mass (circumferential or asymmetric wall thickening), CT enhancement, calcifications, and presence of mesenteric lymph nodes. For unenhanced studies, CT enhancement was not recorded. Lymph nodes were considered positive only if they were localized to the draining mesentery of the CRC on the diagnostic study. These were measured in the longest axis, given these are typically small. The commonly found subcentimeter lymph nodes of the mesenteric root and ileocolic lymph nodes were ignored [13,14]. Specific efforts were made to evaluate these features on the prediagnostic studies at the known location of the primary mass. The original report of the study was then compared to the re-assessment. A report that made no reference of the reassessed findings was considered a undetected, while any mention of the findings were considered detected.
CT Scans
Because the patients that visit our cancer center generally present for second opinion or are self-referred, we do not have control over many of the scan parameters. For our own institution, the CT scans obtained in the ER are scanned on a Toshiba 32 slice scanner with slices reconstructed to 3 mm thick slices. Depending on patient's body size, the contrast dose varies between 75 to 100 cc of Isovue 370 at 75 second delay after initiation of contrast administration. The images were reformatted into axial, sagittal and coronal planes for review. For CT studies obtained at the cancer center, the images are acquired using a 64 slice GE Lightspeed scanner. The images are acquired at 5 mm thick slices and reconstructed to both 2.5 and 5 mm thick slices in axial, sagittal, and coronal planes for viewing. Contrast dose consisted of 100 cc of Isovue 370 injected at 3 cc/s. The scan for the abdomen and pelvis is initiated at 70 seconds after initiation of contrast injection. For images not acquired in our institution, the CT images were more diverse, with a predominance of the images having IV contrast material. The slice thickness in general were at 5 mm in thickness with the available images predominantly in axial plane.
Staging
Staging was evaluated using available CT scan based on the TNM staging system for CRC as well as clinical report when full imaging assessment is not available, as not all patients present with baseline CT of the chest, abdomen, and pelvis. Based on American Cancer Society staging definition, the CT features of the diagnostic and prediagnostic studies were used to determine the stage at each study. The stage was then converted into a numerical scale with 1 being associated with stage 1 and 8 being associated with stage 4B. We did not include stage 2C due to its recent implementation. The differences in stage was tested using Student's T-test with two tails. The average baseline and prediagnostic stage was then compared to historical 5-year survival listed by the Americal Cancer Society informational website [3]; the difference in the historical 5-year survival is reported as the improvement in 5-year survival.
Results
Patient Demographics
A patient list containing 293 unique patients from 1/01/2012 – 12/31/15 was obtained from the Banner MD Anderson Cancer Center tumor registry and 41 patients were identified positive for colon cancer, with available prediagnostic CT scan. From these 41 patients, we identified 51 prediagnostic CT studies for evaluation, of which 16 were performed without intravenous contrast. 20 of the patients were female and 21 patients were male. The ages ranged from 45-90 years old at diagnosis. The mean age at diagnosis was 68 years with a standard deviation of 13.7 years. The average age for males was 70.3 ± 10.8 years (range 50 - 88); the average age for females was 65.6 ± 16.2 years (range 45-90). Table 1 lists the findings (both detected and undetected) from all prediagnostic studies.
Prediagnostic Imaging Features of CRC on CT
With the exception of calcification, the prediagnostic CT scans demonstrated identifiable features of CRC in 31 of the 51 studies (61%). 27 of the 41 (66%) patients had prediagnostic studies with at least one of the features present. Figure 1 gives an example of the various features of early CRC. Figure 2 shows the different identifiable features of all 51 studies at each time point, divided into varying time periods. The two most common prediagnostic features of CRC on CT were the presence of a mass and abnormal lymph nodes. 24 (47%) of all studies demonstrated lymph nodes and 22 (43%) had masses. Additionally, 10 (19.6%) of the studies had mesenteric infiltration. Of the 24 studies with mesenteric lymph nodes, 3 of the studies had stable lymph nodes for at least 33 months while 2 studies were from a patient with lymphoma. Excluding these, 19 of the studies had lymph nodes in the draining station. Of the 21 masses that were identified, asymmetric wall thickening of the colon was the most common with 11 of 21 masses in this category. 7 masses were circumferential and 3 were polypoid. Of these masses, 15 (71%) of them enhanced similar to the adjacent mucosa with one enhancing to a lesser degree. 5 masses were identified in non-contrast studies.
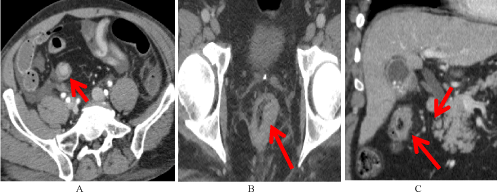
Figure 1. Examples of Undetected Features. Features of early CRC that were present but were unreported during original interpretation of the study. A) Sigmoid polyp (red arrow). B) Asymmetric rectal wall thickening (red arrow). C) Mesenteric infiltration with lymph nodes in the draining mesenteric stations (red arrows).
The prediagnostic studies were obtained from 1 to 112 months prior to the baseline diagnostic images. The combinations of features were plotted against time in Figure 2. In these studies, features were identified at different earliest time points. The earliest mass was identified at 70 months prior to diagnosis while the earliest mesenteric infiltration was seen at 36 months. Although abnormal lymph nodes may be seen, as early as 64 months prior to initial diagnosis, some may be of benign etiology as there was a case with abnormal lymph nodes that remained stable for 57 months.
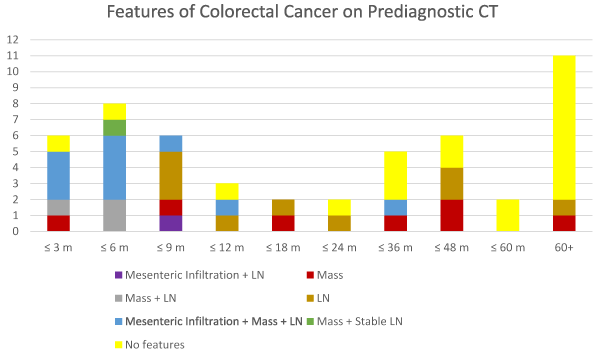
Figure 2. Abnormal Findings on all Prediagnostic CT Scans. A plot of the findings on CT of the abdomen and pelvis according to the time prior to diagnosis. Relevant features generally occur within 2 years of diagnosis.
There are several interesting findings from Figure 2. The most important finding is that CT abnormalities had been present in 23 of 27 (85%) prediagnostic studies within 2 years of diagnosis. The most common feature was abnormal lymph nodes which was seen in 20 (74%) studies. 16 (59%) of the studies had a mass and 10 (37%) had mesenteric infiltration. The second most important finding is that only 8 of 23 (35 %) studies had abnormalities attributable to CRC more than 2 years before diagnosis. Additionally, only 2 studies had CRC features more than 4 years before diagnosis. One study demonstrated a 2 mm mesosigmoid lymph node at 64 months before diagnosis and the other showed a polypoid mass at 70 months before diagnosis. Figure 2 also demonstrates that 10 of the 11 studies with mesenteric infiltration occurred within 1 year of diagnosis with the single exception occurring at 36 months. Furthermore, mesenteric infiltration occurred concurrently with lymph node involvement as there were no cases of isolated mesenteric infiltration.
Undetected Prediagnostic Imaging Features of CRC on CT
21 of the 51 studies reviewed had features of CRC that were not noted during the initial reading. There were undetected findings in 20 of the 41 patients (48.8%) who had prediagnostic studies. The undetected features are listed in Table 1. 9 of the studies had undetected findings in the cecum or ascending colon, 6 in the rectosigmoid, 5 in the rectum, and 1 in the transverse colon. The two most commonly undetected prediagnostic features of CRC on CT were the presence of a mass and abnormal lymph nodes. 15 of the 21 studies (71%) had masses. 9 (60%) of these masses consisted of asymmetric wall thickening of the colon, while 3 (20%) were circumferential, and 3 (20%) were polyps. 13 of the masses demonstrated enhancement. 12 (80%) of the masses exhibited enhancement similar to the adjacent mucosa and one enhanced to a lesser degree. Two masses were found on non-contrast studies. 14 of the 21 (66.7%) studies had lymph nodes. Of these, one study was from a patient with lymphoma and another lymph node had been stable for 37 months. Excluding these, 12 of 21 (57%) studies had lymph nodes in the draining station. 9 (75%) of these lymph nodes were greater than 3 mm in size with 4 lymph nodes greater than 5 mm. 3 (25%) lymph nodes were smaller than 3 mm. The average lymph node size was 3.83±1.64 mm.
Different combinations of undetected CRC features were seen at different times before diagnosis. Figure 3 shows these undetected features of CRC at different time points. Of those studies with mesenteric infiltration, 4 of the 5 studies occurred within one year of diagnosis. The other study occurred 3 years before diagnosis. Within 2 years of diagnosis, only 2 studies had a solitary mass. Only 6 of the 21 studies with undetected findings occurred prior to 2 years. There is a strong predilection for a solitary mass beyond 2 years as 4 of the 6 studies demonstrated this finding. Beyond 2 years of diagnosis, 1 study had the combination of mass, infiltration, and lymph node and the other only had a lymph node.
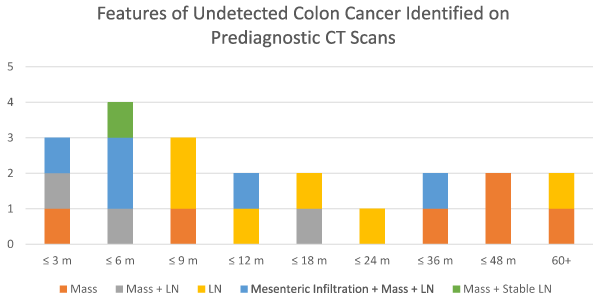
Figure 3. Features of Undetected CRC on Prediagnostic CT Scans. Plot of the undetected features based on the time that prediagnostic CT was obtained. Within 2 years of diagnosis, a variety of features are seen including mass, lymph nodes, and mesenteric infiltration. Prior to 2 years, most common feature is a mass.
Influence on 5-Year Survival of Undetected Findings
Based on historical records, the staging of CRC is important determinant of patient's overall survival. Table 2 shows the prediagnostic and diagnostic staging for each patient. The average prediagnostic stage was 3A, whereas the average diagnostic stage was 3C (p = 0.0015). The historical 5-year survival percentages for stage 3A and 3C are 89% and 53%, respectively [2,3]. This demonstrates an overall 5-year survival improvement of 36% with early detection.
Discussion
Colon cancer is responsible for almost 50,000 deaths every year in the United States [1]. Identification of CRC in its early stages is crucial for long-term survival, with 5 year survival of approximately 97% for stage I disease, 63-87% for stage II disease, 53-89% for stage III disease, and 11% for stage IV disease [2,3]. This is especially important particularly with the rising incidence of CRC in younger patients who are not yet eligible for screening colonoscopy [4,15]. From 1974 onward, the incidence of the colorectal cancer has continued to rise in patients younger than 50 [15]. This has resulted in the lowering of screening age from 50 to 45 for normal risk individuals in order to detect more of the younger patients [16]. This population of patients will likely visit either the emergency room or receive outpatient imaging for nonspecific abdominal complaints. It is during these scans that preclinical CRC may be identified before it becomes metastatic. Even if these cancers are diagnosed at stage 3A, the patients still derive significant survival benefit by preventing stage IV or even late stage III disease.
Our study showed that there are several critical time periods for CRC growth. First, a predominance of early CRC findings occur within 2 years of diagnosis. In review of all studies, 23 of 27 (85%) had positive findings within 2 years of diagnosis while only 8 of 23 (35%) studies had positive findings beyond 2 years of diagnosis. This suggests that CRC growth from an imaging identifiable lesion to a clinically significant lesion may take at most 2 years. Second, mesenteric infiltration and lymph node enlargement tend to be a late phenomenon of tumor growth. This is suggested by the fact that 10 of 11 studies with mesenteric infiltration occurred within one year of diagnosis with a single exception where mesenteric infiltration was seen at 3 years before diagnosis. These findings also suggest that once mesenteric infiltration is identified on imaging, a clinically significant lesion may be seen within a year. Third, mesenteric infiltration does not occur alone as all mesenteric infiltration occurred with lymph nodes in the draining nodal stations. This would suggest that mesenteric infiltration occurs either slightly before or simultaneously with nodal enlargement.
Our study also showed a high percentage of early CRC findings went undetected. Of the 31 studies with positive findings, 21 studies from 20 patients had findings that were undetected during the initial reading. Alarmingly, almost half (48.8%) of all the patients in our study had undetected findings of CRC on CT scan. The two most commonly undetected prediagnositc features of CRC on CT were the presence of a mass and abnormal lymph nodes. The undetected lesions, 20 of the 21 lesions are located on the rectosigmoid and ascending colon, which makes dedicated search of wall thickening, mass, and lymph nodes important in identifying early CRC. The remaining single case involved transverse colon.
Our reported missed rate of 48.8% is higher than reported rates for CT misses [17-19]. In the previous studies, missed CRCs occurred with all modalities, although highest with double contrast barium enema at 27% while CT had a miss rates between 6 to 20% depending on technique [17-19]. These studies evaluated missed cancers within 1 and 3 years of diagnosis for CT and all modalities, respectively [17,19]. In the report from Klang et al., the missed colon cancers were missed because of absence of fat stranding, vascular engorgement, or mesenteric lymphadenopathy [17]. The missed cancer averaged 3.3 cm in length where as the detected cancers measured 5.1 cm [17]. Although the absence of these features with CRC resulted in misidentification, our finding shows that even with these features, misidentification can still occur if they are subtle as the one reported here. Our masses tend to be less conspicuous as many of our CT studies were obtained beyond the one year period studied by Klang et al. [17]. Part of the misses may result from local practice differences. The reported studies in the literature were obtained from single practice group which has more homogeneous practice pattern. Our study gathered CT data from multiple practice groups and regions both within and without the hospital system. This is expected to cause greater practice variation than the reported cases. The combination of these factors may explain why we have higher miss rates on our CT studies.
The significance of enlarged mesenteric lymph nodes depends on etiology. Lucey et al. have observed that mesenteric lymph nodes under 5mm in size and often noted at the mesenteric root are seen in the non-cancer healthy population, with no apparent disease correlation with a 1 year follow up [14]; in another report by the same authors, they note that location, number, and appearance of mesenteric lymph nodes are important in distinguishing the etiology amid a myriad of potential causes [13]. Given the conflicting reports; we limited our evaluation to just the lymph nodes in the draining station of the tumor. 12 of 21 studies with undetected findings of CRC had abnormal lymph nodes that were not stable or associated with lymphoma. Stable lymph nodes occurred on two occasions with one due to lymphoma and the other due to prior perforated diverticulitis. These are consistent with literature as other etiologies of mesenteric lymph nodes [13]. In our study, 57% of the studies with undetected findings had abnormal lymph nodes. We also observed that patients who would later develop CRC had increased mesenteric lymph nodes up to 64 months prior to diagnosis in the draining station of the primary tumor. 75% of the studies with undetected lymph nodes had lymph nodes larger than 3 mm with 33% having lymph nodes larger than 5 mm. Only 25% of studies with undetected findings of CRC had lymph nodes smaller than 3 mm. The various etiologies of these lymph nodes demand that further assessment be obtained, although given their sizes, either colonoscopy or follow up CT would be most suitable.
Calcifications in the abdominal viscera have been described in the literature as fairly rare clinically, and most often related to granulomatous disease [20]. Calcification in the setting of mucinous adenocarcinoma of the colon and rectum has been described as early as the 1950s [21], and are more commonly found in mucinous than nonmucinous carcinomas [22]. There were no colonic calcifications noted on any of the images included in our study. Review of pathology reports revealed that only 3 patients included in our study had mucinous carcinoma. This is in concordance with prior studies regarding the relative rarity of abdominal calcifications as well as that of mucinous colorectal carcinoma.
Our findings describe pre-diagnostic imaging features of CRC on abdominal imaging. Our main limitations include relatively small number of studies, variability in image quality of the outside imaging studies and non-contrast imaging studies. There is also potential bias given that the radiologist analyzing the images had access to imaging from the time of diagnosis and was therefore aware of the location and characteristics of the primary mass. This provided a more limited area in which to look with increased scrutiny, and it is unclear if some of the subtler features identified would be evident without foreknowledge of the eventual lesion location. Nevertheless, our findings are intriguing, and consistent patterns were observed that certainly warrants larger studies to determine the feasibility and utility of this modality to potentially help guide the management of patients with nonspecific abdominal complaints, and eventually may improve patient outcomes as more cases of CRC are diagnosed at an earlier stage. Work is in progress to assess whether the features identified on the present study will be sufficiently sensitive and specific in identifying early CRC in the ED CT studies.
Conclusion
Our study demonstrates that a high percentage of CRC findings are undetected on abdominal CT due to their subtle features, with the most undetected locations in the rectosigmoid and ascending colon. A dedicated search can be performed on the abdominal CT to improve detection by specifically looking for polyps, wall thickening, and small lymph nodes in the draining station. Another significant finding is that most studies had abnormal findings within 2 years of clinical diagnosis suggesting that CRC growth from an imaging identifiable lesion to a clinically significant lesion may take approximately 2 years. Although routine CT may not be able to diagnose earlier stage disease (stages 1 and 2), it can still provide significant survival benefit as early detection was shown to improve survival by lowering stage from 3C to 3A, thus providing 36% improvement in 5-year survival, based on historical survival data. Further prospective study is required to evaluate the sensitivity, negative predictive value, and positive predictive value of screening the colon for the 3 main abnormal features of the present study. Associated downstream costs for the false positive studies that result in unnecessary exams will also need to be evaluated.
Funding
The present research did not receive any external or internal funding.
Disclosures
Dr. Kundranda has served on the advisory board and speaker bureau for Celgene, Amgen, and Bayer.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The local IRB approved waiver of consent for the project.
References
- Siegel RL, KD Miller, A. Jemal (2016) Cancer statistics, 2016. CA Cancer J. Clin 66: 7-30. [Crossref]
- Kniery KN, M Steele SR (2016) Staging, prognosis, and survivorship in colon cancer. Seminars in Colon and Rectal Surgery.
- Society AC (2015) Colorectal Cancer, in American Cancer Society, A.C. Society, Editor., American Cancer Society.
- Alteri, R., et al., Colorectal Cancer Facts & Figures. 2011, American Cancer Society: Atlanta.
- Gindi RM, RA Cohen, WK Kirzinger (2012) Emergency room use among adults aged 18-64: Early release of estimates from the national health interview survey, January-June 2011, CDC, National Center for Health Statistics: Atlanta.
- Mettler FJB, M Faulkner, K Gilley, DB Gray, JE Ibbott, et al. (2009) Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources 1950-2007. Radiology 253: 520-531.
- Zhang X, JP Gaspard, DC Chung (2001) Regulation of vascular endothelial growth factor by the Wnt and KRAS pathways in colonic neoplasia. Cancer Res 61: 6050-6054. [Crossref]
- Lips LC, PT Pickhardt, PJ Cremers, SE janssen-Heijnen, ML de Witte, et al. (2015) Sigmoid cancer versus chronic diverticular disease: differentiating features at CT colonography. Radiology 275: 27-35. [Crossref]
- Padidar AM, et al. (1994) Differentiating sigmoid diverticulitis from carcinoma on CT scans: Mesenteric inflammation suggests diverticulitis. AJR 163: 81-83. [Crossref]
- Horton KM, RA Abrans, EK Fishman (2000) Spiral CT of colon cancer: Imaging features and role in management. Radiographics 20: 419-430. [Crossref]
- Ricci R, Sergiacomi GL, Orlacchio A, Fanucci E, Pocek M et al. (1992) Computed tomography detection of gastrointestinal neoplasms. Ital. J. Gastroenterol 24: 489-493. [Crossref]
- Balthazar EJ, Megibow AJ, Hulnick D, Naidich DP (1988) Carcinoma of the colon: detection and preoperative staging by CT. AJR Am J Roentgenol 150: 301-306. [Crossref]
- Lucey BC, Stuhlfaut JW, Soto JA (2005) Mesenteric lymph nodes seen at imaging: causes and significance. Radiographics 25: 351-365. [Crossref]
- Lucey BC, Stuhlfaut JW, Soto JA (2005) Mesenteric lymph nodes: detection and significance on MDCT. AJR Am J Roentgenol 184: 41-44. [Crossref]
- Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, et al. (2017) Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst 109: 1-3. [Crossref]
- American cancer society guideline for colorectal cancer screening 2018.
- Klang E1, Eifer M2, Kopylov U3, Belsky V2, Raskin S2, et al. (2017) Pitfalls in diagnosing colon cancer on abdominal CT. Clin Radiol 72: 858-863. [Crossref]
- Than M1, Witherspoon J1, Shami J1, Patil P2, Saklani A3 (2015) Diagnostic miss rate for colorectal cancer: an audit. Ann Gastroenterol 28: 94-98. [Crossref]
- Vaughan-Shaw PG, Aung M, Knight H, Williams T, Borley NR, et al. (2015) Systematic analysis of missed colorectal cancer cases and common pitfalls in diagnosis. Frontline Gastroenterol 6: 232-240. [Crossref]
- Stoupis C, Taylor HM, Paley MR, Buetow PC, Marre S, et al. (1998) The Rocky liver; radiologic-pathologic correlation of calcified hepatic masses. Radiographics 18: 675-685. [Crossref]
- SANGSTER AJ (1954) Calcification in carcinoma of the rectum. J Fac Radiol 6: 139-141. [Crossref]
- Ko E, Ha HK, Kim AY, Yoon KH, Yoo CS, et al. (2007) CT differentiation of mucinous and nonmucinous colorectal carcinoma. AJR Am J Roentgenol 188: 785-791. [Crossref]



