Abstract
Background: Recruitment and retention of underrepresented groups in clinical trials is a national challenge and contributes to the high rate of enrollment failures. Decentralization of clinical trials may create opportunities for greater participation from diverse communities. At present, there are several national guidelines to support decentralization (site-less, direct-to-participant, hybrid, remote, or virtual clinical research) efforts. By removing participation access barriers, the flexibility conferred by decentralized clinical trials (DCTs) may result in increased enrollment and retention in clinical trials of underserved and underrepresented populations.
Methods: To explore both clinical trial staff and participant’s perspectives on DCTs, we developed, administered, and analyzed an electronic Qualtrics questionnaire from June 2023 through August 2023 at the New York University Grossman School of Medicine’s Clinical and Translational Science Institute’s Clinical Research Centers in Brooklyn, Manhattan, and Long Island.
Results: There were significant differences between the proportion of staff and participants who identified survey item choices as concerns about the quality, safety, and finances of decentralized trials. Transportation was selected by most respondents as a barrier to centralized clinical studies. Technological access and proficiency were most often selected as barriers to decentralization of clinical studies, by staff and participants respectively.
Conclusion: Our survey identified common concerns, challenges, and benefits of DCTs and can inform a more inclusive study design, operational approach, quality improvements, and best practices to improve recruitment, retention, and enrollment goals.
Keywords
decentralization, clinical, trial, translational, research, recruitment, accessibility
Introduction
Decentralized clinical trials (DCTs) are trials where some or all of the trial’s activities occur at locations other than traditional clinical trial sites [1]. Decentralized research activities can include performing medical procedures at locations other than the central site, providing research encounters through telehealth or home visits, collecting and monitoring data with digital health devices, providing investigational products, and outsourcing research procedures and laboratory and radiological testing to local health providers. The flexibility of DCTs can improve recruitment and retention of participants and further serve to increase diversity in trials [2, 3]. At present, the American Society of Clinical Oncology (ASCO) is the only entity that published guidelines to approach implementing DCTs in the United States [4], despite a national draft guidance recently released by the U.S. Department of Health and Human Services Food and Drug Administration (FDA) in 2023. Gathering thoughts and opinions from various stakeholders, from different regions, especially in underserved areas will provide insight on advantages, disadvantages, and prioritization that should be considered when decentralizing clinical research.
Participation in clinical trials from underserved and underrepresented groups is limited due to several barriers including but not limited to financial barriers, transportation, misinformation and distrust, and accessibility [5, 6]. This lack of diverse representation hinders the ability for some populations to receive novel treatments and impacts generalization of trial findings [6]. Moreover, as only about 5% of eligible participants participate in all clinical research, a further decrease of participation from underrepresented groups will impact the understanding of a potential therapy on sub-groups [5,6]. The rate of participation may vary within different areas of clinical research and different time periods, such as trials during COVID-19, but still show a pattern of underrepresentation from certain populations. A 2020 cross-sectional study surveying a group of adults found that of the 9% of adults invited to participate in a clinical trial, only 47% elected to participate [6]. Respondents who were non-Hispanic Black, college educated, single, or urban-dwelling or had medical conditions had higher odds of clinical trial participation, while non-Hispanic Black respondents had lower odds, highlighting the need for strategies to increase equity in clinical research participation. These barriers to participation also result in low recruitment rates and ineffective enrollment in clinical trials, which hinder the ability of studies to produce statistically significant results. DCTs can therefore enhance participation from diverse communities, improve research rigor, and ultimately accelerate research translation by supporting enrollment goals and increasing generalizability.
Differences in opinions and concerns from various stakeholders can be yet another barrier to decentralizing clinical trials. Discordances in participant and healthcare worker perspectives on safety have been noted, due to the discrepancies in knowledge and awareness of risks involved [7-9]. Varied levels of healthcare literacy and inefficient communication techniques can create a difference in opinions regarding healthcare choices [9]. Thus, it is vital that individualized communication be employed by healthcare workers, so that participants are fully aware of risks, benefits, and other aspects involved in their care.
Here, we present survey results from clinical research staff and participants at an urban, underserved clinical research center network. These perspectives will serve to address the differences in opinions between key stakeholders and identify and clarify barriers and advantages to DCT participation. In doing so, we can continue to build a path towards standardization of operations in decentralization to improve recruitment and retention and enable attainment of targets for enrollment and outcomes [5].
Materials and methods
We developed a quality improvement project to determine the barriers and facilitators for decentralized clinical trials among participants and research staff. A structured, anonymous, Qualtrics DCT survey was created as informed by literature review regarding the decentralization process, considerations, limitations, best practices, federal guidance, and by clinical research content experts that included clinical research nurses, clinical research coordinators, principal investigators, researchers, leadership, evaluation science experts, and a biostatistician. The DCT survey was designed to examine opinions regarding decentralization of clinical trials of those involved in clinical research within the NYU Langone Health research network (Manhattan, Brooklyn, and Long Island) including research participants, NYU Clinical and Translational Science Institute (CTSI) Clinical Research Center (CRC) staff, other research teams, investigators, research nurses, coordinators, and pharmacy personnel [9]. The survey was distributed to these key stakeholders across the health system to identify congruences and discrepancies.
Participant-facing (Appendix A) and research staff-facing (Appendix B) surveys were created and differed in terms of health literacy, specifically there were differences in wording and language to make the surveys more understandable to participants [10]. These surveys are available in the Supplementary file. In the staff-facing survey, general research processes were stated, while the participant-facing survey used first person language to describe the activities from the participant’s point of view. Respondents received an email with a link to the survey or offered to fill out the survey on an iPad based on their predefined cohorts: participants and clinical research staff. Furthermore, research respondents were provided an option to complete the survey after completion of their clinical visit. Post clinic survey respondents completed surveys on ipad devices.
A total of 17 staff-survey questions and 18 participant-survey questions were developed, with some employing branching logic. These questions were created to assess familiarity with DCTs, gather demographic information from respondents, and to capture perspectives on DCTs from six domains of healthcare quality: safety, effectiveness, participant-centered, timeliness, efficiency and equity [11]. A voluntary consent portion was also included. Both participant-facing and research staff-facing surveys were reviewed by an NYU biostatistician and other survey experts to determine the sample size needed for statistical power and the optimal survey design to maximize response rate. These anonymous surveys were part of an NYU CTSI CRC quality, safety, and efficiency improvement initiative.
Both the participant-facing and research staff-facing surveys provide background on DCTs, collect demographic information, and ask for the participant’s research background. Respondents were asked 3 questions about safety, quality, and financial concerns of DCTs, with multiple drop-down response options, where one could select multiple responses. Respondents were then asked another 3 questions regarding barriers to participation in centralized and decentralized clinical research, including indicating which populations would benefit from decentralization, again with multiple drop down response options where one could select multiple responses.
Survey respondents were categorized into two cohorts, participants and research staff, for comparison. Staff and participant respondent demographics are detailed in Appendix C and D, respectively. Survey responses were analyzed by stakeholder perspectives on equivalent questions grouped by motifs: Barriers and concerns about decentralization. Answer choices were excluded from analysis if there was not an equivalent answer choice between the two surveys.
Statistical software, Excel 2024, Version 2502 64-bit was used to collect and analyze data. Descriptive statistics were used to determine the proportion of each cohort that indicated an aspect of clinical research as a barrier to participation in centralized or decentralized clinical studies, as well as which populations would gain the most from decentralization. Fisher’s Exact tests were used to compare the proportion of staff and participants who selected an answer choice (answered “yes” to a concern) for questions assessing whether the respondent had concerns regarding the safety, quality, and finances of DCTs. The multiple testing method was not used to adjust p-values in our analysis.
Results
There were 125 survey respondents (80 participants, 45 staff). 17 participant and 13 staff survey submissions were excluded due to incomplete responses. 5 additional participant submissions were excluded due to erroneous completion by staff respondents. 12 of the 17 incomplete participant respondents and 12 of the 13 incomplete staff respondents did not complete items beyond the demographics item, which was the first question of the survey. 58 participant (77.3% response rate) and 32 staff (71.1% response rate) survey submissions were included in the final data analysis.
In response to the question, “Which participant populations do you think stand to gain the most from decentralization,” of the complete staff survey submissions, most staff respondents (81.25%) answered that “Immunocompromised/chronically ill” populations will benefit most from decentralization (Figure 1). Of the complete participant survey submissions, most participant respondents indicated that “Geriatric” (75.86%) and “Rural” (74.14%) populations will benefit the most.
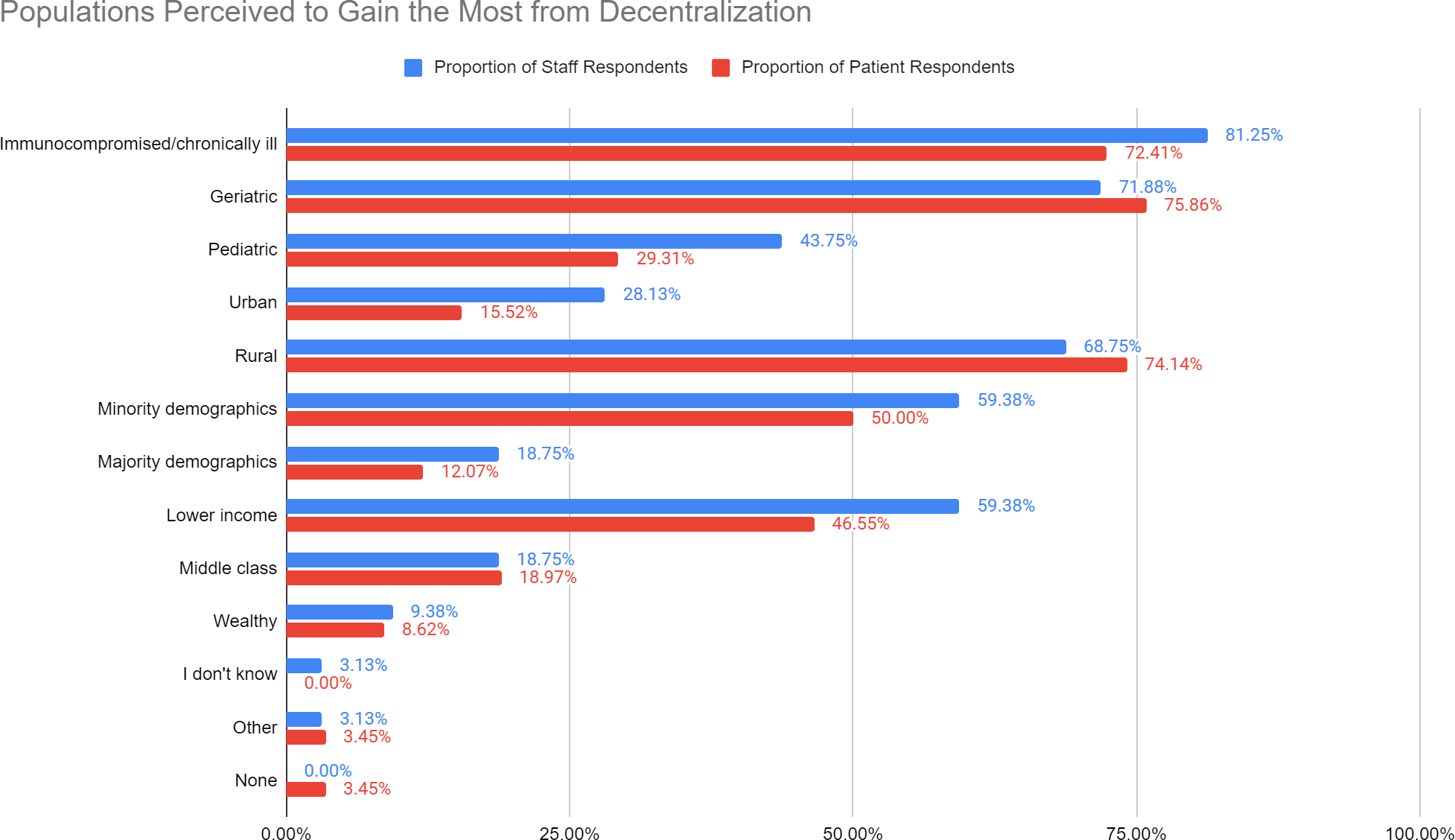
Figure 1. Staff and participant responses regarding populations to benefit from decentralization. Analysis of staff and participant responses to the question, “Which participant populations do you think stand to gain the most from decentralization?” Responses were stratified as staff (blue) and participant (red) and included, Immunocompromised/chronically ill, Geriatric, Pediatric, Urban, Rural, Minority Demographics, Majority Demographics, Lower Income, Middle Class, Wealthy, “I don’t Know”, Other, None - response rates are included. Options listed above were displayed in a drop down menu pattern with multiple overlapping options for selection
In response to the question, “What barriers do you foresee preventing participant enrollment in fully onsite research studies,” of the complete survey submissions, the majority of staff (75.00%) and participants (72.41%) perceive “Transportation” as a barrier to enrollment in centralized studies (Figure 2). There were no statistically significant differences between the proportion of staff and participants that selected “Finances/cost,” “Transportation,” “Location,” “Technological proficiency,” “Technological access,” “Time spent for participation,” “Medical mistrust,” “Support system,” “I don’t know,” “Other,” and “None” as a barrier. However, there was a statistically significant difference between the proportion of staff and participants that selected “Language/interpreter availability” (p=0.0099).
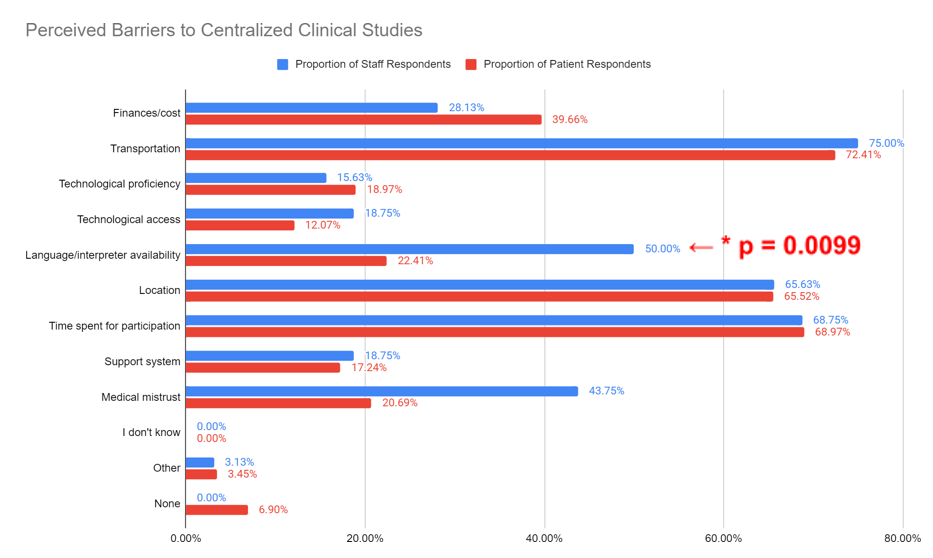
Figure 2. Staff and participant responses regarding barriers to participation in centralized clinical studies. Analysis of staff and participant responses to the question, “What barriers do you foresee preventing participant enrollment in fully onsite research studies?” Answers included finances/cost, transportation, technological proficiency, technological access, language/interpreter availability, location, time commitment, support system, medical mistrust, “I don’t know”, Other, and None. Answers were stratified by staff (blue) and participants (red), response rates are included. A statistically significant difference was identified between the proportion of staff and participants that selected “Language/interpreter availability” (p=0.0099)
Additionally, in response to, “What barriers do you foresee preventing participant enrollment in decentralized research studies (including hybrid DCT)?”, 68.75% of staff report “Technological access” as a barrier to enrollment in decentralized studies (Figure 3). However, the percentage of participant responses (68.25%) show that most respondents perceive “Technological proficiency” as a barrier to enrollment in decentralized studies. However, there was a statistically significant difference between the proportion of staff and participants that selected “Language/interpreter availability” (p=0.036).
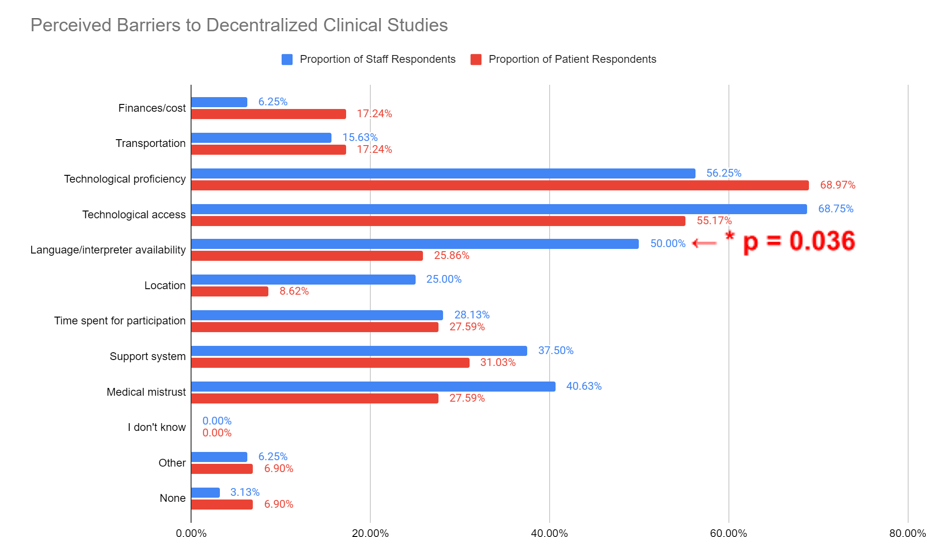
Figure 3. Staff and participant responses regarding barriers to participation in decentralized clinical studies. Analysis of staff and participant responses to the question, “What barriers do you foresee preventing participant enrollment in decentralized research studies (including hybrid DCT)?” Answers included finances/ cost, transportation, technological proficiency, technological access, language/interpreter availability, location, time commitment, support system, medical mistrust, “I don’t know”, “Other” and “None”. Answers were stratified by staff (blue) and participants (red) with response rates included. A statistically significant difference was identified between the proportion of staff and participants that selected “Language/interpreter availability” (p=0.036)
Additional survey questions queried staff and participant’s concerns about DCT safety, quality, and financial impact. P-values from a Fisher’s Exact Test analysis to determine differences between staff and participant responses are outlined in Table 1. Statistically significant differences between groups were seen in 2 responses to the question, “Which of the following safety concerns (if any) do you have about decentralized clinical trials?” and include “Appropriate drug storage”/”Being able to store the drug (e.g., at specific temperatures, in a safe location)” and “None.” In terms of the quality of decentralized studies, there were statistically significant differences between the number of staff and participants in regards to the responses, “Accurate data monitoring”/”Collecting and reporting data by yourself (e.g., blood pressure, sleep schedule, steps),” “Protocol adherence”/”Being able to follow research instructions by yourself,” “Staff communication with participant”/”Communicating with staff,” and “None.” Statistically significant differences were demonstrated between staff and participant responses regarding DCT financial concerns with “Additional resources”/”Buying the necessary equipment,” and Additional trainings/“Having to attend additional trainings” responses.
doi
|
Staff response selected “Yes” |
Participant response selected “Yes” |
P-Value |
Which of the following safety concerns (if any) do you have about decentralized clinical trials? (Select all that apply). If none, please explain why. |
Adverse effects |
Experiencing undesired drug effects |
0.51 |
Appropriate drug storage |
Being able to store the drug (e.g. at specific temperatures, in a safe location) |
0.008* |
Appropriate drug transportation |
Receiving the drug in a safe and timely manner |
0.4501 |
Appropriate drug administration |
Taking the drug by yourself |
0.18 |
participant privacy |
Maintaining privacy of your health information |
0.75 |
I don't know |
I don't know |
0.12 |
Other |
Other |
0.11 |
None |
None |
0.0031* |
Which of the following quality concerns (if any) do you have about decentralized clinical trials? (Select all that apply). If none, please explain why. |
Accurate data monitoring |
Collecting and reporting data by yourself (eg. blood pressure, sleep schedule, steps) |
0.012* |
Specimen quality |
Sample quality (eg. blood, urine, saliva) |
1.00 |
Protocol adherence |
Being able to follow research instructions by yourself |
0.0013* |
Participant experience |
Your personal experience |
0.16 |
Participant recruitment |
Getting enough participants to participate |
0.71 |
Staff communication with participant |
Communicating with staff |
0.031* |
I don't know |
I don't know |
0.12 |
Other |
Other |
1.00 |
None |
None |
0.020* |
Which of the following financial concerns (if any) do you have about decentralized clinical trials? (Select all that apply). If none, please explain why. |
Additional resources |
Buying the necessary equipment |
0.030* |
Additional trainings |
Having to attend additional trainings |
0.042* |
Third party contracts |
Traveling to off-site locations |
0.59 |
Participant reimbursement |
Change in compensation |
0.41 |
I don't know |
I don't know |
0.082 |
Other |
Other |
1.00 |
None |
None |
0.095 |
* indicates statistical significance with P ≤ 0.05 |
Table 1. Differences in staff and participant responses regarding concerns with decentralized clinical trials by item
Discussion
Differences between staff and participant responses are expected due to differences in clinical research knowledge, individual’s perceptions, and differences between groups in terms of their expectations regarding clinical research and DCTs. As seen in Figure 1, the majority of staff respondents answered that “Immunocompromised/chronically ill” populations will benefit most from decentralization (81.25% of staff respondents) while most participant respondents selected “Geriatric” and “Rural” populations will benefit the most (75.86% and 74.14% of participant respondents, respectively). This could reflect the difference in insights staff and participants have on each population. For instance, staff may interface more often with participants who are immunocompromised/chronically ill in an acute setting and have the knowledge to contextualize the consequences of such conditions. The NYU CRCs conduct clinical trials from a variety of specialities, so staff may have a better understanding of risk benefits for immunocompromised/chronically ill participants. Thus, this could make staff inclined to overestimate the limitations these participants experience as opposed to the participants themselves. Staff also have years of experience and understand the nuances of conducting clinical research and multitude of barriers that exist in conducting complex trials. On the other hand, participants may have more insight into geriatric and rural populations since these are geographic, environmental factors that directly impact their ability to participate in clinical trials.
Of the common barriers to participation in centralized clinical studies, staff and participant respondents selected “Transportation,” “Location,” and “Time spent for participation” most often and at similar rates (75.00% and 72.41%, 65.63% and 65.52%, and 68.75% and 68.97%, respectively) with the majority of both staff and participants selecting “Transportation” (75.00% and 72.41%, respectively) exhibiting the highest barrier. This congruency suggests that transportation to the clinical research site, location of the clinical research site, and time invested in clinical research participation are salient considerations from the perspectives of both groups that should be targeted when decentralizing clinical research activities. Significantly more staff (50.00%) than participants (22.41%) selected “Language/interpreter availability” as a barrier to centralized, decentralized, and hybrid studies. This may reflect participant underestimation of the impact of language challenges. Research staff indicated that language barriers hinder participant understanding and enrollment in centralized studies, while this was not as much of a concern to participants. This could suggest that measures should be taken to ensure that participants completely understand the research process, including asking for and providing for interpretation into participant’s preferred language at the initial encounter, as well as providing research consents and educational materials that are properly translated to their preferred language. Medical interpreters are utilized during all encounters for participants who report language other than English as preferred. NYU has access to Voyce and phone service interpreters with EHR embedded software.
There were significant differences in the proportion of staff and participants who indicated “None” as a safety concern and “None” as a quality concern. This could indicate a lack of participant knowledge about decentralization and need for more thorough participant education prior to enrollment. “Appropriate drug storage”/”Being able to store the drug (e.g., at specific temperatures, in a safe location)” and “None” were significantly different between staff and participant responses to safety concerns, which further support a difference in staff and participant knowledge about research study processes and procedures. Participant education could focus on investigational product safety to address this gap. In regards to financial concerns, “Additional resources”/”Buying the necessary equipment” and “Additional trainings”/”Having to attend additional trainings” differed significantly in terms of selection. This suggests a difference in staff and participant understanding of potential costs associated with decentralization. Expected financial costs and compensation should be addressed during the development of the research study and consent process, so staff and participants can be fully aware of any disadvantages and advantages of decentralization.
Several quality concerns were significantly different between the two cohorts, including “Accurate data monitoring”/”Collecting and reporting data by yourself (e.g., blood pressure, sleep schedule, steps),” “Protocol adherence”/”Being able to follow research instructions by yourself,” “Staff communication with participant”/”Communicating with staff,” and “None.” These differences may signify discordant understanding of important quality aspects between staff and participants and should be addressed to ensure that the quality of clinical studies is not compromised when moving necessary clinical trial activities off-site and reducing face-to-face time, or opportunities for clarification, between the participant and research staff. For example, a difference in the selection rate for “Protocol adherence”/”Being able to follow research instructions by yourself” could mean that one group underestimates the challenges of following the protocol when off-site. Thus, proper training, introducing research protocol and health literacy, and monitoring should be implemented to prevent compromising study quality.
This survey response supports previous findings from a literature review on DCTs conducted in Europe that found that DCTs were self-reported by participants to be more convenient than centralized clinical trials due to the possibility of the studies being conducted at home, which improved transport and time barriers [12]. The findings from this survey also support results from another study that analyzed interviews from European clinical research regulators, which suggested that investigators believe that DCTs benefit trial participants by reducing travel burden [13]. However, investigators from this same study also note that challenges to DCTs are the potential exclusion of digitally illiterate participants and lack of personal contact that may be needed for clinical judgment [13]. Based on these identified barriers to participation in centralized research trials, increasing accessibility to clinical research through decentralization may be most effective if focused on removing transportation, location, and time barriers.
Survey Limitations and Next Steps
The quantity of information that could be collected from our survey was limited by the use of multiple choice questions, which allows for better standardized data analysis and shorter time commitment; however, it is difficult to gather more detailed and nuanced data. Focus groups can help interrogate the idea of decentralized clinical research and present solutions to our approaches. Further limitations of the study include selection bias as the survey was conducted within a clinical research center, so responses were sampled from participants already involved in research and enrolled in a study. These participants may not be reflective of the underrepresented community. Participant populations that are underrepresented in clinical research already face barriers to participation and access as mentioned, so additional considerations should be taken to expand the reach of surveys to this group. As a next step, physician-investigators could administer the survey to their clinic participants and community-based organizations and volunteers could also aid in the distribution of the survey within the community. Furthermore, surveys were administered in English. Non-English speakers may have a different perspective on clinical research accessibility as compared to those that speak English. In going forward, we plan to use translated versions of the survey or will utilize an interpreter for participants who have non-English language preferences. And lastly, focus group qualitative interviews with key stakeholders will allow us to obtain more specific details on a more personal level as it relates to research experiences and perspectives.
Despite these limitations and selection biases, several important concerns, barriers, and perspectives regarding DCTs and clinical research in general were captured by both staff and participants. In response, we aim to suggest change in processes and practices to reduce barriers to participation in clinical research including the translation of study materials, use of interpreter services for all participants whose primary language is other than English at every study visit, and health literacy training for participants and staff. Moreover, we plan to expand the use of community health worker training on research to ensure direct community involvement and trust building with clients, community and our research enterprise [14].
Conclusion
There is no standardized approach for the design and implementation of DCTs outside of the 2023 FDA draft guidance and the ASCO editorial [4]. DCTs may serve to increase access to clinical research especially for underserved and underrepresented populations for many reasons including costs associated with transportation, accommodation, time off work, and childcare [15] but may also require additional resources, safety, and quality considerations.
The perspectives gathered here, from key stakeholders involved in clinical research, including participants and staff, offer valuable insights into the benefits, facilitators, barriers, and concerns pertaining to DCTs and clinical research in general. By analyzing these perspectives, we can plan a better approach and a more comprehensive strategy to inform the clinical research workforce on how to effectively design and implement DCTs with the overarching goal of increasing diverse enrollment of participants and ensuring retainment of underserved populations. A comparative analysis of participant and staff survey responses reveal disparities in knowledge and perspectives, underscoring the importance of addressing knowledge and access gaps to promote health equity in clinical research. Understanding perspectives on DCTs can identify preemptive measures necessary to ensure inclusivity and equity in trial participation, including recruitment and retainment of participants. The inherent flexibility of DCTs allows for a more personalized research experience, thereby mitigating some of the barriers faced by underrepresented populations and facilitating increased engagement in research. By leveraging insights from stakeholders in the design and implementation of DCTs, we can advance towards a more equitable and inclusive landscape in clinical research.
Declarations
Ethics approval and consent to participate
IRB approval was not needed since the project was designed to collect anonymous data for quality improvement purposes. Participants were asked to voluntarily consent to the survey before submitting any responses.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest
The authors declare that they have no competing interests.
This work was supported by The Robert A. Winn Diversity in Clinical Trials: Clinical Investigator Pathway Program, VCU and NYU Grossman School of Medicine. This research is supported in part by an NYU CTSA grant UL1TR001445 from the National Center for Advancing Translational Sciences, National Institutes of Health.
Authors’ contributions
AN, BD, JV, and LD analyzed and interpreted the anonymous survey data regarding perspectives on decentralized clinical trials. AN, BD, JV, and LD were major contributors in writing the manuscript. All authors contributed to project conception and design as well as manuscript writing and editing. All authors have read and approved the final version of this manuscript.
Acknowledgements
We acknowledge the NYU Langone Health research network (Manhattan, Brooklyn, and Long Island) staff who provided valuable support.
References
- Center for Drug Evaluation and Research. Decentralized Clinical Trials for Drugs, Biological Products, and Devices. FDA. 2023.
- Miyata BL, Tafuto B, Jose N (2023) Methods and perceptions of success for patient recruitment in decentralized clinical studies. J Clin Transl Sci 7: e232. [Crossref]
- Weber D, Nøhr C (2023) Decentralized clinical trials: Potentials for equity in digital health. Stud Health Technol Inform 304: 91-95. [Crossref]
- Adesoye T, Katz MH, Offodile AC (2023) Meeting trial participants where they are: Decentralized clinical trials as a patient-centered paradigm for enhancing accrual and diversity in surgical and multidisciplinary trials in oncology. JCO Oncol Pract 19: 317-321. [Crossref]
- Goodson N, Wicks P, Morgan J, Hashem L, Callinan S, et al. (2022) Opportunities and counterintuitive challenges for decentralized clinical trials to broaden participant inclusion. NPJ Digit Med 5: 58. [Crossref]
- Bae AS (2022) Key barriers against racial and ethnic minority participation in US clinical trials. Int J Clin Trials 9: 227-233. [Crossref]
- Williams CP, Everson NS, Shelburne N, Norton WE (2021) Demographic and health behavior factors associated with clinical trial invitation and participation in the United States. JAMA Netw Open 4: e2127792. [Crossref]
- Porter M, Bhattacharya S (2005) Investigation of staff and patients’ opinions of a proposed trial of elective single embryo transfer. Hum Reprod 20: 2523-2530. [Crossref]
- Dauer LT (2019) Patient perspectives on dialogue and shared decision making. Health Phys 116: 212-213. [Crossref]
- de Las Heras B, Daehnke A, Saini KS, Harris M, Morrison K, et al. (2022) Role of decentralized clinical trials in cancer drug development: Results from a survey of oncologists and patients. Digit Health 8: 20552076221099997. [Crossref]
- Institute of Medicine (US) committee on quality of health care in America. Crossing the quality chasm: A new health system for the 21st century. Washington (DC): National Academies Press (US). 2001. [Crossref]
- Dimitrova M, Hristov R, Djemadan A, Gaytandzieva I (2023) Decentralized clinical trials–current environment, potential barriers and facilitators for implementation and risk mitigation: A review of the literature. Acta Medica Bulgarica 50: 73-78.
- De Jong AJ, Van Rijssel TI, Zuidgeest MG, Van Thiel GJ, Askin S, et al. (2022) Opportunities and challenges for decentralized clinical trials: European regulators’ perspective. Clin Pharmacol Ther 112: 344-352. [Crossref]
- Yakubov A, Pimenova D, Ahmed A, Corvacho R, Madigan J, et al. (2023) The development of a clinical research educational training for community health workers using the joint task force for clinical trial competency framework. Front Pharmacol 14: 1295281. [Crossref]
- Chino F, Zafar SY (2019) Financial toxicity and equitable access to clinical trials. Am Soc Clin Oncol Educ Book 39: 11-18. [Crossref]



