Purpose: Daidzin, an isoflavone found in the Japaneese Kudzu root, has been found to exhibit dose dependent responses towards Jurkat leukemia cell viability after 16 hours, and caspase-3 enzymatic activity after 14 hours. The purpose of this experiment was to develop an understanding of the influence of peroxidase activity on daidzin-induced apoptosis.
Materials and methods: An H2O2 standard curve was established via a fluorometric assay, measuring the optical density at 570 nm against H2O2 concentration. Treated & untreated lysates were assayed in ascending concentrations in order to acquire their optical density at 570 nm and peroxidase activity.
Results: The results demonstrated no statistically significant difference in peroxidase activity between untreated and treated Jurkat leukemia cell lysates. Additionally, an inverse relationship between lysate concentration and peroxidase activity was observed.
Conclusion: Results suggest that an apoptotic pathway was used independent of the presence of high concentrations of H2O2 within the transduction pathway.
leukemia, daidzin, peroxidase, apoptosis, ROS
Daidzin, an isoflavone and 7-O glucoside of daidzein, is found in the Japanese Kudzu plant (Pueraria lobata, Fabaceae), and soybean leaves has been used in many cancer cells studies for its antioxidant, and potentially anti-cancer properties [1]. The Kudzu vine has been used in traditional Chinese medicine for millennia to reduce emetic, diuretic, and pyretic conditions. It has been used experimentally as a potential treatment for breast and colon cancers, as well as leukemias [2,3]. Speculated mechanisms of action for daidzin’s anti-cancer properties include induction of apoptotic cycle, cell cycle arrest, as well as anti-inflammatory, anti-angiogenic, and anti-metastatic effects [4].
Soy isoflavones in the form of aglocones (daidzein), and glucosides (daidzin) on highly invasive breast cancer cells MDA-MB-231 have shown an ability to suppress cell adhesion and motility by inhibiting transcription factors NF-kB and AP-1, resulting in the suppression of uPA (urokinase-type plasminogen activator) [5]. Other trials treating colon cancer HT-29 cells with Puerarin, the 8-C glucoside of daidzein, exhibited induced apoptosis via increased expression of bax and decreased expression in bcl-2 and c-myc. Furthermore, the treatment greatly increased the activation of caspase-3, a regulatory protein that plays a central role in the execution-phase of induced cell death [3]. Kundu et al. demonstrated daidzin efficacy against human leukemia cells via apoptosis induction and increased oxidative stress [6]. The objective of this study is to compare peroxidase and caspase-3 activity of daidzin treated jurkat leukemia cell lysate compared to untreated cell lysate to determine the apoptotic pathway induced by daidzin.
Storage and handling: The Peroxidase Activity Fluorometric Assay Kit was stored at -20˚C and protected from light. The Assay was warmed to room temperature before use. Vials were appropriately mixed prior to opening.
Reagent preparation and storage
H2O2 substrate: diluted to 12.5 mM by adding 5 ul of H2O2 substrate (0.88 M) to 347 ul of Assay Buffer. The diluted H2O2 substrate was stable for one day at 4˚C and one month at -20˚C.
HRP positive control: 1 ml of Assay Buffer was added into lyophilized HRP to prepare HRP solution. The HRP solution was stable for one day at 4˚C and one month at -20˚C.
OxiRed™ probe: Before use, the DMSO solution was warmed to 37˚C for 1-2 minutes to allow for it to liquefy, then mixed well. It was stored at -20˚C.
Standard curve preparations
Fluorometric assay: The H2O2 substrate solution was diluted to 0.1 mM by adding 10 ul of H2O2 substrate solution (12.5 mM) to 1240 ul of assay and mixed well. The H2O2 substrate solution was diluted again to .01 mM by adding 100 ul of the 0.1 mM H2O2 solution to 900 ul of Assay Buffer. The substrate solution (0.01 mM) was added into a series of wells in ten ul increments from 0-50 ul. Lastly, the final volume of each well was adjusted to 50 ul with Assay Buffer, which generated 0, 100, 200, 300, 400, 500 pmol/well of H2O2 standard.
Standard curve measurement: HRP positive control was diluted to a ratio of 1:199 in assay buffer. For each well, a total 50 ul reaction mix containing 2 ul of OxiRed™ Probe and 48 ul of diluted HRP positive control solution were prepared. Six wells were designated for the fluorometric assay, each with 50 ul, generating 300 ul of reaction mix per trial.
Positive control preparation: 1 ul of the diluted HRP positive control solution was pipetted into a single well. The final volume was adjusted to 50 ul with assay buffer.
Reaction mix: For each well, a 50 ul reaction mix was prepared by adding 46 ul of assay buffer, 2 ul of OxiRed™ Probe, and 2 ul of H2O2 substrate solution together. The reaction mix was added to each test sample and HRP positive control and incubated for 3 minutes at 37˚C.
Measurements: The optical density was measured at 570 nm for the fluorometric assay, and at 15-minute intervals until a period of linear range, which fell within H2O2 standard curve, was determined to calculate the peroxidase activity of the samples. To measure the optical density at 570 nm, the assay was placed into a micro-plate reader. A receipt with the appropriate data was then produced by the apparatus, which was immediately labeled, and time stamped. The 45-minute time point was established by observing the fluorometric assay’s standard curve, and stagnation of optical density at 570 nm from that point forward.
Calculations: The H2O2 standard curve was plotted. The peroxidase activity was calculated by finding the difference in optical density (∆A=A1-A0), and then applying it to the fluorometric assay’s standard curve to calculate the peroxidase activity of the samples.
Peroxidase Activity=B/(T x V)*Sample Dilution Factor=mU/ml
The results of the experiment demonstrated no statistical significance between peroxidase activity of untreated and daidzin treated lysate. A paired t-test was conducted on both trials of the 50 ul concentration for untreated and treated lysate. Trials one and two both produced p-values above the chosen significance (p=0.15 & 0.29 respectively). This suggests that the daidzin treated cell lysate did not have a higher H2O2 activity, or optical density at 570 nm than the untreated cell lysate. The peroxidase activities illustrated in Figures 1 support the conclusion that H2O2 activity was not higher in the daidzin treated lysate sample (Figure 1). The mean optical densities at 570 nm of untreated and daidzin treated lysates as displayed in table 1 display an inverse relationship (Table 1).
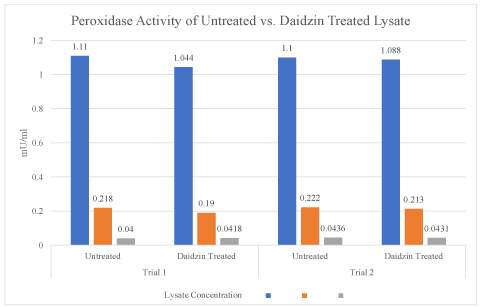
Figure 1. The peroxidase activities of untreated and daidzin treated lysate from trial 1 and trial 2 are illustrated side by side. The amount of activity (mU/ml) is displayed in relation to lysate concentration (ul). As in the optical density chart, no significant change between the two sample groups is evident, in addition to the inverse relationship of lysate concentration and peroxidase activity
Table 1. The peroxidase activities of untreated and daidzin treated lysate from trial 1 and trial 2 are illustrated side by side. The amount of activity (mU/ml) is displayed in relation to lysate concentration (ul). As in the optical density chart, no significant change between the two sample groups is evident, in addition to the inverse relationship of lysate concentration and peroxidase activity
Peroxidase assay: Raw data
Daidzin treated Jurkat leukemia cell lysate did not demonstrate a statistically significant increase in peroxidase activity relative to the untreated lysate samples. Both sample groups produced nearly equivalent levels of peroxidase activity. Additionally, results demonstrated an inverse relationship between the lysate concentration and peroxidase activity. The absent increase in peroxidase activity in the daidzin treated lysates was an unexpected finding.
Apoptosis is a required function for most multi-cellular organisms to survive and efficiently perform their intended functions without significant physiological & biochemical consequences. A minimum of three phases have been identified in the apoptotic process: induction, effector, and execution. Multiple studies have found oxidative stress, brought on by reactive oxygen species (ROS), to be a vital component in the induction of programmed cell death. Thiol reducants and antioxidants operate to ultimately inhibit apoptosis at this phase. This balance between ROS effects and antioxidant responses is comprehensively known as the oxidation response, often measured by increases in H2O2 within the cell. One of the oxygen species’ characteristics is the ability to act as an intracellular messenger molecule, allowing it to induce changes via phosphorylation of specific regulatory proteins [7]. Ultimately, this leads to activation of signaling pathways, transcription factors, and specific genes to alter cellular function.
The lack of increase in peroxidase activity indicates that the daidzin induced apoptosis may occur through a transduction pathway not reliant on reactive oxygen species. A previous study has established a relationship between the concentration of H2O2 and its correlation to apoptosis induction [8]. However, the effects of H2O2 on cancer cells at the lower micro-molar range could be associated with its intrinsically higher ROS content than normal cells [9]. In leukemia cells, H2O2 and other reactive oxygen species have demonstrated the ability to induce both pro-survival and pro-apoptotic transduction pathways [10]. Hydrogen peroxide increases the levels of activated caspase-8 sequestered within the mitochondria, thereby reducing apoptosis via the extrinsic apoptotic pathway. FasL stimulation via MAPK7, activated by H2O2, inhibits tyrosine phosphatase and ultimately confers resistance to leukemia cells against regulated cell death. The absent increase in peroxidase activity found in the daidzin treated lysates could potentially be due to the use of an apoptotic pathway independent on production of H2O2 in high concentrations. However, the role of other reactive oxygen species to induce regulated cell death is still possible (i.e. superoxide, hydroxyl radical, & nitric oxide, etc.), due to their prevalence in apoptotic pathways.
We found that peroxidase activity was not significantly different between untreated and daidzin treated lysate, and that an inverse relationship existed between peroxidase activity and lysate concentration. Conducting further research on ROS levels and protein expression could determine the apoptotic pathway being used (i.e. Bradford Assay). Once the apoptotic pathway has been discovered, the results may be applied to a clinical setting to enhance the treatment of specific sub-types of leukemia by being able to effectively & precisely induce apoptosis in the malignant cells. Additionally, the experiment could be improved by extending established time point of the assay in order to improve the reliability that peroxidase activity would not increase over an extended period of incubation. Future directions include performing the peroxidase fluorometric assay with a larger spectrum of lysate and peroxide concentrations and conducting multiple trials for greater experimental accuracy.
- Osman SF, Fett WF (1983) Isoflavone glucoside stress metabolites of soybean leaves. Phytochemistry 22: 1921-1923.
- Jin S, Zhang QY, Kang XM, Wang JX, Zhao WH (2010) Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann Oncol 21: 263-268. [Crossref]
- Yu ZY, Li WL (2006) Induction of apoptosis by puerarin in colon cancer HT-29 cells. Cancer Lett 238: 53-60. [Crossref]
- Tuli HS, Tuorkey MJ, Thakral F, Katrin S, Manoj K, et al. (2019) Molecular mechanisms of action of genistein in cancer: Recent advances. Front Pharmacol 10: 1336. [Crossref]
- Leong SP (2011) The role of the lymphovascular system in cancer metastasis. Lymphology 44: 42-44. [Crossref]
- Kundu T, Dey S, Roy M, Siddiqi M, Bhattacharya RK (2005) Induction of apoptosis in human leukemia cells by black tea and its polyphenol theaflavin. Cancer Lett 230: 111-121. [Crossref]
- Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, et al. (2005) Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol 17: 183-189.
- Chen P, Hu YF, Wang L, Xiao WF, Bao XY, et al. (2015) Mitochondrial apoptotic pathway is activated by H2O2-mediated oxidative stress in BmN-SWU1 cells from bombyx mori ovary. PLoS One 10: e0134694. [Crossref]
- Nogueira-Pedro A, Cesário TA, Dias CC, et al. (2013) Hydrogen peroxide (H2O2) Induces leukemic but not normal hematopoietic cell death in a dose-dependent manner. Cancer Cell Int 13: 123. [Crossref]
- Liou GY, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44: 479-496. [Crossref]

