Introduction: Pancreatic metastases from soft tissue sarcomas is rare.
Case presentation: A woman known for high grade intimal sarcoma presented during her follow a solitary metastatic relapse of the head of pancreas. The MRI evidenced a 3.5 cm mass of the head of pancreas, hyper-intense on T1, T2 and diffusion weighted images. A new 18F-FDG PET/CT confirmed the presence of a solitary hypermetabolic mass of the head of the pancreas (SUVmax 11.4), highly suspicious of an isolated pancreatic relapse.
Results: Following multidisciplinary discussion, a pancreaticoduodenectomy with resection of whole gallbladder and locoregional lymph nodes dissection was performed.The patient had survived > 6 years after the pancreatectomy.
Conclusions: Pancreatic metastases from STS are quite rare and no standard approach has been established yet. Multidisciplinary approach and evaluation of a surgical resection of isolated metastases can achieve long disease-free survival interval and be of curative intent.
intimal sarcoma, pancreatic metastases, pancreaticoduodenectomy, soft tissue sarcoma
Primary sarcomas of large systemic arteries are rare neoplasms with only a few hundred cases reported at the literature. Intimal sarcomas are soft tissue sarcomas arising from intima or associated with a great vessel. Most commonly, they are arising from a pulmonary artery or the thoracic aorta and are usually found with thromboemboli and misdiagnosed as pulmonary thromboembolism [1]. Metastatic sites include lungs, brain, lymph nodes and bones. In metastatic disease, palliative chemotherapy remains the standard treatment approach. A few reports show that repeated surgical interventions can be associated with prolonged survival in patients with metastatic pulmonary artery sarcoma [2,3]. Pancreatic metastaseses from soft tissue sarcoma (STS) are quite rare and no standard approach has been established yet. The largest data available published by Wiltberger and al. [4] comprise a retrospective study including over 650 patients who underwent resection of pancreatic metastases of different tumor types. The data shows that surgical resection for pancreatic metastases is feasible and provides long-term survival, while associated with an acceptable morbidity and mortality, however no soft tissue sarcoma patients were included. In this report, we present the first case of pancreatic metastases from intimal sarcoma treated curatively and a review of literature on pancreatic metastases of STS.
In April 2012 a 46-year-old woman, had a left pneumectomy with R1 resection at the left pulmonary artery for an 11 cm high grade intimal sarcoma. Pathology reported a proliferation of polymorphic spindle cells with pleomorphic, hyperchromatic nuclei, combined with multiple vascular invasion. Immunohistochemical staining results were positive for desmin, α-smooth muscle actin and MDM2, and negative for CD31, CD34, and p63 (Figure 1). This surgery was followed by a new resection one month later in R0. Twenty lymph nodes were resected and found negative. In February 2014, during her follow up Fluorodeoxyglucose positron emission tomography (18F-FDG PET/CT) showed an hypermetabolic adrenal left mass (maximum Standard Uptake value, SUVmax 8.6) and a mass of the head of pancreas (SUVmax 6) with no other hypermetabolic lesion noted, highly suspicious of metastatic relapse (Figure 2). The patient was asymptomatic and laboratory tests of liver and pancreatic biochemical parameters (AST, ALT, ALP, GGT, bilirubin, lipase) were within their respective normal range. Tumor biomarker for adenocarcinoma of hepatobiliary origin CA 19-9 was within normal range as well.
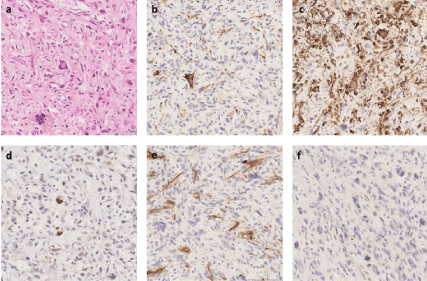
Figure 1. Pheumectomy pathology images. a. Hematoxylin and eosin (HE) stain showing pleomorphic spindle cell proliferation (x200) b. Immunohistochemistry (IHC) showing partial desmin positivity (x200). c. IHC showing SMA positivity (x200). d. IHC showing partial MDM2 positivity (x200). e. IHC showing CD34 negativity (x200). f. IHC showing p63 negativity (x200)
The metastatic nature of adrenal mass was confirmed by biopsy, showing similar pleomorphic spindle cells, with a matching immunohistochemical profile, consistent with an intimal sarcoma origin. In this context, the patient was treated with systematic chemotherapy of doxorubicine/ifosfamide. After 6 cycles, a new 18F-FDG PET/CT revealed a metabolic complete response of pancreatic lesion and a partial metabolic response of adrenal (residual SUVmax 4.7) (Figure 2). The Magnetic Resonance Imaging (MRI) confirmed the complete response of the intrapancreatic lesion. After multidisciplinary discussion we decided to proceed to left adrenal resection. The pathology showed a 4.5 cm metastases. Six months later, the follow up imaging revealed a new lesion at the right adrenal compatible with metastases. A contralateral adrenalectomy was agreed and confirmed the metastases. Since replacement therapy by glucocorticoid started.
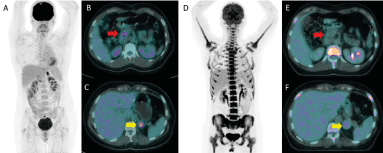
Figure 2. 18F-FDG PET/CT before (A-B-C) and after (D-E-F) chemotherapy. Complete metabolic response of the pancreatic lesion (red arrow) and partial metabolic response of the left adrenal lesion (yellow arrow)
One year later, 3-month imaging follow up by MRI evidenced a 3.5 cm mass of the head of pancreas, hyper-intense on T1, T2 and diffusion weighted images (Figure 2). The patient was asymptomatic and laboratory tests were normal. A new 18F-FDG PET/CT confirmed the presence of a solitary hypermetabolic mass of the head of the pancreas (SUVmax 11.4), in the same localization as compared to the previous 18F-FDG PET/CT, highly suspicious of an isolated pancreatic relapse (Figure 3).
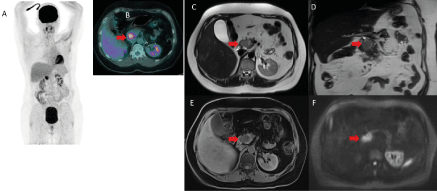
Figure 3. 18F-FDG PET/CT (A-B) and MRI (C-D-E-F) for the diagnosis of the isolated pancreatic relapse. Solitary pancreatic mass associated with a high hypermetabolism on !8F-FDG PET/CT (SUVmax 11.4) and on MRI 3.5cm mass at the head of the pancreas with a hypersignal on axial (C) and coronal(D) T2-weighted MRI without compression on biliary or wirsung duct. This mass appeared as hypo-intense on unenhenced T1-weighted MRI (E) with significant restriction of diffusion at b800 (F). (red arrow)
Following multidisciplinary discussion, a pancreaticoduodenectomy with excision of whole gallbladder and locoregional lymph nodes dissection was performed. The resected tumor was a mass of 40 x 35 x 29mm with 30% tumor necrosis totally resected (R0). Microscopic examination revealed a pleomorphic spindle cell proliferation invading pancreatic and peri-pancreatic adipous tissue, combined with multiple extra vascular invasion. Neither lymph node invasion was found nor adjacent organs infiltration exempt pancreas. The final histologic diagnosis confirmed the metastatic origin of the high-grade intimal sarcoma of PA.
Since the patient continues a close follow up imaging. At the time of issue of this case report, the patient is doing well with no sign of relapse 6 years after her surgery.
Pancreatic metastases are rare and represent only 1-2% of pancreatic malignancies. Most metastases to pancreas originate from renal cell carcinoma, lung cancer, melanoma and gastrointestinal cancer. Usually, they are not discovered until widespread systemic disease appears, therefore no curative treatment is applicable. The symptoms of pancreatic metastases usually include jaundice, abdominal pain and weight loss, similar as in pancreatic cancer.
Primary pulmonary artery sarcomas are also an uncommon thoracic malignancy which was first described by Moritz Mandelstamm in 1932. The clinical and radiological findings are often confused with those of thromboembolic disease, leading to delays in confirming the diagnosis. The standard treatment remains surgery and may include pulmonary endarterectomy (PEA), lobectomy and pneumonectomy [5]. The prognosis is poor since complete surgical resection is rarely possible due to its diagnosis at advanced stage with locally advanced and\or metastatic disease with a median OS of 17 months [6] and currently, chemotherapy including doxorubicin, gemcitabine, ifosfamide, or vinorelbine may provide some disease control in advanced or metastatic intimal sarcoma. Some reports claimed that repetitive surgical interventions may be associated with prolonged survival in patients with advanced pulmonary artery sarcoma. To our knowledge, no case of solitary metastases of intimal sarcoma has been presented to literature [7].
To the best of our knowledge pancreatic metastases of STS are rare and few cases have been reported so far (Table 1). The role of surgical resection of isolated metastases, including pancreatic in STS has not been established yet due to their rarity. For the moment, surgical resection of limited pulmonary metastases is known to result to an important survival benefit [8,9]. Wiltberger et al. suggested a survival benefit after surgical resection of pancreatic metastases in a retrospective analysis of 676 patients treated between 1994-2012 at the University Hospital of Leipzig, with 50% presented 5year OS but no sarcoma patient was included [10-12]. Here we report for the first time a case of a pancreatic metastases of a sarcoma treated with radical surgery following multidisciplinary evaluation and shared decision-making with the patient herself [13]. In some cases, like ours, pancreatic resection of isolated metastases of STS may be associated with favorable survival [14].
Table 1. Cases of surgical resection of pancreatic metastases in STS in the English literature
Abbreviations: F = female; M = male; CT = chemotherapy; DOD = dead of disease; DOOC = dead of other causes; DP = duodenopancreatectomy; TP = total pancreatectomy
In conclusion, we report a first case of intimal sarcoma with metastases at the pancreas [15]. On the basis of a review of the literature, pancreatic metastasectomy may be a useful treatment option for patients with limited sarcoma metastases [16]. Currently there are no studies addressing the outcomes of patients with mestastatic sarcoma isolated to the pancreas and more evidence is necessary to confirm findings in favour of metastasectomy [17]. In this context, we emphasize the necessity of meticulous investigation of lesions of the hepatobiliary system of patients with soft tissue sarcoma and a multidisciplinary approach and evaluation of possible surgical resection of isolated metastases.
- Fukuda W, Morohashi S, Fukuda I (2011) Intimal sarcoma of the pulmonary arthety-diagnostic challenge. Acta Cardiol 66: 539-541. [Crossref]
- [1]Tanaka A (2014) Aggressive multiple surgical interventions to pulmonary artery sarcoma. European Journal of Cardio-Thoracic Surgery 47: 384-385.
- Choi YM, Jang EK, Ahn SH (2013) Long-term survival of a patient with pulmonary artery intimal sarcoma after sequential metastasectomies of the thyroid and adrenal glands.Endocrinol Metab (Seoul) 28: 46-49.
- Wiltberger G (2015) Extended resection in pancreatic metastases: feasibility, frequency, and long-term outcome: a retrospective analysis. BMC surgery 15: 126-126. [Crossref]
- Dima SO (2014) Pancreatic metastases originating from uterine leiomyosarcoma: a case report. World J Surg Oncol 12: 405.
- Makino Y (2016) A case report of pancreatic metastasis from synovial sarcoma successfully treated by metastasectomy with adjuvant chemotherapy. Medicine (Baltimore) 95: e4789.
- Yokoyama Y (2004) Pancreatic metastasis of dermatofibrosarcoma protuberans. J Gastroenterol 39: 798-800.
- Iwamoto I (2005) Metastasis of uterine leiomyosarcoma to the pancreas. Journal of Obstetrics and Gynaecology Research 31: 531-534.
- Falconi M (2006) Pancreatic metastasis from leiomyosarcoma of the broad ligament of the uterus. Lancet Oncol 7: 94-95. [Crossref]
- Koh YS (2007) Pancreatic metastasis of leiomyosarcoma in the right thigh: a case report. World J Gastroenterol 13: 1135-1137.
- Carboni F (2006) Isolated pancreatic metastasis of extremity myxoid liposarcoma: Report of a case. Jpn J Clin Oncol 36: 662-664.
- Akatsu T (2007) Pancreatic metastasis from musculoskeletal sarcoma: a case report with malignant fibrous histiocytoma and review of the literature. Dig Dis Sci 52: 1958-1963.
- Burke JP (2012) Whipple's procedure for an oligometastasis to the pancreas from a leiomyosarcoma of the thigh. Ir J Med Sci 181: 361-363.
- Alonso Gómez J (2012) Uterine Leiomyosarcoma Metastasis to the Pancreas: Report of a Case and Review of the Literature. Journal of Gastrointestinal Cancer 43: 361-363.
- Robert PE (2012) Resectable pancreatic metastasis of left thighbone leiomyosarcoma: case report and literature review. J Gastrointest Cancer 43: 40-43.
- Yamamoto H (2001) Surgical Treatment for pancreatic metastasis from soft-tissue sarcoma: Report of two cases. Am J Clin Oncol 24: 198-200. [Crossref]
- Li S (2017) Pancreatic metastasis of sarcoma: two case reports. J Xiangya Med 2.



