The acquired immunodeficiency syndrome (AIDS) patients can scarcely be cured completely because of the Human Immunodeficiency Virus (HIV) provirus existence post-infection in body, though cocktail of the highly active antiretroviral therapy (HAART) has achieved great effects on controlling and decreasing HIV clinically. Recently, the genome editing strategies are developing by leaps and bounds. Among three generation of technologies, the clustered regularly interspaced short palindromic repeat (CRISPR)-CRISPR-associated protein 9 (Cas9) is a newborn to former zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) but grows fast into the most popular at present, because of its advantages in less time- and cost-consuming. Eradicating HIV by disrupting the viral co-receptor C-C chemokine receptor type five (CCR5) or C-X-C chemokine receptor type four (CXCR4) gene, excising the provirus segments integrated into human genome, or activating/inactivating the replication of the virus genome by using these technologies are several current strategies to reach the goal of AIDS prevention and treatment. It seems hard, however, to arrive the keen anticipation exactly by using them respectively. After a comprehensive literature review, it concluded that the combination of those interventions, as together co-receptor with provirus genome interference as a cocktail edition, may lead us to an eventual cure for the horrible disease.
HIV/AIDS, HAART, cocktail, gene editing, CRISPR/Cas9
Abbreviations
AIDS: acquired immunodeficiency syndrome; HIV: Human Immunodeficiency Virus; HAART: highly active antiretroviral therapy; ZFNs: zinc finger nucleases; TALENs: transcription activator-like effector nucleases; CCR5: C-C chemokine receptor type five; CXCR4: C-X-C chemokine receptor type four; CRISPR: clustered regularly interspaced short palindromic repeat; Cas9: CRISPR-associated protein 9; NRTI: nucleoside reverse transcriptase inhibitor; AML: acute myelogenous leukemia; sgRNA: single guide RNA; SaCas9: Staphylococcus aureus Cas9; AAV: adeno-associated virus; iPSCs: induced pluripotent stem cells; SIV: simian immunodeficiency virus; HSPCs: hematopoietic stem and progenitor cells.
In 1981, the acquired immunodeficiency syndrome(AIDS) was first reported by the Centers for Disease Control and Prevention in USA [1]. Then it was found to be caused by the virus belonging to the retrovirus in the family of lentivirus, which was spread by blood, sexual behavior and mother-to-child transmission, damaging the immune system of the patients and thus named as Human Immunodeficiency Virus(HIV). The infection mechanism of HIV in human has been fairly studied since its discovery. In 1984, Klatzmann D and his colleagues discovered that HIV could combine the CD4+ T cells specifically and then intrude into the cells with inhibiting the cell replication or killing them forthright, compromising the acquired immune system of patients [2]. Meanwhile, the scientists successfully cloned and sequenced the HIV genome [3]. And in 1996, it was found that the C-C chemokine receptor type five(CCR5) and C-X-C chemokine receptor type four(CXCR4) were the co-receptors of HIV [4].
The chemotherapy for suspending viral replication and transcription came along with the etiological studies. In 1987, nucleoside reverse transcriptase inhibitor(NRTI) was approved for use as antiretroviral agent and it was proved to reduce viral load and delay disease progression [5]. However, using the single agent could not suppress the virus enduringly. In 1996, highly active antiretroviral therapy(HAART) was put forward by Dr. David Ho. Itombined three or more antiviral agents to inhibit the replication of virus and slow the progress of illness, which effectively reduced the morbidity and mortality of AIDS clinically. Since then, HAART became the mainstay of therapy for HIV/AIDS. However, HAART failed eradicating the virus completely with the existence of virus reservoirs, in which the provirus genome was integrated into host genome. Thus far, AIDS patients need to take the drugs in the rest of life time under the shadow of discrimination and the risk of viral rebound or drug-resistant, bearing the side effects of the drug in long term. But it indicates that the idea of combining several methods of therapy into one, like cocktail just as the principle of HAART then, is practicable, arriving to an expected effect.
In 2007, “Berlin patient” who had AIDS and acute myelogenous leukemia (AML) was treated with an allogeneic hematopoietic stem cell(HPSC) transplant and the donor was homozygous for the CCR5Δ32 deletion [6]. Then the HAART was stopped after transplantation and there was no any viral rebound for 8-year follow-up. All trials show that “Berlin patient” has been given a sterilizing cure [7].
The “Berlin patient” case indicates that it seems applicable to completely cure AIDS patients by transplanting allogenic HPSC with CCR5Δ32 deletion. However, with the small chance of successful cross-match, it is almost no possibility to find the matched donor. Besides, co-receptor CXCR4 is unneglectable, that 46% of individuals who are treated by HAART have both CCR5 and CXCR4 dependent virus tropics and another 4% of them only harbor CXCR4-HIV strains. The virus depending on CCR5 can evolve to CXCR4 dependent strains [8]. The “Essen Patient” who suffered from anaplastic large T-cell lymphoma and HIV, received the similar treatment of the “Berlin patient”. Unfortunately, the virus rebounded after transplantation as the type of HIV-1 changed into CXCR4 tropic. The inducement of the virus variation were not explicit, but it suggested the importance of CXCR4 mutant in HIV resistance [9]. Anyway, it has provided reference for a promising therapeutics to disrupt CCR5 or/and CXCR4 gene of healthy cells, then to transfuse them back to patients.
Host cells with HIV genome integrated, which are called HIV reservoirs, confers the risk of virus proliferation to AIDS patients. They produce minimal viral proteins under normal circumstances, thus escape the guard of immune system [10]. However, when HAART is suspended or stimulations are given, the latent infected cells will be activated along with virus gene replication and expression. Then the HIV proliferates and makes the host morbid. Therefore, eliminating the HIV reservoirs completely is quite important to achieve the goal of curing AIDS.
In recent years, gene editing technologies, including zinc-finger nucleases(ZFNs), transcription activator-like effector nucleases(TALENs) and the clustered regularly interspaced short palindromic repeat(CRISPR)/CRISPR-associated protein 9(Cas9), leaped forward and scientists have applied them into curing AIDS in several ways. ZFNs are discovered in 1996 [11] and then widely used to manipulate the genomes since they can recognize specific DNA domains and cleave them with double-strain breaks(DSBs). TALENs are similar to ZFNs in working mechanisms, but TALENs behave better in specificity to DNA domains [12]. However, both ZFNs and TALENs were limited to be applied intensively because of the difficulty in their construction and susceptibility to induce genomic rearrangements [13]. As a novel technology, the CRISPR/Cas9 system, which plays a major role in archaeal and bacterial adaptive immunity, has been successfully applied for genome editing in diverse biological fields, with the advantages of less time- and cost-consuming than ZFNs and TALENs [14-16]. Great advancements are achieved in a short period, such as mutating the two independent DNA cleavage modules in Cas9 to compromise its cleavage activation (dcas9) and replacing the terminal loops of sgRNA with two hairpins to render itself the ability in recruiting protein [17]. Within the CRISPR system, not only to compromise target gene could people do, but also artificially activating a specific function scientists are capable of. A series of freestanding studies narrated below prove it a better choice and provide evidence that navigates future exploration in this field.
Editing CCR5 gene
Tebas, et al. disrupted CCR5 gene of CD4+ T cells of 12 AIDs patients by ZFNs in vitro, then re-transfused the cells to the patients, and the HIV levels of the ones who suspended the HAART with 11–28% disruption frequency rebounded but more slowly than normal patients, suggesting the progress of illness was impeded indeed [18]. The higher editing efficiency could be yielded to ~45% by TALENs [19,20]. In comparison of targeting effect within piggy/Bac, TALENs only achieved ~14 % biallelic modification while CRISPR/Cas9 enforced to 33%, though both of them attained 100 % targeting of CCR5Δ32 monoallelic in induced pluripotent stem cells(iPSCs). All the modified iPSCs could differentiate into monocytes/macrophages and gain resistance to HIV-1 challenge [21]. Dual gRNAs resulted in doubled efficiency from 12.5% to 27% of cell colonies and 22.2% to 41% biallelic editing [22]. Delivery systems were recently discussed about in a comprehensive study on primary CD4+ T cell manipulation [23].
Editing CXCR4 gene
It was proved that lentivirus-mediated CXCR4 biallelic ablation gained the resistance to HIV-1 infection [8]. Electroporation of Cas9/sgRNA heterodimer led to the loss of high-level cell-surface CXCR4 expression in ∼40% of CD4+ T cells [24,25]. The very trial in co-modification of CCR5 and CXCR4 was performed by ZFNs in primary human CD4+ T cells, gaining resistant to co-harboured HIV-1 virus strains in vitro. Using CRISPR/Cas9 system to disrupt both CCR5 and CXCR4 should present a better result, however, co-receptor mutants resistant to infection are not able to clean up the virus in patients substantially, which drives scientist to focus on the provirus.
Activating then eliminating HIV reservoir
The strategy “shock and kill” is to eliminate virus reservoir by reactivating latently infected cells and killing them by viral cytotoxicity and/or host immune defense [26]. The combination of Cas9 activators and latency-reversing compounds could promote latent HIV-1 gene activation, which has been proved by dCas9-VP64 and a superior dCas9-SAM systems [27-29]. Compare to chemical activating agents, CRISPR/Cas9 system is safer and more efficient though, the balance of “shock” and “kill” is difficult to master. A recent study reveals a severe inflammatory response in central neural system after chemical activation of simian immunodeficiency virus (SIV) reservoirs in the pigtailed macaques (ongoing HAART) [30]. Therefore, the clinical application should be considered deliberately to avoid catastrophic consequence of extensive activation without proper eradication.
Restricting the replication of HIV-1
The virus restriction factors APOBEC3G (A3G) and APOBEC3B (A3B) are normally absent in human cells, and the latter can inhibit wild-type HIV-1 by dCas9-SAM system activation [31]. The endogenous viral restriction indicates the significance of restriction factors mining, which could be accomplished by sgRNA library screen study. It is promising but there is a long way to translation, and viral latency cannot be eliminated completely in this way to the best of our knowledge.
Excising the provirus
The intrinsic property of CRISPR system, a targeted genomic high-efficient DSB, provides an inspiration of provirus excising or vital element destruction. Multiplex gRNAs achieved to completely excise a 9709-bp fragment of HIV-1 5’-3’ LTR-spanning viral genome, at 85% efficiency of viral inhibition in targeted cells with low off-target toxicity. The whole genome excision proved Cas9/gRNA the ability of inter-chromosomal multisite interference simultaneously [32]. Not only could integrated provirus but also free viral segments be disrupted by CRISPR, which supplied a longer period of time for engineered cells to defend against HIV-1 infection. Generation of anti-HIV-1 hPSC lines expressing Cas9 protein differentiated to a relatively homogenous population of monocytes/macrophages, presenting safety to be an artificial immune strategy [33].
However, using only one single gRNA to edit HIV gene may lead to the acceleration of viral escape due to mutation of the target sequence. Triple combination of 10 gRNAs suggested the accessibility of multi-targeted intensive sites [34]. Recently, researchers combined two antiviral gRNAs which targeted different regions of HIV-1 genome and demonstrated the prevention of viral escape and powerful inhibition of HIV replication [35,36]. Provirus deletion in latency could get rid of the risk of viral rebound, and the prospect is promising when several gRNAs are combined. Table 1 summaries the advantages/disadvantages of the methods and challenges for blocking the cell intrusion of HIV-1 and eradicating HIV reservoirs as well.
Table 1. HIV-1/AIDS gene therapy by CRISPR/Cas9.
Strategies |
Advantages |
Disadvantages |
Chanlleges |
Edit CCR5 gene
22, 23, 24 |
High efficiency in blocking the HIV-1 |
The infection of CXCR4 HIV strain can’t be blocked. Cas9 can lead to cleavage mutants. |
Appropriate locus and delivery system are needed. The clinical safety remains to be evaluated. |
Edit CXCR4 gene
8, 25 |
High efficiency in blocking the HIV-1 |
The infection of CCR5 HIV strain can’t be blocked. Cas9 can lead to cleavage mutants. |
The same as above. |
“Shock and kill”
28, 29, 30 |
More efficiency and safer than chemical activating agents. dCas9 won’t lead to cleavage mutants. |
The balance of “shock” and “kill” is difficult to manipulate. |
How to deliver the system into infected cells such as CD4+ T cells should be considered. |
Induce the expression
of restriction factors
32 |
Induce the expression of endogenous viral restriction factors not expressed in normal cells. |
The efficiency is not explicit and latency virus can’t be eliminated completely. |
Powerful restriction factors and efficiency delivery system need to be considered. |
Excise the provirus
33, 34, 35, 36, 37 |
The virus reservoir can be destroyed entirely. CRISPR/Cas9 system could be an intracellular defense against HIV-1 |
Cas9 can lead to cleavage mutant and the acceleration of viral escape exists. |
Appropriate locus is needed. Using two sgRNAs or more is necessary. |
Edit both CCR5 and
CXCR4 genes
26 |
The approach has not been reported in CRISPR, while the research has been done by using ZFNs. |
From the above, it is able to edit the co-receptors which assists HIV-1 entrance the predisposing cells and excise the HIV-1 provirus gene integrated into the host genome in latent reservoirs by CRISPR/Cas9. In this way, should it feasible to combine various strategies together as the spirit of HAART to a molecular cocktail to interfere HIV-1 infection?
It is worth noted that CCR5 and CXCR4 are both chemokine receptors in human body, playing significant roles in immune system. For example, CCR5 is the receptor of CCL3, CCL4, CCL5, etc. And it is expressed in T cells, macrophages, dendritic cells chronically [37]. Analogously, CXCR4 refers to the ligands such as SDF-1 and ubiquitin, etc [38,39]. And it is also widely expressed in cells as CD4+ T cells, dendritic cells, microglia and glomerular podocytes, etc [40]. Mutating the two receptors’ gene would cause a side effect on the patients’ body health in some way. But according to the observation so far, the people with CCR5Δ32 gene, mono-allelic or bi-allelic have been existing for a long time and distributing widely in the world [37,41], which suggests that the CCR5Δ32 gene do no harm to health in a large population. As for CXCR4, its function could be replaced by other pathways as SDF-1 could also work by binding to CXCR7 [42] and to achieve the best results, using the milder strategies to reconstruct immune system partially should be taken into consideration.
In the first step, it is thought applicable to isolate hematopoietic stem and progenitor cells (HSPCs) from the blood of HIV-1 patients and use the combined strategy with CRISPR/Cas9 to disrupt the targeted CCR5, CXCR4 in these cells. Then the stem cells are induced to differentiate into the precursors of T cells and monocytes, such as pre-T cells and monoblasts, which are later transplanted into the patients’ body. The pre-T cells will be able to differentiate into the CD4+ T cells and arrive lymphatic organs by lymphocyte homing while the monoblasts differentiate into monocytes. The other CD4+ cell subsets still come from the non-modified stem cells existed in patients’ body whose CCR5 and CXCR4 gene are wild type as usual. Therefore, in the partial reconstructed immune system, the function of CCR5 and CXCR4 won’t be affected greatly.
Then it is essential to eliminate the HIV-1 reservoirs in the patients’ body, which is supported by the methods of excising integrated provirus gene or “shock and kill”. The steps of excising the provirus gene in vitro involve: a) screening and selecting CD4+ cells of patients, b) constructing delivery vectors and inducing the Cas9/sgRNAs (targeting important regions of provirus gene) into the cells, c) transfusing the edited cells into the patients’ body. When the method is used in vivo, adeno-associated virus (AAV) vector should be adopted for the safety. Staphylococcus aureus Cas9 (SaCas9) with smaller size, was found easier in delivery by AAV in vivo [43] and the Cas9 which was split into several parts can be packed in AAV for in vivo research and therapeutic applications [44,45]. Accordingly, constructing the AAV-SaCas9-sgRNAs which targets provirus gene is an available way. The method of “shock and kill” by CRISPR in vivo need the construction of AAV containing split dCas9-SAM (or VP64)-sgRNAs, which activate the virus gene. The main two steps of the process are shown in Figure 1.
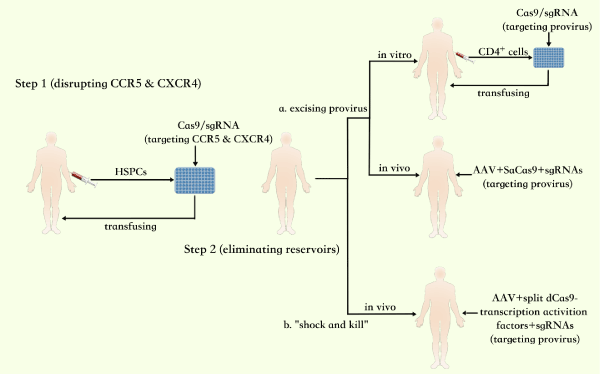
Figure 1. The steps of molecular cocktail based on CRISPR for clinical application to cure AIDS.
In the above curing process, there are periods that patients should always yield to the treatment of HAART [9] When they are under severe side effects of HAART, the method of restricting the replication of HIV-1 by CRISPR can be considered to share responsibility for the drug therapy. The construction of AAV containing split dCas9-SAM (or VP64)-sgRNAs can activate the expression of restriction factors.
In this molecular cocktail therapy, cells differentiated from the modified part of the patients’ HSPCs gain the ability to resist the invasion of HIV-1, and the patients’ immune system won’t be devastated when virus develop explosively with stimulations. On the other hand, eliminating the HIV-1 reservoirs is the 2nd frontline for patients, lowing the risk of the latent infected cells, which will infect the CD4+ cells derived from non-modified hematopoietic stem cells. And it may take several courses to ensure that the reservoirs are wiped out. After the two main steps of molecular cocktail therapy, the patients could reduce the dose of drugs stepwise under monitoring the viral load in real time, there is great possibility to reach the goal of cuing the HIV-1 eventually.
There are many challenges remaining in the strategy, in which the stem cells are enrolled for genome editing. Due to the cleavage activation of Cas9 which can mutate the homologous host genome, the risk of molecular cocktail seems to be inevitable and the side effect is uncertain. Researchers found that CRISPR/Cas9 which was used to edit a gene of blindness in the mice could lead to hundreds of unexpected mutations [46]. Then, in the process of screening and culturing the cells, there are also problems. For example, establishing the large-scale culture systems to yield HSPCs is needed and maintaining the undifferentiated state also requires great cost of labors and resources. And in the step 2 eliminating the reservoirs in vitro, it’s hard to collect all reservoirs cells which should be modified. The challenges also exist in transporting mechanisms. When using in vivo treatment, the effective method which transports the CRISPR system into specific reservoirs cells is defective. The strategy of “shock and kill” in vivo also depends on efficient transporting system and the strategy may be harmful to the patients with an exactly opposite effect. Furthermore, in the therapy process, the time of stopping HAART can influence the result of therapy totally in view of the sharp contrast between “Berlin patient” and the “Essen Patient” [9], which should be considered seriously. Finally, the ethical problems are also unneglectable. The modification of gene in HSPCs with possibilities of gene mutant may cause unpredictable results and when the technology is advanced, it might be inappropriately used for various purposes out of medicine.
The opportunities, indeed, ride on with challenges. First of all, the rapid development of CRISPR system provides new prospect continually. In addition to the standard SpCas9 and SaCas9, there is blowout-like discovery of its siblings, enumerating that Cpf1 has different PAM and smaller size with low off-target characteristic [47,48] and that CasX and CasY were recently found [49] etc. Besides, with the modification works of sgRNA and Cas9 protein, multiplex sgRNAs and the homologues of Cas9 can be used to target distinct sites on genome, improving the gene editing efficiency and preventing viral escape. Furthermore, the powerful CRISPR is not only used in directly gene editing, but also provide original inspiration and choices for the revelation of HIV-1 etiology. Recently, researchers identified three novel factors for HIV-1 entry by genome-wide CRISPR/Cas9-based screening, which suggested the new target sites for curing AIDS [50]. Eventually, the two steps and three ways of molecular cocktail therapy provide multiple selections for patients, even though the best “one-two punch” has not drew a charge. And HAART targets on the several sites of the life circle of virus while the molecular cocktail herein only focuses on the two respects - infection blockade and reservoir elimination, which suggests that the approaches can be extended to be more multidimensional [10] Therefore, as technologies march at a breathless pace, the content of molecular cocktail can be more plentiful and the superior combination of methods will present.
Throughout decades of struggling against HIV along lab bench to bed, it’s a certain fact that depending on single clinical interference is hard to cure the disease thoroughly. CRISPR/Cas9 is widely focused on because of its apparent advantages in economic and manipulation. Not long since the technology was put up, several strategies have been attempted to fight against AIDS at the genetic level and show great prospects. Combining strategies based on CRISPR/Cas9 to form a set of program, similar to HAART, may be an eventual cure for HIV/AIDS. In our opinion, using the CRISPR/Cas9 system to mutate the co-receptors CXCR4 and CCR5 in the stem cells, in addition with the provirus elimination together as a molecular cocktail should be practicable, and within sight soon. To be sure, personal and precision medicine with the clinical application of gene editing technology is like a tiger with wings, and dawn of the victory in conquering AIDS/HIV would come with might redoubled.
This work was supported by the National Natural Science Foundation of China (grant No. 81171977 and No. 31500614).
2021 Copyright OAT. All rights reserv
The authors declare that the research was reviewed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, et al. (1981) Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med 305: 1425-1431.
- Klatzmann D, Barre-Sinoussi F, Nugeyre MT, Danquet C, Vilmer E, et al. (1984) Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science 225: 59-63.
- Alizon M, Sonigo P, Barre-Sinoussi F, Chermann JC, Tiollais P, et al. (1984) Molecular cloning of lymphadenopathy-associated virus. Nature 312: 757-60.
- O'Brien SJ, Moore JP (2000) The effect of genetic variation in chemokines and their receptors on HIV transmission and progression to AIDS. Immunological reviews 177: 99-111.
- Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrman SN, et al. (1985) 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A 82: 7096-100.
- Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, et al. (2009) Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 360: 692-698. [Crossref]
- Yukl SA, Boritz E, Busch M, Bentsen C, Chun TW, et al. (2013) Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS pathogens 9: e1003347.
- Hou P, Chen S, Wang S, Yu X, (2015) Genome editing of CXCR4 by CRISPR/cas9 confers cells resistant to HIV-1 infection. Sci Rep 5: 15577. [Crossref]
- Kordelas L, Verheyen J, Esser S (2014) Shift of HIV Tropism in Stem-Cell Transplantation with CCR5 Delta32 Mutation. The New England Journal of Medicine 371: 880-882.
- Deeks SG, Overbaugh J, Phillips A, Buchbinder S (2015) HIV infection. Nature reviews Disease primers 1: 15035.
- Kim YG, Cha J, Chandrasegaran S (1996) Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proceedings of the National Academy of Sciences of the United States of America 3: 1156-1160.
- Holkers M, Maggio I, Liu J, Janssen JM, Miselli F, et al. (2013) Differential integrity of TALE nuclease genes following adenoviral and lentiviral vector gene transfer into human cells. Nucleic acids research 41: e63.
- Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, et al. (2011) Genetic engineering of human pluripotent cells using TALE nucleases. Nature biotechnology 29: 731-4.
- Gasiunas G, Barrangou R, Horvath P, Siksnys V (2012) Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A 109: E2579-86.
- Wiedenheft B, Sternberg SH, Doudna JA (2012) RNA-guided genetic silencing systems in bacteria and archaea. Nature 482: 331-338. [Crossref]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, et al. (2013) RNA-guided human genome engineering via Cas9. Science 339: 823-826. [Crossref]
- Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, et al. (2015) Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517: 583-588.
- Tebas P, Stein D, Tang WW, Frank I, Wang SQ, et al. (2014) Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. The New England journal of medicine 370: 901-910.
- Mussolino C, Alzubi J, Fine EJ, Morbitzer R, Cradick TJ, et al. (2014) TALENs facilitate targeted genome editing in human cells with high specificity and low cytotoxicity. Nucleic acids research 42: 6762-6773.
- Mussolino C, Morbitzer R, Lutge F, Dannemann N, Lahaye T, et al. (2011) A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic acids research 39: 9283-9293.
- Ye L, Wang J, Beyer AI, Teque F, Cradick TJ, et al. (2014) Seamless modification of wild-type induced pluripotent stem cells to the natural CCR5Delta32 mutation confers resistance to HIV infection. Proc Natl Acad Sci U S A 111: 9591-9596.
- Kang H, Minder P, Park MA, Mesquitta WT, Torbett BE, et al. (2015) CCR5 Disruption in Induced Pluripotent Stem Cells Using CRISPR/Cas9 Provides Selective Resistance of Immune Cells to CCR5-tropic HIV-1 Virus. Molecular therapy Nucleic acids 4: e268.
- Li C, Guan X, Du T, Jin W, Wu B, et al. (2015) Inhibition of HIV-1 infection of primary CD4+ T-cells by gene editing of CCR5 using adenovirus-delivered CRISPR/Cas9. J Gen Virol 96: 2381-2393. [Crossref]
- Schumann K, Lin S, Boyer E, Simeonov DR, Subramaniam M, et al. (2015) Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc Natl Acad Sci U S A 112: 10437-10442.
- Didigu CA, Wilen CB, Wang J, Duong J, Secreto AJ, et al. (2014) Simultaneous zinc-finger nuclease editing of the HIV coreceptors ccr5 and cxcr4 protects CD4+ T cells from HIV-1 infection. Blood 123: 61-69. [Crossref]
- Archin NM, Margolis DM (2014) Emerging strategies to deplete the HIV reservoir. Curr Opin Infect Dis 27: 29-35. [Crossref]
- Saayman SM, Lazar DC, Scott TA, Hart JR, Takahashi M, et al. (2016) Potent and Targeted Activation of Latent HIV-1 Using the CRISPR/dCas9 Activator Complex. Mol Ther 24: 488-98.
- Limsirichai P, Gaj T, Schaffer DV (2015) CRISPR-mediated Activation of Latent HIV-1 Expression. Molecular therapy 24: 499-507.
- Zhang Y, Yin C, Zhang T, Li F, Yang W, et al. (2015) CRISPR/gRNA-directed synergistic activation mediator (SAM) induces specific, persistent and robust reactivation of the HIV-1 latent reservoirs. Scientific reports 5: 16277.
- Gama L, Abreu CM, Shirk EN, Price SL, Li M, et al. (2017) Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. Aids 31: 5-14.
- Bogerd HP, Kornepati AV, Marshall JB, Kennedy EM, Cullen BR (2015) Specific induction of endogenous viral restriction factors using CRISPR/Cas-derived transcriptional activators. Proc Natl Acad Sci U S A 112: E7249-56.
- Hu W, Kaminski R, Yang F, Zhang Y, Cosentino L, et al. (2014) RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci U S A 111: 11461-11466.
- Liao HK, Gu Y, Diaz A, Marlett J, Takahashi Y, et al. (2015) Use of the CRISPR/Cas9 system as an intracellular defense against HIV-1 infection in human cells. Nature communications 6: 6413.
- Zhu W, Lei R, Le Duff Y, Li J, Guo F, et al. (2015) The CRISPR/Cas9 system inactivates latent HIV-1 proviral DNA. Retrovirology 12: 22. [Crossref]
- Wang G, Zhao N, Berkhout B, Das AT (2016) A Combinatorial CRISPR-Cas9 Attack on HIV-1 DNA Extinguishes All Infectious Provirus in Infected T Cell Cultures. Cell Rep 17: 2819-2826. [Crossref]
- Lebbink RJ, de Jong DC, Wolters F, Kruse EM, van Ham PM, et al. (2017) A combinational CRISPR/Cas9 gene-editing approach can halt HIV replication and prevent viral escape. Scientific reports 7: 41968.
- Barmania F, Pepper MS (2013) C-C chemokine receptor type five (CCR5): An emerging target for the control of HIV infection. Appl Transl Genom 2: 3-16.
- Pavlasova G, Borsky M, Seda V, Cerna K, Osickova J, et al. (2016) Ibrutinib inhibits CD20 upregulation on CLL B cells mediated by the CXCR4/SDF-1 axis. Blood 128: 1609-1613. [Crossref]
- Saini V, Marchese A, Majetschak M (2010) CXC Chemokine Receptor 4 Is a Cell Surface Receptor for Extracellular Ubiquitin. The Journal of Biological Chemistry 285: 15566-76.
- Israel F. Charo, Rebecca Hills, Richard Horuk, Kouji Matsushima, Philip M, et al. (2016) Chemokine receptors: CXCR4. Guide to pharmacology.
- Libert F, Cochaux P, Beckman G, Samson M, Aksenova M, et al. (1998) The deltaccr5 mutation conferring protection against HIV-1 in Caucasian populations has a single and recent origin in Northeastern Europe. Human molecular genetics 7: 399-406.
- Cruz-Orengo L, Holman DW, Dorsey D, Zhou L, Zhang P, et al. (2011) CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J Exp Med 208: 327-39.
- Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, et al. (2015) In vivo genome editing using Staphylococcus aureus Cas9. Nature 520: 186-191. [Crossref]
- Ma D, Peng S, Xie Z (2016) Integration and exchange of split dCas9 domains for transcriptional controls in mammalian cells. Nat Commun 7: 13056. [Crossref]
- Zetsche B, Volz SE, Zhang F (2015) A split-Cas9 architecture for inducible genome editing and transcription modulation. Nature biotechnology 33: 139-42.
- Schaefer KA, Wu WH, Colgan DF (2017) Unexpected mutations after CRISPR-Cas9 editing in vivo. Nat Methods 14: 547-548. [Crossref]
- Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, et al. (2015) Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163: 759-771. [Crossref]
- Gao L, Cox DBT (2017) Engineered Cpf1 variants with altered PAM specificities. Nat Biotechnol 35: 789-792. [Crossref]
- Burstein D, Harrington LB, Strutt SC, Probst AJ, Anantharaman K, et al. (2017) New CRISPR-Cas systems from uncultivated microbes. Nature 542: 237-241. [Crossref]
- Park RJ, Wang T, Koundakjian D, Hultquist JF, Lamothe-Molina P, et al. (2017) A genome-wide CRISPR screen identifies a restricted set of HIV host dependency factors. Nature genetics 49: 193-203.

