We report an emergency patient with a first-time seizure due to open lip schizencephaly demonstrating the value of IR T1 and T2-weighted 3D SPACE data sets in the evaluation of cortical malformations in comparison to well-known standard MR imaging techniques. “SPACE” (Sampling Perfection with Application optimized Contrasts using different flip-angle Evolution) is an MR technique based on a 3D turbo spin-echo sequence allowing acquisition of 3D data sets with whole brain coverage in acceptable measurement times providing different contrasts.
magnetic resonance, 3D – SPACE, cortical malformations, schizencephaly
Concerning visualization of structural brain lesions in patients with epilepsy, magnetic resonance (MR) imaging is the imaging method of choice due to its high tissue contrast and sensitivity for lesions [1].
Congenital brain malformations, such as schizencephaly including heterotopia require images providing both, high contrast and high spatial resolution. The latter, especially, is necessary to detect also small lesions of heterotopia.
Up to now, MR imaging of epilepsy is done with standard 2D sequences providing good soft tissue contrast, e.g. fast inversion recovery (IR) sequences. Some centers apply T1-weighted 3D gradient-echo data sets, such as magnetization prepared rapid gradient-echo (MP-RAGE) with isotropic 1 mm spatial resolution in order to allow arbitrary reformations additionally to source images. However, overall image quality with this kind of sequences is not as good as with 2D IR sequences with appropriately chosen parameters.
A recently developed technique called “SPACE (Sampling Perfection with Application optimized Contrasts using different flip-angle Evolution)” allows the acquisition of high-contrast 3D data sets with T1-, T2- or T2-contrast with dark CSF (FLAIR) in acceptable measurement times [2-5]. Based on a 3D turbo spin-echo (TSE) sequence with a long echo train and different flip angles of the refocusing pulses SPACE produces regular as well as stimulated spin-echo (flip angle and signal evolution of T2-weighted 3D SPACE are given in Figure 1).
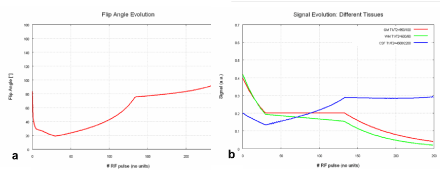
Figure 1. T2-weighted 3D SPACE. In SPACE, a T2-weighted 3D turbo spin-echo sequence with stimulated echoes, there is evolution of flip angle and tissue signal depending on the number of RF pulses. a) flip angle evolution b) signal evolution
Adding an inversion pulse at the beginning of the sequence T1 contrast or FLAIR contrast can be generated.
In the following we report a case of a 24-years-old female epilepsy patient suffering from open lip schizencephaly examined with standard MR imaging incl. T1-weighted 3D MP-RAGE and T2- and T1-weighted SPACE data sets, demonstrating the value of this new sequence type for the evaluation of such cerebral cortical malformations.
A 24-year-old female patient was admitted to our emergency department immediately after a first-time seizure, observed by her mother. Furthermore, the patient’s history revealed right-sided hemiparesis since birth. Due to our standard practice, cranial computed tomography (CT) was passed on, and dedicated MR imaging was performed in an acute setting. The examination was done on a 1.5 T whole body scanner (Magnetom Avanto, Siemens, Erlangen, Germany) with a 12-channel phased-array head coil applying our standard brain protocol: T2 (incl. FLAIR)-weighted TSE, diffusion-weighted imaging, T1-weighted spin-echo (SE), T2*-weighted GRE sequences before and a T1-weighted SE sequence after i.v. gadolinium administration in transverse orientation as well as sagittal T1-weighted 3D MP-RAGE. Additionally, a T1-weighted 2D turbo IR sequence perpendicular to the Sylvian fissure, as well as T2-weighted and T1-weighted IR SPACE data sets were applied before gadolinium administration.
Following pathological findings were observed: left-sided open lip schizencephaly, lined out by polymicrogyric cortex, in typical location, accompanied by absence of the septum pellucidum. Furthermore, alterations of the normal gyration were found also in the right hemisphere (Figures 2 and 3).
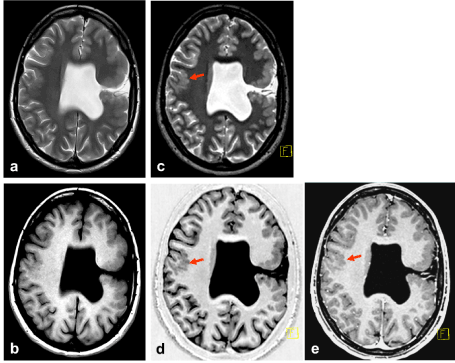
Figure 2. a) TSE T2 5 mm, b) SE T1 5 mm, c) SPACE T2 1 mm, d) SPACE IR T1 1mm, e) MPRAGE Gd 1 mm. Open lip schizencephaly on the left side. Cortical abnormalities on the contralateral hemisphere (arrow) are most obvious in 3D data sets, especially SPACE
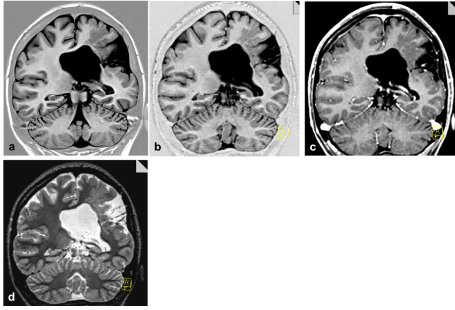
Figure 3. a) 2D IR T1 3 mm, and coronal reformations (1 mm isotropic slice thickness) from b) SPACE IR T1, c) MPRAGE Gd, d) SPACE T2. Accompanying band heterotopia is excellently depicted in the reformations of the sagittal 3D data sets (b-d) in comparison to (only in plane) high resolution 2D IR T1 (a)
The latter was definitely more conspicuous on T1-weighted 2D IR and especially in the SPACE data sets. For this case of polymicrogyria the findings were more obvious on the T1-weighted images than on T2-weighted images.
Congenital cortical malformations, such as heterotopia and schizencephaly are rare morphological alterations of the central nervous system, for the first time described in the late 19th century. The modern definition was given by Yakovlev and Wadsworth in 1946.
Generally, patients manifest by seizures. This lesion is characterized by a cleft extending from the pial surface extending to the lateral ventricle with (open lip) or without (closed lip) connection to the ventricle. Thereby, the cleft is lined by polymicrogyric cortex. The septum pellucidum may be absent. Usually, the insula and the pre- and postcentral gyri are involved. Furthermore, the tissue surrounding the cleft as well as other regions of the brain may show areas of heterotopia [6-8].
Schizencephaly may be accompanied by motor deficits: “congenital” hemiparesis [9].
All these signs were found in the patient described in this report.
To detect – especially small areas of – heterotopia, high soft tissue contrast is required as well as high spatial resolution. A recently developed technique based on 3D TSE is able to fulfill these requirements in an acceptable measurement time in a clinical setting: 3D SPACE data sets are available in different contrast behaviours (T1-weighting, T2-weighting without and with fluid suppression). Excellent T1-weighting is achieved by the inversion recovery technique. SPACE data sets provide thin isotropic slices at measurement times from 4 to 7 minutes (depending on the inversion pulse) with high signal-to-noise ratio. Another, extremely important advantage of SPACE in comparison to conventional 3D TSE is the coverage of the whole brain with SPACE in a single slab.
On the described case of a young patient suffering from complex schizencephaly, we could show the impressive value of this technique for the detection of altered cortical tissue. The cortical malformations on the right- hemisphere were found only in the 3D data sets and in the 2D IR sequence with excellent T1 contrast. However the latter lacks of the potential to perform thin-sliced isotropic, arbitrarily chosen reconstructions.
Because SPACE IR T1 is a spin-echo sequence, whereas MP-RAGE is a gradient-echo sequence, SPACE could be superior to MP-RAGE in the differentiation between cortical malformations, such as small heterotopic isles within the white substance, versus other lesions, such as presumed microangiopathic disease and other. This has to be evaluated in another prospective study.
In conclusion, the SPACE technique may be of value for MR imaging of structural abnormalities in epilepsy patients.
No conflicts of interest. No grants or financial supports.
- Deblaere K, Achten E (2008) Structural magnetic resonance imaging in epilepsy. Eur Radiol 18: 119-129.
- Viallon M, Vargas MI, Jlassi H, Lövblad KO, Delavelle J (2008) High-resolution and functional magnetic resonance imaging of the brachial plexus using an isotropic 3D T2 STIR (Short Term Inversion Recovery) SPACE sequence and diffusion tensor imaging. Eur Radiol 18: 1018-23. [Crossref]
- Komada T, Naganawa S, Ogawa H, Matsushima M, Kubota S, et al. (2008) Contrast-enhanced MR imaging of metastatic brain tumor at 3 tesla: utility of T(1)-weighted SPACE compared with 2D spin echo and 3D gradient echo sequence. Magn Reson Med Sci 7: 13-21.
- Kato Y, Higano S, Tamura H, Mugikura S, Umetsu A, Murata T, Takahashi S (2009) Usefulness of contrast-enhanced T1-weighted sampling perfection with application-optimized contrasts by using different flip angle evolutions in detection os mall brain metastasis at 3T MR imaging: comparison with magnetization-prepared rapid acquisition of gradient echo imaging. AJNR Am J Neuroradiol 30: 923-929.
- Baumert B, Wörtler K, Steffinger D, Schmidt GP, Reiser MF, Baur-Melnyk A (2009) Assessment of the internal craniocervical ligaments with a new magnetic resonance imaging sequence: three-dimensional turbo spin echo with variable flip-angle distribution (SPACE). Magn Reson Imaging 27: 954-60. [Crossref]
- Hayashi N, Tsutsumi Y, Barkovic AJ (2002) Morphological features and ssociated anomalies of schizencephaly in the clinical population: detailed analysis of MR images. Neuroradiology 44: 418-27.
- Oh KY, Kennedy AM, Frias AE, Byrne JL (2005) Fetal schizencephaly: pre- and postnatal imaging with a review of the clinical manifestations. RadioGraphics 25: 647-57. [Crossref]
- Barkovich AJ, Kjos BO (1992) Schizencephaly: correlation of clinical findings with MR characteristics. AJNR Am J Neuroradiol 13:85-94. [Crossref]
- Osborn A. Diagnostic imaging – Brain (2004) Salt Lake City: Amirsys: I-1-71 – I-1-72.



