Abstract
This study investigated the utility of computerized tomographic assisted biomodel fabrication in Australia, which was enlisted as an aid to undertake either the resection or reconstruction of elements of the maxillofacial skeleton. The study sample was derived from the in-house database held by Anatomics® in relation to the patient cohort referred to them for maxillofacial biomodel fabrication. The primary predictor variable was the biomodel construct. The secondary outcome variables were the range and various applications that the biomodel engendered within the requesting surgical community. Other variables included the requesting surgical subspecialty. The study identified a total of 956 requests made by specialist surgeons which resulted in biomodel construction. The biomodels were fabricated in a range of facial anatomical combinations or as individual subsites. Biomodel applications included surgical simulation, mirror imaging, pre-bending of internal fixation reconstruction plates, the provision of implant positioning/fabricating templates and cutting guides and the production of PoreStar® porous polyethylene implants fabricated for contour reconstruction. The requests predominantly came from the discipline of Oral and Maxillofacial Surgery; though Plastic Surgery, Neurosurgery and Otorhinolaryngology also contributed to the mix of specialty demand. This national specific institutional review supports the prevailing consensus on the growing role that computer tomographic assisted biomodelling has in the surgical resection and/or reconstruction of the maxillofacial skeleton.
Key words
3D printing, stereolithographic, biomodel, maxillofacial, virtual, complex surgery
Introduction
There are very few procedures in surgical practice more challenging than the repair of a cranio-maxillofacial (CMF) deformity. Elements of the craniofacial skeleton may be either developmentally absent, damaged either by trauma, infection or inflammation, or destroyed by an infiltrative neoplastic process.
As a result of these deleterious pathologies, the hard (and soft) tissue components of the face can require intricate and accurate surgical reconstruction. Such procedures are considered in the context of restitution of the form (facial harmony and symmetry), function and, in addition, must often consider the psychological well-being of the patient.
Facial structure is both idiosyncratic and complex. The task of recreating its inherent three-dimensional characteristics often presents a monumental challenge to the surgeon. It relies on a very artistic appreciation of facial aesthetics, proportion, symmetry and balance.
Difficult, often protracted, “eye match” manual techniques have been historically employed in order to obtain, at best, satisfactory results. Local and regional tissue recruitment is the preferred method of general reconstructive surgery. This is because of the proximity and similarity of neighbouring tissue.
However, two fundamental problems occur when considering this philosophy in its application to head and neck reconstruction. The maxillofacial region is the cephalic extent of the axial skeleton and, it comprises 10% of the body quantitative surface area. As such, there intrinsically is a lack of tissue available to recruit for reconstructive purposes. This is reflected in a lack of potential loco-regional donor sites.
The reconstruction of a bony contour, discontinuity or a composite defect is generally based on the placement of either an autogenous graft on an allogenic implant. Various elements of intramembranous, endochondral or ectomesenchymal autogenous bone have been incorporated into skeletal defects by free (onlay-inlay), pedicled or free vascularized techniques.
It is widely acknowledged that computerised tomographic assisted biomodelling has evolved over the last 20 years as a useful adjunct to both plan and execute CMF corrective bone surgery. In essence, the notion of CT biomodelling can be considered as the intersection of biology, engineering and medicine.
The purpose of this study was to investigate the utility of computerized tomographic (CT) assisted biomodel fabrication in the setting of resection and/or reconstruction of elements of the maxillofacial skeleton.
Our hypothesis was that since its inception, CT assisted biomodelling has and continues to remain a useful adjunct to aid in the planning of the surgical resection and/or reconstruction of a deficiency or deformity of the maxillofacial skeleton.
There were three specific aims to this study. First, to identify the number of biomodels that have been produced annually by request by the Anatomics® company, Melbourne, Australia, between 2000 and 2015. Second, to review the range and the applications of biomodels as utilized by the respective requesting surgical teams. Third, to document and consider available patient demographic and surgeon specific variables (patient age, sex, pathology, and requesting surgical subspecialties).
Methods
To address the research purpose, we designed and implemented an ambispective descriptive cohort study of the requests made to Anatomics® for the construction of a maxillofacial biomodel.
The study sample was derived from the in-house database details held by Anatomics® in relation to the patient cohort referred to them for biomodel fabrication. The period of study was 2000 to 2015. The inclusion criteria were the fabricated maxillofacial (viscero-cranial) biomodels. The exclusion criteria were neuro-cranial and extra cranio-facial biomodels.
The study also collected data on facial and cranial PoreStar® porous polyethylene implants from May 2014 to November 2015, pre-bent plates, and Anatomics® surgical planning aids. The numbers of nationally registered surgeons per subspecialty was retrieved from the Australian Institute of Health and Welfare database through the Australian Health Practitioner Register Authority.
CT imaging protocol
Anatomics® request that a high resolution Three Dimensional (3D) Helical CT scan be performed according to a strict protocol. Fine slice acquisition (0.5 mm slice thickness) with 0.4 mm spacing is required for facial bones imaging. Lower resolution of 1.0 mm with 0.8 mm spacing is also accepted but not as desirable for modelling fine features of the facial skeleton. The scans should be secured on a bone algorithm, with a zero-gantry tilt and with a low milliAmp dose. The patient must remain completely still during the scan. The fine slice acquisition data is archived in DICOM format to CD, DVD or electronic transmission via Anatomics® ordering portal software.
Biomodel fabrication protocol
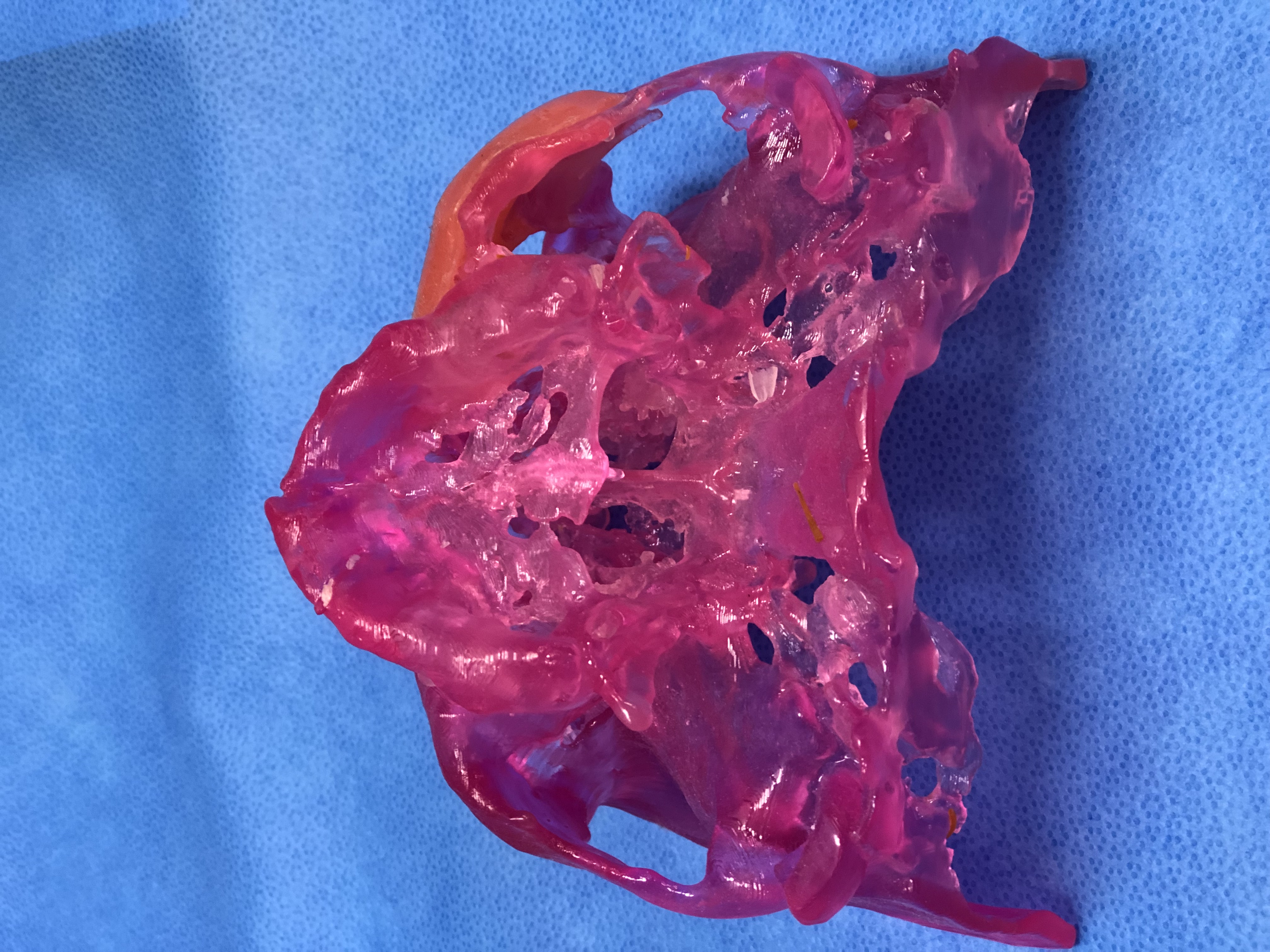
Following CT image acquisition and importation, the 3D data sets were interfaced through an Anatomics-specific software manufacturing chain in order to create a virtual 3D model (stereolithographic file). This in turn is exported and via 3D Printing (specifically stereolithography) converted into a high-resolution 3D resin biomodel.
In essence, an Ultraviolet (UV) laser is guided to selectively polymerize a photosensitive liquid resin contained in a processing chamber enabling the fabrication of a stereolithographic model. A recoater blade moves across the surface and successive layers are incrementally added as the build platform is lowered into the vat of liquid resin. Selective red colouration can be introduced into the biomodels by passing the laser over specific features a second time with increased laser power. Specially formulated resin is utilized for this purpose. The resin is also USP Class VI capable for medical applications ensuring suitability for use in the surgical field. The completed UV solidified Photopolymer model is then cured in a UV oven for one hour. Biomodels then undergo quality assurance to ensure they match the scanned anatomy and post-processing to clean and lacquer finish.
Anatomics® subsequently supplies the biomodel to the requesting surgeon or utilizes the biomodel along with the provided 3D imaging to create patient specific implants (PSIs). Implants for cranial vault reconstruction are manufactured by Anatomics® from heat-cured preformed polymethylmethacylate (PMMA). As of 2011, Anatomics® also provide PoreStar® polyethylene implants. These implants are manufactured from star-shaped particles of high-density porous polyethylene (HPDE). The particles are bound together into a 3D physical topology that approximates that of trabecular bone. The design aim is to achieve bony ingrowth into the implant.
Implant Positioning Templates® (IPTs) are devices that serve as adjuncts to a biomodel and/or PSI. In the context of facial surgery, IPTs® are typically “proxies” or “implant analogues” that can be used by the operating surgeon to pre-shape the implant tissue pocket in preparation for implant placement. Sterile IPTs® are supplied optionally on request along with the sterile PoreStar® implant.
PoreStar® PSI design iterations can be achieved using AnatomicsC3D® software. This is an on-line planning solution that allows surgeons to connect remotely to Anatomics® via any web browser to view 3D implant designs in real time and collaborate on implant design.
The entire manufacturing process for biomodels, PoreStar® implants and IPTs® at Anatomics® is controlled by an ISO 13485 certified quality management system for medical device design and manufacture.
Variables
The primary predictor variable was the biomodel construct. The secondary outcome variables were the range of applications that the biomodel engendered within the requesting surgical community. Other variables that were considered included patient demographics and the requesting surgical subspecialty.
Data collection methods
The records were electronically retrieved from the Anatomics® database and entered into a spreadsheet. A descriptive analysis of the relevant data was undertaken by the authors.
Ethics
We adhered to the privacy and confidentiality of patient data, clinical information, and surgeon data in the conduct of this research. Such retrospective data was treated anonymously by the authors. The study was granted an exemption in writing by the Hunter New England Health Research Ethics Committee (Institutional Review Board, HNEHREC 16/03/16/5.01). All patients that were included in the study had their treatment plan decided by their respective surgeons or surgical multi-disciplinary teams. The research did not receive any specific grant from funding agencies in the public, commercial or non-for-profit sectors.
Results
The study sample consisted of 956 requests of Anatomics® for the fabrication of a CMF biomodel. The period of study was sixteen years from 2000 to 2015. The topographical characteristics of the biomodels produced reflected a range of upper, mid and lower facial anatomical combinations and individual subsites. Face and mandible (31%, n=287), mandible (16%, n=149), and mid-face (13%, n=124) biomodels were the commonest requested (Figures 1 and 2).
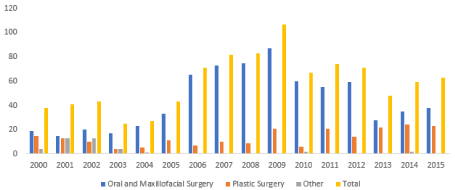
Figure 1. Yearly biomodel requests by surgical discipline
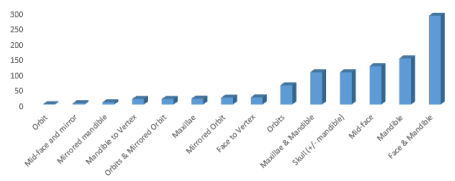
Figure 2. Upper, mid and lower facial biomodel combinations and sub-sites
The total number of biomodels requested per annum steadily increased from 2000 to 2009, at which time they peaked. The numbers remained relatively constant from 2010 to 2015. Requests for biomodel fabrication came predominantly from Oral and Maxillofacial surgeons (n=702). Biomodels requested on behalf of male patients (n=539) exceeded those for female patients (n=417) for most years, although this was not clinically significant. (Figure 3).
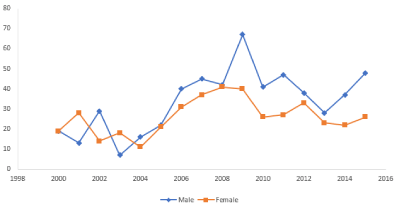
Figure 3. Biomodel requests by gender
The average age of patients for whom biomodel requests were made ranged from a low of 16 years of age in 2001 to a high of 38 years of age in 2012. The average patient age per year was similar between the sexes for most years. (Figure 4).
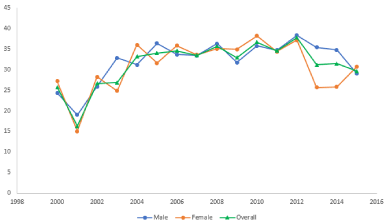
Figure 4. Average age of patient per year
From May 2014 to November 2015, requests for facial PoreStar® PSIs were led by the Plastic Surgeons. All cranial implant requests were made exclusively for Neurosurgeons (Table 1). The average implants per case for facial biomodels exceeded cranial biomodels (1.5 vs. 1.1). Biomodel implant volume data was incompletely available (14/26 facial, 17/19 cranial). However, there was still a difference between the volumes of facial and cranial implants (5.5 cm3 vs. 68.3 cm3). Requests to Anatomics® for surgical planning aids (AnatomicsC3D Online Implant Design® and IPTs®) were exclusively associated with facial implant requests. Figures relating to neurosurgery cranial implants were not considered in this study.
Table 1. Customized PoreStar® Implants
|
Facial |
Cranial |
Discipline |
OMFS † |
10 |
0 |
Plastics |
16 |
0 |
Neurosurgery |
0 |
19 |
Gender |
Male |
19 |
14 |
Female |
7 |
5 |
Age (years) |
Male |
37 |
44 |
Female |
35 |
39 |
Implants Per Case |
1.5 |
1.1 |
C3D † †® |
6 |
0 |
IPT † † †® |
9 |
0 |
Volume (cm3) |
5.5 |
68.3 |
†Oral and Maxillofacial Surgery
† †AnatomicsC3D Online Implant Design®
† † †Implant Positioning Template® |
A total of 6 requests were made for pre-bent plates (3 in 2008, and 1 in 2013, 2014 and 2015), and these were evenly spread between the Oral Maxillofacial Surgery and Plastic Surgery sub-specialties.
Pathology from each of the 956 cases was not able to be obtained from the database.
From 2010 to 2015, the number of surgeons requesting biomodels as a fraction of surgeons registered nationally in Australia was Ear, Nose and Throat Surgery 2/495 (0.004%), Neurosurgery 3/245 (0.012%), Plastic Surgery 27/448 (6.03%), and Oral and Maxillofacial Surgery 73/202 (36.1%).
Discussion
This study identified a total of 956 CMF biomodels that were produced by the Anatomics® company over 16 years. The total biomodel requests per year have significantly increased over the decade of 2000 to 2009, advanced predominantly by the sub-specialty of Oral Maxillofacial Surgery. Plastic Surgery has increased its yearly biomodel requests from 2010 to 2015, and has in recent years reached levels comparable to Oral and Maxillofacial Surgery. Nevertheless, an analysis of request numbers proportionate to number of registered surgeons affirms Oral and Maxillofacial Surgery as the dominant requesting sub-specialty. These trends may be explained by an increasing evidence base supporting the utility of biomodels in maxillofacial surgery.
An expanding scope for the use of CMF biomodels is indicated by the adoption of biomodels amongst different surgical sub-specialties and a rising average patient age over the study period. The mid face and mandible was the dominant region for which biomodels were requested, in keeping with the prevailing view that biomodels are most useful in the execution of anatomically complex surgery that demands surgical precision. Specifically, the exacting considerations of posterior facial height and glenoid fossa articulation combined with anterior facial projection and occlusal table support underpins the absolute accuracy that is required in mandibular reconstruction. There were no notable patient sex differences in requests for biomodels.
Anatomics have recently introduced a standardized observational proforma assessing the accuracy and fit of Porestar patient specific implants based on biomodel fabrication. The questionaire specifically includes a detailed analysis of a variety of demographic variables including surgeon (specialty and hospital), patient (age, sex, pathology and site), biomodel and implant information (design, characteristics, placement, method of fixation, size and fit). Whilst early data supports the efficacy of biomodels, it will require greater statistical power than is cureently available for more meaningful interpretation, and therefore may be the subject for future study.
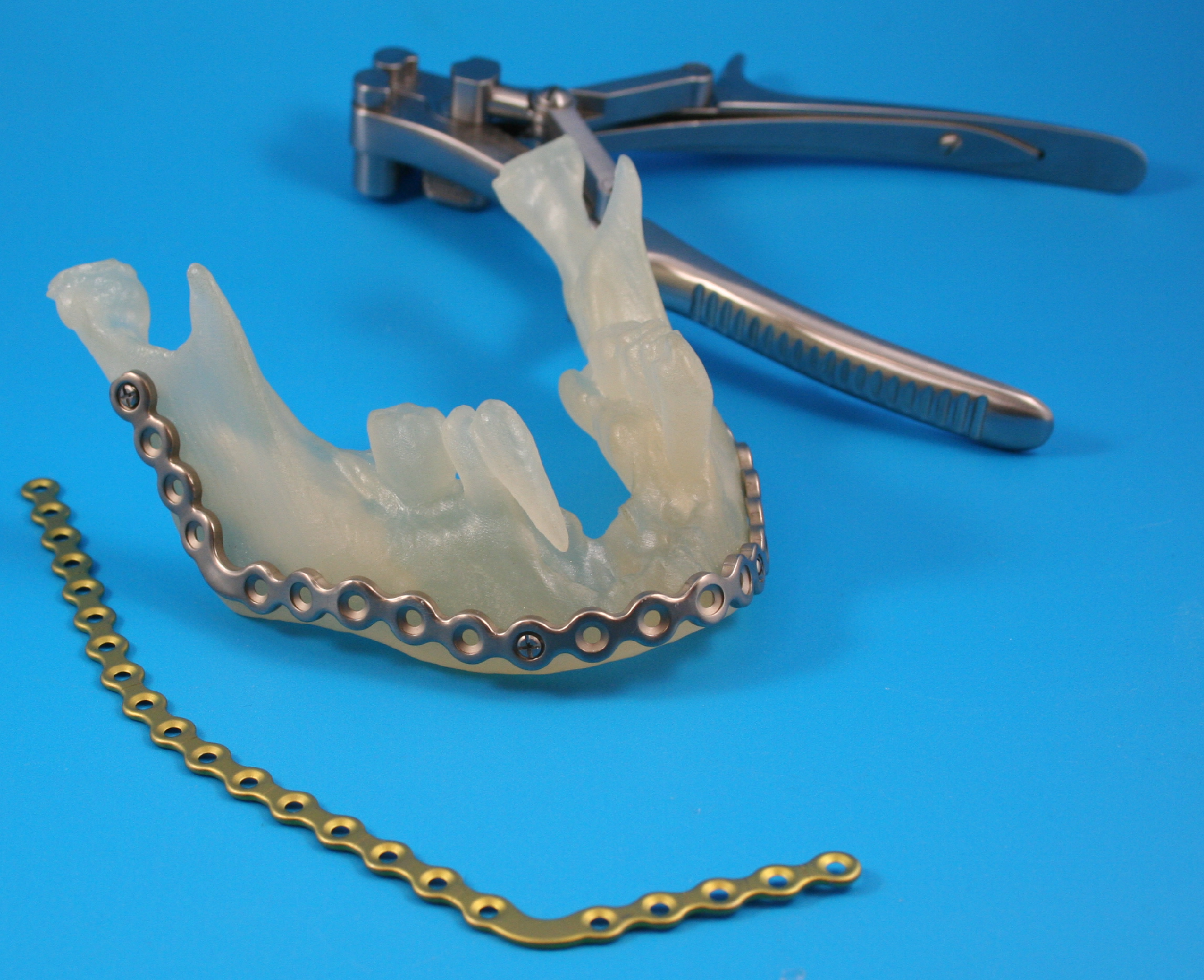
The Anatomics® biomodelling system (after D’Urso) [1] has proven pre-operative, intra-operative, and diagnostic benefits [2] due to its morphologic accuracy [3,4]. Virtual biomodels have since been utilised in order to manufacture resection guides [5], bone graft cutting guides [5], and pre-bent plates and can also outline native maxillofacial anatomy using mirroring technology [6-8]. Interestingly, the ‘hands on’ physical biomodel can better facilitate surgical simulation, technical evaluation of distance, volume and angulation as well as an exchange of ideas between surgeons [9].
The current drawback of biomodel technology is high costs [5] and lengthy production times [10]. Guides, splints and plates can be manufactured within 2 to 7 days given the right hospital infrastructure [11]. Decreases in additional corrective procedures [11], operative time [8], blood loss [8], and improved patient outcomes [10,11] may render biomodel technology cost effective long term. Hospital owned 3D printers [12], with centralized digital access and applications into related surgical fields such as prosthetics [13], will likely improve the cost-benefit ratio.
The current status and likely future directions of medical-surgical 3D printing has been succinctly reviewed by Fox and Peregrin [14]. These authors reaffirm that the principal advantage of biomodel technology is to allow surgeons to pre-operatively plan procedures (ie: osteotomy, ostectomy, osteo-augmentation) down to the millimetre. In essence, it allows for the consideration of complex operative undertakings on patient specific (one-to-one) models in addition to removing the trial and error element (that often plagues such procedures) from the patient to a disposable model. The pre-operative fabrication of a 3D printed biomodel provides a more tangible experience for the surgical team by improving their abiliity to accurately visualize the extent of pathology instead of purely relying on a cognitive re-assembly of 2D coronal, axial, and sagittal screen imagery. Emergent applications of this technology include:
- a likely increase in uptake by soft tisue-organ specific surgeons,
- a rising demand for 3D models (ie: provide millemetre to millimetre exact correlations and manipulate the model while performing a procedure) due to paradigm shift from open to minimally invasive access surgery, and
- a surgical education and training tool for both the trainee and the experienced surgeon (as well as the patient) and to immortalize rare cases (particularly those unencountered during routine training), leading to the establishment of a biomodel library or museum.
Although it is generally accepted that biomodel technology contributes to better outcomes, there currently is no way to uphold this notion. In particular, no studies to date have been able to quantify meaningful reductions in complications rates, operating times, and operative costs. There also remains three more defined ongoing limitations to biomodel uptake and usage: (i) slow build times, (ii) a lack of autonomy due to handing over aspects of the case workup to information technology expertise and (iii) expensive infrastructure (equipment, soft ware, technical expertise), particularly for occasional ‘one off’ usage.
The future lies in the potential evolution of bio-printing and hybrid technology, as an example, using a stem cell base as an alternative or in addition to the resin polymer or allied scaffolds.
Reports of biomodel usage and applications have been variously detailed in the literature for the last 20 years. Our study has documented an extremely large number of biomodels that were produced by a single source. Our experience in biomodel request and application has predominantly been in the planning and execution in cases of complex trauma and pathology. A review of our own records not unexpectedly reveals a case mix that reflects maxillary and mandibular segmental resection for cytologically benign but biologically aggressive disease (ameloblastoma, odontogenic keratocystic tumors, expansile cemento-ossifying fibromas), cytologically malignant disease, (including invasive intra-osseous squamous cell carcinoma), osteoradionecrosis and mid facial primary and secondary trauma repair (complex zygomatico-orbital fractures, orbital floor revision, onlay autogenous or alloplastic repairs for post traumatic contour deformity). We believe that our experience is likely to be reflective of other similar units both nationally and internationally.
This study’s strengths included a large number of cases, a long study period, and a single large company focus that represents a national ‘snapshot’ of current trends in Australia. The weaknesses of the study include exclusion of other companies that may produce biomodels in Australia or any ‘in-house’ 3D printing that may be undertaken, and an inability to globally consider the types of pathology that underpins requests for biomodel fabrication.
The results of this study confirm that computer tomographic assisted biomodel fabrication is playing an increasingly important role to aid in both the plannng and undertaking of surgical resection and/or reconstruction of segments of the maxillofacial skeleton. It is likely that as the technology continues to evolve, the demand by surgeons should continue to increase presently. It is the exacting requirements of posterior facial height and glenoid fossa articulation combined with anterior facial projection and occlusal table support that most often necessitates biomodel fabrication technology in the context of complex mandibular resection and reconstruction.
Disclosure statement
Mr Robert Thompson is employed by Anatomics®. His contribution to the paper was to retrieve relevant information from their in-house database. He also contributed to the outline of the biomodel fabrication protocol. Dr Umair Ansari has no conflict of interest. Prof. Gary Hoffman has no conflict of interest. No author received any funding for the conduct of this research. All authors have agreed to the submission.
The study was granted an exemption in writing by the Hunter New England Health Research Ethics Committee (Institutional Review Board, HNEHREC 16/03/16/5.01). The research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.
References
- D'urso PS (1998) Stereolithographic Biomodelling in Surgery, PhD Thesis, The University of Queensland, Australia.
- D'Urso P, Barker T, Earwaker W, Bruce LJ, Atkinson RL, et al. (1999) Stereolithographic biomodelling in cranio-maxillofacial surgery: a prospective trial. J Craniomaxillofac Surg 27: 30-37.
- Arvier J, Barker T, Yau Y, D'Urso P, Atkinson R, et al. (1994) Maxillofacial biomodelling. Br J Oral Maxillofac Surg 32: 276-283. [Crossref]
- Barker T, Earwaker W, Lisle D (1994) Accuracy of stereolithographic models of human anatomy. Australas Radiol 38: 106-111.
- Wilde F, Cornelius CP, Schramm A (2014) Computer-assisted mandibular reconstruction using a patient-specific reconstruction plate fabricated with computer-aided design and manufacturing techniques. Craniomaxillofac Trauma Reconstruction 7: 158-166. [Crossref]
- Cohen A, Laviv A, Berman P, Nashef R, Abu-Tair J, et al. (2009) Mandibular reconstruction using stereolithographic 3-dimensional printing modeling technology. Oral Surg Oral Med Oral Pathol Oral Radiol 108: 661-666.
- Feng F, Wang H, Guan X, Tian W, Jing W, et al. (2011) Mirror imaging and preshaped titanium plates in the treatment of unilateral malar and zygomatic arch fractures. Oral Surg Oral Med Oral Pathol Oral Radiol 112: 188-194.
- Khalifa G, El Moniem N, Elsayed S, Qadry Y (2016) Segmental Mirroring: Does It Eliminate the Need for Intraoperative Readjustment of the Virtually Pre-Bent Reconstruction Plates and Is It Economically Valuable?. J Oral Maxillofac Surg 74: 621-630.
- Robiony M, Salvo I, Costa F, Zerman N, Bazzocchi M, et al. (2007) Virtual reality surgical planning for maxillofacial distraction osteogenesis: the role of reverse engineering rapid prototyping and cooperative work. J Oral Maxillofac Surg 65: 1198-1208.
- Hanasono M, Jacob R, Bidaut L, Robb G, Skoracki R, et al. (2010) Midfacial reconstruction using virtual planning, rapid prototype modeling, and stereotactic navigation. Plast Reconstr Surg 126: 2002-2006.
- Tepper O, Sorice S, Hershman G, Saadeh P, Levine J, et al. (2011) Use of virtual 3-dimensional surgery in post-traumatic craniomaxillofacial reconstruction. J Oral Maxillofac Surg 69: 733-741.
- Schwam Z, Chang M, Barnes M, Paskhover B (2016) Applications of 3-Dimensional Printing in Facial Plastic Surgery. J Oral Maxillofac Surg 74: 427-428.
- Sykes L, Parrott A, Owen C, Snaddon D (2004) Applications of rapid prototyping technology in maxillofacial prosthetics. Int J Prosthodont 17: 456-459. [Crossref]
- Fox M, Peregrin T (2016) 3-D printing: Revolutionizing pre-operative planning, resident training, and future of surgical care. Bulletin: Am Coll Surg 101: 9-18.