We present the history of 60-years-old woman with mitral and aortic bioprosthesis that have been implanted together with left atrial appendage closure. The patient had been treated with VKA and low dose of acetylsalicylic acid since surgery. On 28th day of postoperative period an ischemic stroke occurred. In transoesophageal echocardiography we have discovered non-obstructive thrombosis of the artificial mitral annulus and a gap in left atrial appendage (LAA) patch. There was rapid outflow through the gap in LAA directing to mitral annulus in close proximity to visible thrombus. The pathologic flow might have had the contribution in thrombus mobilization and stroke occurrence. We have decided to change anticoagulation to low molecular weight heparin. In control echocardiography, during heparin therapy, thirteen days later, there was remarkable worsening of annulus thrombosis with multiple thrombi present. Re-exchange anticoagulation to warfarin yielded in almost complete resolution of thrombosis after eleven days. We decided to continue anticoagulation over recommended three months not only due to incident of thrombosis but also suspicion of history of atrial fibrillation and presence of disrupt-ed LAA patch.
Prosthetic thrombosis, embolic stroke, left atrial appendage closure, thrombus
A 60-years-old woman with a history of caseous mitral annulus calcification, severe mitral regurgitation, moderate aortic stenosis and regurgitation, have had implantation of mitral and aortic bioprosthesis together with left atrial appendage closure. In postoperative period a typical anticoagulant was implemented - low molecular weight heparin followed by vitamin K antagonist under international normalized ratio (INR) control. Due to coexisting coronary artery disease and venous graft implanted an antiplatelet treatment (75 mg of acetylsalicylic acid) was also administered. On 28th day after the surgery a symptom of right disparation, ocular ataxia, facial numbness and balance disorders occurred. In ultrasonography and computed tomography angiogram (angioCT) there was obstruction of right vertebral artery revealed. No visible ischemic focus was discovered in brain computed tomography. Laboratory tests revealed therapeutic level of INR.
In transthoracic echocardiography we have discovered turbulent flow through mitral prosthesis with no other dysfunction. Appearance and function of aortic bioprosthesis and other heart structures were normal. In transoesophageal imaging there was small thrombus in lateral part of mitral annulus. Patch on left atrial appendage was disrupted and a flow through the leak was visible. This outflow was rapid, turbulent and directed straight to mitral annulus, in close proximity to present thrombus. We suspect mobilization of a thrombus resulting in stroke by rapid left atrial appendage outflow. After the diagnosis of non-obstructive prosthetic thrombosis anticoagulation with low molecular heparin was implemented. In control echocardiography thirteen days later, we have confirmed previously diagnosed thrombus and four new mobile thrombi present. Our next decision was to implement vitamin K antagonist (VKA) with rigid INR control. After two weeks of the VKA therapy we have observed resolution of annulus thrombosis. We plan to continue VKA with target INR 3,0 together with low dose of acetylsalicylic acid for three months and continue further anticoagulation with VKA alone.
Patients after cardiac surgery are high risk group of ischemic strokes [1]. The risk is even higher after valve surgery [2]. Mitral valve replacement presents a 1,62% threat of stroke in postoperative period [3]. Current recommendations direct us to use vitamin K anticoagulants for three months after mitral valve replacement (bioprosthesis) [4,5]. Taking into account prevalence of atrial fibrillation in patients subjected to mitral valve surgery and specific surgical access, closure of left atrial appendage emerged as almost a routine [6]. Dispatching of the LAA patch took is rare [7]. In our patient it took place probably to high pressure generated by contracting LAA muscle. Unfortunately outflow through the crack was directing to mitral annulus and probably mobilized thrombus that was present in this place. The bare metal of mitral annulus is highly thrombogenous. After period of epithelialization, (ap-proximately 3 months), thromboembolic risk grows low and the anticoagulation can be stopped.
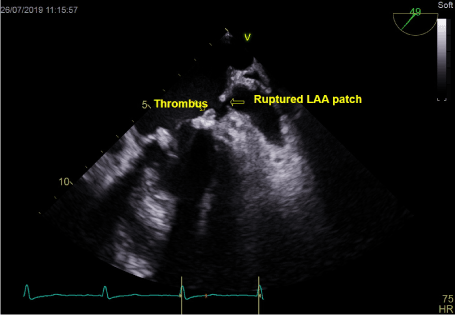
Figure 1. Thrombus on mitral annulus and gap in LAA patch
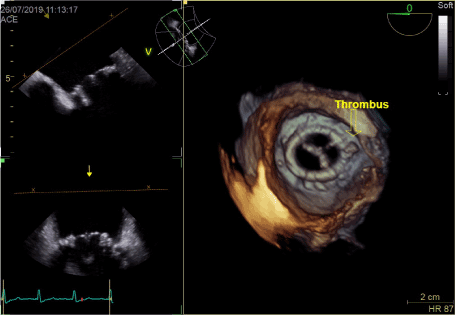
Figure 2. Thrombus on mitral annulus close to LAA in 3D
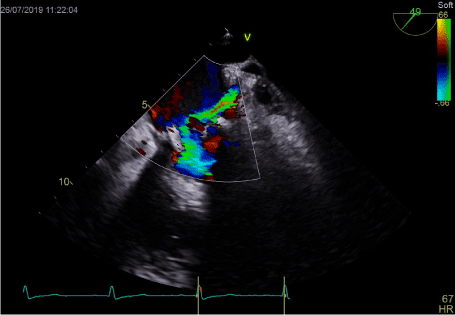
Figure 3. Turbulent LAA outflow directing to mitral annulus
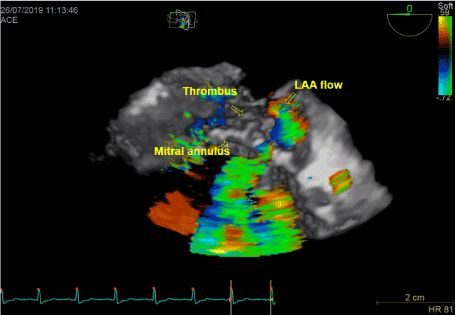
Figure 4. 3D reconstruction pf lateral part of mitral annulus
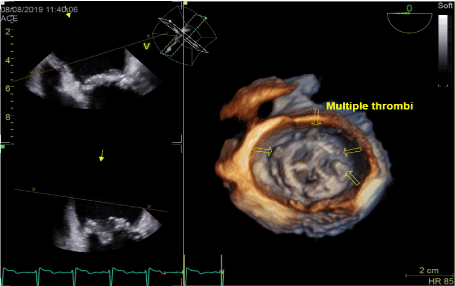
Figure 5. Worsening of annulus thrombosis on low molecular heparin therapy
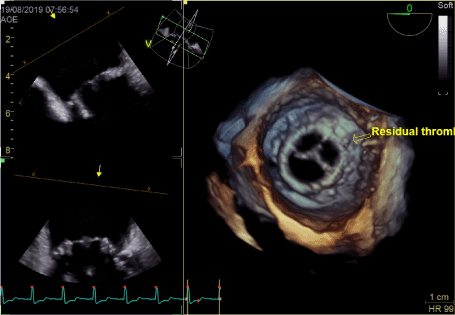
Figure 6. Resolution of thrombosis on vitamin K antagonist therapy
In this case we think, that continuing of anticoagulation over recommended 3 months is good choice. Arguments for further anticoagulation are: thrombembolic event in postoperative period, unclosed left atrial appendage, presence of malign LAA flow, calcification of native mitral annulus [8] and not clear history of atrial arrythmia. In this specific case there was no documented atrial fibrillation but the risk of the arrhythmia in patient with history of severe mitral regurgitation is very high [9]. Taking into account history of the patient, despite of lower risk of both bleeding and stroke of NOACs [10] we decided to continue anticoagulation with vitamin K antagonist.
- Hogue CW Jr , Murphy SF, Schechtman KB, Dávila-Román VG. (1999) Risk Factors for Early or Delayed Stroke After Cardiac Surgery. Circulation 100: 642–647. [Crossref]
- Ruel M, Masters RG, RubensDF, Bedard PJ, Pipe AL, et al. (2004) Late incidence and determinants of stroke after aortic and mitral valve replacement. Ann Thorac Surg 78: 77–83. [Crossref]
- Udesh R, Natarajan P, Jeevanantham V, Gleason T.G, Badhwar V, et al. (2017) Perioperative Strokes Following Surgical Correction of Mitral Valves: A Systematic Review and Meta-Analysis. Eur Neurol 78: 63-70. [Crossref]
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, et al. (2017) 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 38: 2739–2791. [Crossref]
- Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, et al. (2013) Dabigatran versus Warfarin in Patients with Mechanical Heart Valves. N Engl J Med 369: 1206-1214. [Crossref]
- Squiers JJ, Edgerton JR. (2018) Surgical Closure of the Left Atrial Appendage: The Past, The Present, The Future. J Atr Fibrillation 10: 1642. [Crossref]
- Lynch M, Shanewise JS, ChangG, Martin RP, Clements SD. (1997) Recanalization of the Left Atrial Appendage Demonstrated by Transesophageal Echocardiography. Ann Thorac Surg 63: 1774-1775. [Crossref]
- De Marco M, Gerdts E, Casalnuovo G, Migliore T, Wachtell K, et al. (2013) Mitral Annular Calcification and Incident Ischemic Stroke in Treated Hypertensive Patients: The LIFE study. Am J Hypertens 26: 567–573. [Crossref]
- Grigioni F, Avierinos J-F, Ling LH, Scott CG, Bailey KR, et al. (2002) Atrial fibrillation complicating the course of degenerative mitral regurgitation. J Am Coll Cardiol 40: 84-92. [Crossref]
- Bennaghmouch N, de Veer AJWM, Bode K, Mahmoodi BK, Dewilde WJM, et al. (2018) Efficacy and Safety of the Use of Non–Vitamin K Antagonist Oral Anticoagulants in Patients with Nonvalvular Atrial Fibrillation and Concomitant Aspirin Therapy A Meta-Analysis of Randomized Trials. Circulation 137: 1117-1129. [Crossref]






