Background: While reducing the escape of pneumoperitoneum gas has always been desirable in terms of avoiding exposure to surgical smoke and its contents, the concern over leakage has increased during the COVID-19 pandemic. This study was undertaken to compare insertion and retention forces and leak rates of commercially-available trocars and provide recommendations on methods to minimize exposure to pneumoperitoneum gas.
Methods: Trocars of 5 and 12-mm diameter from three commonly-used brands were evaluated. A preclinical model was used to assess insertion and retention forces, and a benchtop model was used to measure gas leakage under four conditions; with the obturator fully inserted, without a probe, with a probe, and with a probe under a bending stress as might occur during instrument manipulation.
Results: All 5-mm trocars displayed similar insertion and retention forces, whereas among the 12-mm trocars the XCEL brand had significantly lower insertion and higher retention forces. With regard to gas leakage, the XCEL trocars displayed low levels under all four conditions. High levels of leakage (>1000 ml/min) either with the obturator inserted or during bending with the probe inserted were found in two of the brands.
Conclusions: Differences between commercial trocars were observed for insertion and retention forces and for rates of leakage under conditions that occur during laparoscopy. High retention forces are necessary to protect against accidental slippage of the trocar during surgery and release of pneumoperitoneum gas. Although it is currently unknown whether coronavirus may be transmitted via laparoscopic gas, it is considered a best practice to minimize the escape of gas that may occur.
trocar, COVID-19, laparoscopy, insufflation, pneumoperitoneum
The SARS-CoV-2 virus is responsible for the worldwide epidemic of COVID-19 disease, which has severely limited elective surgical activity throughout the world. The main source of transmission of SARS-CoV-2 appears to be through respiratory droplets [1] (particles >5-10 μm in diameter) from infected people and through contact with contaminated surfaces [2-7]. The possible transmission through aerosolization during laparoscopy is currently unknown [8,9].
Even though the presence of SARS-CoV-2 in the peritoneal cavity is still debated [10,11], as well as its presence in surgical smoke [12], concerns about the possible release of SARS-CoV-2 with high-pressure CO2 leaks around or through the trocars have been raised [13]. There is a risk that coronavirus as well as other viral particles and micro-organisms could be aerosolized in the pneumoperitoneum, and escape via a trocar either during the procedure (e.g. instrument change or extraction of a specimen) or during desufflation, especially if energy devices are used. Several surgical societies (e.g., SAGES/EAES [14], ESGE [15], AAGL[16]) have issued recommendations regarding the use of trocars in laparoscopy to help reduce the risk of virus transmission to the operating room (OR) personnel from patients that are potentially infected with COVID-19.
Trocar systems have a cannula, which is used to insert medical devices into the body cavity. During a procedure, surgeons frequently manipulate and exchange the instruments in the cannulas causing frictional forces between the instrument and the cannula, and this can result in movement of the cannula in an inward or outward direction within the body wall. If the cannula is not fixed in place, there is a potential the cannula may slip out of the body wall. If the cannula does not have the desired retention it may tilt or fall out, allowing gas to flow into the operating room. Trocar retention can also present problems as instruments and specimens are removed from a body cavity through the cannula and the associated seal systems of the trocar may let gas escape into the operating room. Ideally the cannulas remain in a fixed position once placed in the patient’s body.
Valveless trocars allow pneumoperitoneum to escape during procedures if there is an increase in intra-abdominal pressure, or when instruments are exchanged. This “open-circuit” type of trocar may produce a jet-like stream of aerosolized particles within the pneumoperitoneum, so is recommended against [13]. A “closed-circuit” valved trocar may help decrease the release of gas. With laparoscopy or robot-assisted laparoscopy, sudden release of trocar valves, non-air-tight exchange of instruments or specimen extraction via abdominal or vaginal incisions may potentially expose the health care team to aerosolized viral particles. While it is important to acknowledge these concerns, at present, they remain theoretical in relation to risk of transmission of COVID-19 to operating room personnel. Other practical measures for safely maintaining pneumoperitoneum have been outlined by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) [14].
This study on preclinical models was undertaken to compare insertion force, retention force, and relative leakage between the XCEL trocars (Ethicon, Inc., Cincinnati OH) and other commercially-available trocars. In addition, recommendations specific to the use of trocars in laparoscopic procedures with consideration given to precautions required during the current pandemic are provided.
For this study, several of the most-widely used 5-mm and 12-mm trocars were evaluated. For the insertion and retention force experiments, the 5-mm trocars evaluated were ENDOPATH XCEL® Bladeless 5-mm trocar (B5LT, Ethicon, Inc., Cincinnati OH); Kii Fios® First Entry Advanced Fixation 5-mm Trocar (CFF03), and Kii Fios First Entry Z-Thread 5-mm Trocar (CTF03, Applied Medical Resources Corporation, Rancho Santa Margarita CA ); and Versaport™ Plus 5-mm Trocar (NB5STF) and Versaport Optical 5-mm Trocar (ONB5STF, Medtronic, Fridley MN). The retention force for CFF03 could not be determined because of the saline-inflated balloon located at the distal tip of the trocar.
The 12-mm trocars evaluated for insertion and retention force were ENDOPATH XCEL® Bladeless 12-mm trocar (B12LT, Ethicon); Kii Fios® First Entry Advanced Fixation 12-mm Trocar (CFF73) and Kii Fios First Entry Z-Thread 12-mm Trocar (CTF73, Applied Medical); and Versaport Plus 12-mm Trocar (NB12STF) and Covidien Versaport Optical 12-mm Trocar (ONB12STF, Medtronic). The retention force for CFF73 could not be determined because of the saline-inflated balloon located at the distal tip of the trocar.
Trocar insertion and retention forces were evaluated in a porcine model, with animals weighing 36 to 54 kg. Since insertion force may increase with weight of the animal, comparisons were performed within individual subjects. Based on internal testing, placement in the upper or lower abdomen is not a significant factor for force, as long as insertions are performed above the arcuate line. Insertions were blocked by animal and distance from midline (proximal, middle, distal).
Peak insertion force was measured with a Daytronic 3570 load cell (Daytronic Inc., Miamisburg OH) located in a thin shroud placed around the trocar housing. Retention force was measured with a Mark-10 force gauge (Mark-10 Corp., Copiague NY) attached with a custom adapter for each trocar design.
The anesthetized pig was placed in dorsal recumbency and insufflated with a target CO2 pressure of 12-15 mmHg. For 5-mm trocars, 7 insertion sites were located between the costal arch and umbilicus, and 2 sites between the umbilicus and arcuate line. For 12-mm trocars, 4-5 insertion sites were located between the costal arch and umbilicus, and one site between the umbilicus and arcuate line. Incisions were made 4 mm longer than the cannula diameter with a #11 scalpel blade. After placing the trocar in its dedicated fixture, insertion was performed perpendicularly to the body wall as the insertion force was being monitored. After full insertion, the trocar was withdrawn perpendicularly to the body wall as the retention force was monitored. After removal of the trocars, plugs were used to maintain cavity insufflation.
Animal procedures were approved by the Institutional Animal Care and Use Committees and conducted in accordance with the Guide for the Care and Use of Laboratory Animals and applicable animal welfare regulations in AAALAC-accredited facilities.
For leak testing, the 5-mm trocars were ENDOPATH XCEL® Bladeless Trocar (B5LT, Ethicon), VersaOne™ Optical Trocar with Fixation Cannula (ONB5STF, Medtronic), Kii Optical Access System with Advanced Fixation (CFR03, Applied Medical), and Kii Optical Access System with Z-Thread (CTR03, Applied Medical). The 12-mm trocars were ENDOPATH XCEL® Bladeless Trocar (B12LT, Ethicon), VersaOne™ Optical Trocar with Fixation Cannula (ONB12STF, Medtronic), Kii Optical Access System with Advanced Fixation (CFR73, Applied Medical) and Kii Optical Access System with Z-Thread (CTR73, Applied Medical). For each test, a sample of 28 to 30 trocars was used.
Leak rates were evaluated with a Cosmo Air Flow Tester (Cosmo Instruments Co, Tokyo, Japan) or a TSI Flowmeter (TSI, Inc., Shoreview MN) under four conditions; 1) with the obturator fully inserted, 2) with no obturator or probe, 3) with a probe inserted, and 4) with a probe inserted and under a bending stress. The probe used was a 4.7-mm rod that simulated a laparoscopic device shaft. The 4.7-mm probe represents the smallest instrument size specified for both the 5 and 12-mm XCEL trocars. Without the obturator or probe inserted a test pressure of 9 mmHg was used, and with either the obturator or probe inserted the test pressure was 17 mmHg. A bending stress moment of 1.27 N.m was applied perpendicularly to the probe; this represents a force of 24.0 N on an XCEL trocar, a typical force used to manipulate trocars during a procedure.
Statistics: Peak insertion and retention forces were analyzed via ANOVA with location in body wall as a co-factor. Comparison of the XCEL trocar to the other trocars was performed using Fisher’s protected Least Significant Difference (LSD, i.e, only perform individual comparisons if the factor of product code had a significant effect) method of pairwise comparison using 95% LSD confidence limits, with an alpha of 0.05. Leak rates were evaluated by the Kruskal-Wallis test followed by multiple comparisons versus a control using a family alpha of 0.20 and a Bonferroni individual alpha of 0.10. Statistical calculations were performed with Minitab v17 (Minitab LLC, State College PA).
Peak insertion and peak retention force
For both the peak insertion force and peak retention force, the ANOVA was significant for product for both 5-mm and 12-mm trocars (p<0.001 for all), as well as for the adjusting factor of location (p<0.05). Results are provided in Table 1 and shown in Figures 1 and 2.
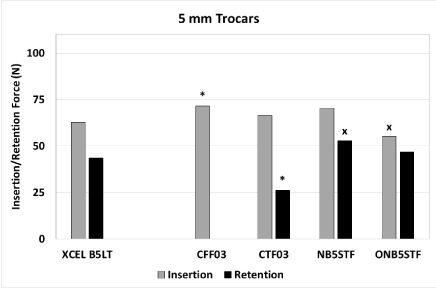
Figure 1. Peak insertion and retention forces for 5 mm trocars. An asterisk indicates the peak insertion force is significantly lower or peak retention force is significantly higher for XCEL relative to the compared trocar (p<0.05). An ‘x’ indicates that the peak insertion force is significantly higher or peak retention force is significantly lower for XCEL relative to the compared trocar (p<0.05)
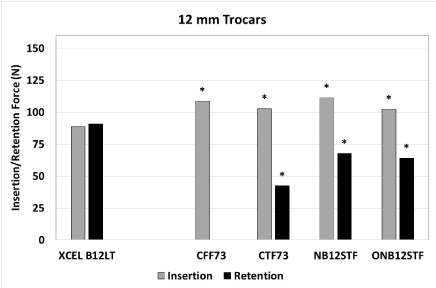
Figure 2. Peak insertion and retention forces for 12 mm trocars. An asterisk indicates the peak insertion force is significantly lower or peak retention force is significantly higher for XCEL relative to the compared trocar (p<0.05)
Table 1. Peak insertion and retention forces for 5 mm and 12 mm trocars.
5 mm Trocars |
Peak Insertion Force (N) |
|
p-value |
|
Peak Retention Force (N) |
p-value |
Ethicon XCEL B5LT |
62.7 |
|
- |
|
43.6 |
- |
| |
|
|
|
|
|
|
Applied Medical CFF03* |
71.6 |
|
0.013 |
|
NA |
NA |
Applied Medical CTF03 |
66.3 |
|
0.615 |
|
26.2 |
<0.001 |
Medtronic NB5STF |
70.3 |
|
0.057 |
|
52.9 |
0.005 |
Medtronic ONB5STF |
55.2 |
|
0.046 |
|
46.7 |
0.644 |
12 mm Trocars |
Peak Insertion
Force (N) |
p-value |
|
Peak Retention Force (N) |
p-value |
Ethicon XCEL B12LT |
88.5 |
- |
|
90.7 |
- |
| |
|
|
|
|
|
Applied Medical CFF73* |
108.5 |
<0.001 |
|
NA |
NA |
Applied Medical CTF73 |
102.8 |
0.001 |
|
42.3 |
<0.001 |
Medtronic NB12STF |
111.2 |
<0.001 |
|
67.6 |
<0.001 |
Medtronic ONB12STF |
102.3 |
0.001 |
|
64.1 |
<0.001 |
*The retention force for the CFF03 and CFF73 could not be determined because of the saline-inflated balloon located at the distal tip of the trocar.
For the 5-mm trocars, the XCEL trocar had a peak insertion force that was significantly lower than CFF03 by 12%, but higher than ONB5STF by 14%. No difference was observed in comparison with NB5STF. The XCEL trocar had a peak retention force that was 40% significantly higher than CTF03, and lower than NB5STF by 21%. No difference was observed in comparison with ONB5STF.
For the 12-mm trocars, the XCEL trocar had a peak insertion force that was significantly lower than all other trocars tested: CFF73 by 18%, CTF73 by 14%, NB12STF by 20%, and ONB12STF by 13%. The XCEL trocar had a peak retention force that was significantly higher than all other trocars tested: CTF73 by 115%, NB12STF by 34%, and ONB12STF by 42%.
Gas leak tests
Because the Medtronic and XCEL trocars had similar designs, the Medtronic trocars were not tested with the obturator inserted. For the 5-mm trocars (Table 2 and Figure 3), with the obturator fully inserted, the CFR03 and CTR03 had significantly higher leak rates than the XCEL trocar. Without probe insertion the ONB5STF had a significantly higher leak rate than the XCEL trocar, while CFR03 and CTR03 did not show a significant difference in comparison with XCEL. With probe insertion and with bending of the probe, the ONB5STF, CFR03 and CTR03 all had higher leak rates than the XCEL trocar.
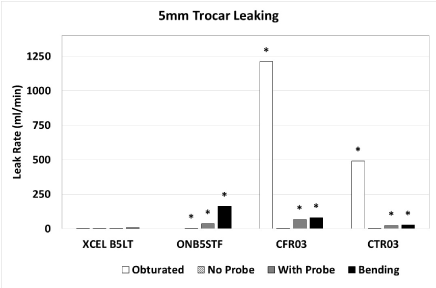
Figure 3. Leak rate for 5-mm trocars with obturator fully inserted, no probe, with probe, and with probe bent at an angle. An asterisk indicates a significantly higher leak rate compared to the XCEL trocar
Table 2. Leak rates for 5 mm and 12 mm trocars
5-mm Trocars |
State |
Mean ± St Dev
(ml/min) |
Median Leak (ml/min) |
p-value |
Ethicon XCEL B5LT |
Obturated |
0.4 ± 1.0 |
0.0 |
- |
Applied Medical CFR03 |
Obturated |
1210 ± 164 |
1227 |
<0.001 |
Applied Medical CTR03 |
Obturated |
490 ± 105 |
485 |
<0.001 |
|
|
|
|
|
Ethicon XCEL B5LT |
No Probe |
0.13 ± 0.43 |
0.0 |
- |
Medtronic ONB5STF |
No Probe |
3.20 ± 9.71 |
0.0 |
0.018 |
Applied Medical CFR03 |
No Probe |
0.00 ± 0.00 |
0.0 |
0.212 |
Applied Medical CTR03 |
No Probe |
0.00 ± 0.00 |
0.0 |
0.220 |
|
|
|
|
|
Ethicon XCEL B5LT |
With Probe |
1.50 ± 7.10 |
0.0 |
- |
Medtronic ONB5STF |
With Probe |
34.4 ± 39.7 |
16.0 |
<0.001 |
Applied Medical CFR03 |
With Probe |
67.0 ± 100.6 |
3.0 |
<0.001 |
Applied Medical CTR03 |
With Probe |
23.3 ± 60.1 |
0.0 |
0.044 |
|
|
|
|
|
Ethicon XCEL B5LT |
Bending Load |
5.00 ± 9.88 |
3.00 |
- |
Medtronic ONB5STF |
Bending Load |
162 ± 218 |
60.0 |
<0.001 |
Applied Medical CFR03 |
Bending Load |
79.5 ± 96.1 |
11.0 |
<0.001 |
Applied Medical CTR03 |
Bending Load |
26.2 ± 44.0 |
6.50 |
<0.001 |
12-mm Trocars |
State |
Mean ± St Dev
(ml/min) |
Median Leak (ml/min) |
p-value |
Ethicon XCEL B12LT |
Obturated |
166 ± 60.3 |
160 |
- |
Applied Medical CFR73 |
Obturated |
1881 ± 132 |
1918 |
<0.001 |
Applied Medical CTR73 |
Obturated |
1973 ± 49.4 |
1999 |
<0.001 |
|
|
|
|
|
Ethicon XCEL B12LT |
No Probe |
0.33 ± 0.88 |
0.0 |
- |
Medtronic ONB12STF |
No Probe |
0.23 ± 0.63 |
0.0 |
0.912 |
Applied Medical CFR73 |
No Probe |
0.13 ± 0.35 |
0.0 |
0.616 |
Applied Medical CTR73 |
No Probe |
0.21 ± 0.42 |
0.0 |
0.768 |
|
|
|
|
|
Ethicon XCEL B12LT |
With Probe* |
100.3 ± 47.7 |
96.5 |
- |
Medtronic ONB12STF |
With Probe |
0.60 ± 1.19 |
0.0 |
<0.001 |
Applied Medical CFR73 |
With Probe |
16.9 ± 44.8 |
0.0 |
<0.001 |
Applied Medical CTR73 |
With Probe |
16.2 ± 48.7 |
1.0 |
<0.001 |
|
|
|
|
|
Ethicon XCEL B12LT |
Bending Load |
173 ± 43.3 |
177 |
- |
Medtronic ONB12STF |
Bending Load |
1086 ± 256 |
1105 |
<0.001 |
Applied Medical CTR73 |
Bending Load |
82 ± 111 |
14.9 |
0.010 |
* 12-mm trocars were tested with a 4.7mm probe, off-center.
For the 12-mm trocars (Figure 4), with obturator fully inserted, the CFR73 and CTR73 had significantly higher leak rates than the XCEL trocar, while there was no significant difference in leak rate between trocars without probe insertion. With the probe inserted, the ONB5STF, CFR03 and CTR03 all had significantly lower leak rates than the XCEL trocar. With bending of the probe, the CTR73 had a lower leak rate, while the ONB12STF had a significantly higher rate than the XCEL trocar. The CFR73 could not be tested with bending because the device did not fit into the testing fixture.
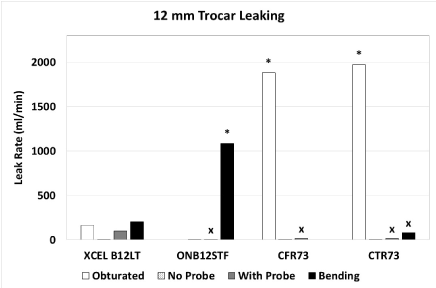
Figure 4. Leak rate for 12-mm trocars with obturator fully inserted, no probe, with probe, and with probe bent at an angle. An asterisk indicates a significantly higher leak rate compared to the XCEL trocar, and an ‘x’ indicates a significantly lower leak rate compared to the XCEL trocar
This study evaluated insertion and retention forces and leakage of commonly-used commercial trocars with consideration of the current COVID-19 pandemic. For the 5-mm trocars, the XCEL trocar was found to have similar insertion and retention forces compared to competitive brands, whereas for the 12-mm size, the XCEL trocar had significantly lower insertion and higher retention forces in comparison with the other trocars. Given the current environment, the differences observed for the 12-mm trocars could provide extra assurance of lower gas leak during insertion, and for reduced risk of accidental discharge of pneumoperitoneum gas via unintended ejection of the trocar during surgery.
All trocars exhibited low rates of leakage when there was no probe or obturator inserted, and when a probe was inserted without any bending stress. When the obturator was inserted in the Applied Medical products high levels of leakage occurred (>1500 ml/min for the 12 mm size), significantly higher than with XCEL trocars. Increased leakage rates were also observed with the 12-mm Medtronic product, where a rate of over 1000 ml/min was observed when the trocar was under a bending stress. This may be a concern as manipulation of the inserted device is common practice during a procedure. In contrast, both the 5-mm and 12-mm XCEL trocars exhibited low rates of leakage under all four conditions tested.
As major vessel or tissue injury may occur during trocar insertion if too much force must be applied [17], the design of the ENDOPATH™ XCEL™ Bladeless Trocars allows it to have the lowest peak insertion force compared to the other trocars examined in this study. Reducing the risk of accidental trocar dislodgement decreases the chance of sudden loss of pneumoperitoneum and release of laparoscopic gas into the OR environment [18]. In this study the XCEL trocars demonstrated higher peak retention forces than competitive devices.
Leaking of gas through the trocar was noticeably less for 5 mm trocars than for 12 mm trocars, and in general less for no probe than with probe inserted. For some trocars, slight bending of the trocar at an angle substantially increased the rate of leak. Since change in the angle of trocar is necessary for routine maneuvering, leaking during bending can be a disadvantage. Similarly, for some trocars, leaking increased when the obturator was inserted. To avoid leakage with these trocars, surgeons may feel rushed in needing to remove the obturator quickly. The XCEL trocars overall demonstrated the best combination of low insertion force, high retention force and low leak rates with or without a obturator or probe inserted, even with bending.
Few studies have compared the leakage rate of trocars during use. One study compared the effect of reprocessed XCEL trocars to unused devices [19]. Reprocessed trocars were found to have more imperfections, required higher insertion forces and lower retention forces, and had significantly higher leakage rates. In a comparison of valveless to standard trocars [20], the valveless trocar was found to have fewer episodes of pressure loss greater than 8 mmHg, but there was no overall difference in leakage as measured by CO2 consumption. Another comparison of valved and valveless found that the valveless trocars were associated with a reduction of intraoperative CO2 consumed and eliminated [21]. A recent study evaluated three brands of trocar in light of the COVID-19 pandemic, showing the various mechanisms by which leakage may occur, but did not provide performance comparisons between the trocars [22].
There has long been a concern that escaping laparoscopic gas may contain surgical smoke and its contents, such as potentially hazardous chemicals, bacteria and viruses. Today, among surgeons worldwide, there is the additional concern with the use of minimally invasive surgery that operating room staff may be exposed to COVID-19 due to the creation of pneumoperitoneum. It has been hypothesized that bursts of pneumoperitoneum gas from the trocar during exchange of instruments, specimen retrieval, or venting, for example to clear smoke, can permit transmission of virus to OR personnel [23]. In addition, at the conclusion of the procedure, a large quantity of the gas may flow into the OR if the pneumoperitoneum is deflated through a trocar port. These practices are not thought to have jeopardized staff previously, but with the advent of COVID-19, recommendations are to desufflate the abdomen using a smoke evacuation device and to avoid opening a trocar valve to clear smoke.
Laparoscopy has many advantages over open surgery, such as less trauma to the patient and decreased length of stay [24]. Body fluids and tissues are contained within the operative field, so there is less risk to the staff. For these reasons, laparoscopy was strongly recommended over open surgery during the acquired immunodeficiency syndrome (AIDS) epidemic in patients infected with human immunodeficiency virus (HIV) [25].
Current concerns center on the possibility of transmission of the SARS-CoV-2, the virus responsible for COVID-19. The naked virus itself is small, less than 100 nm, but is thought to be transmitted in much larger droplets, 10-20 µm in diameter [26]. Transmission has been proposed to be by either direct human-to-human contact (especially coughing and sneezing) or via contaminated surfaces [23]. Aerosols generated by infected individuals may travel a meter or more and cause infection, thus surgical procedures that generate aerosols are also suspected of being a possible means of viral transmission. Enhanced precautions are being recommended for procedures that may generate aerosols such as laryngoscopy, bronchoscopy and upper endoscopy [27].
Whether SARS-CoV-2 or any other virus can be transmitted via laparoscopy has not yet been determined. A study in patients with hepatitis B virus (HBV) undergoing laparoscopy found that the surgical smoke contained fragments of HBV, but no testing was performed to confirm whether the virus was still viable [28]. In open procedures, human papillomavirus (HPV) has been detected in laser surgical smoke, however, whether virus transmission is likely to occur during laparoscopic surgery is currently a matter debate [29-31].
Recommendations have been made to use balloon trocars without any supporting evidence that these promote less leakage [32-34]. A balloon on a trocar is an approach to abdominal wall retention; this type of trocar was designed to increase operating efficiency by reducing the number of times a surgeon may have to reposition the trocar. There is no evidence that a balloon port reduces airflow leakage from the incision site, although a relatively common thought process in the medical community is that the balloon may fill space and prevent leaking from an incision that is too large. Moreover, there may be additional considerations with various fixation methods. Adding a balloon to a trocar necessarily adds puncture depth within the abdomen and may reduce the trocar’s angles of movement within the working area. Since the clinical goal of a trocar is to maximize surgical access to relevant anatomy, restricting a trocar’s movement angle could potentially hinder the surgeon.
Using ordinary photography, it is possible to show that nebulized gas escapes both through the trocar, and around the device when inserted or removed [35]. Near infrared and Schlieren photography can provide better visualization, confirming the concerns with certain insufflation and access systems [36,37]. In particular, some advanced bipolar devices have been observed to allow leakage through the shaft and out the handle, similar to robotic devices, however Harmonic ultrasonic shears limit the amount of gas from passing through and escaping from the instrument [38].
The coronavirus disease 2019 pandemic has place unprecedented challenges on the medical community. Several surgical societies have made recommendations of best practices in performing minimally invasive surgery during the COVID-19 pandemic.14-16 Key recommendations relative to pneumoperitoneum maintenance and trocar usage are presented below. The provided optimal use of trocar recommendations, which may evolve over time, can be used as guidance for surgical staff. By following these recommendations, and careful choice of surgical instrumentation, surgeons may be able to lower the risk of exposure to pneumoperitoneum gas and its contents.
Recommendations for Optimal Use of Trocars
- The trocar chosen to be used should have a low insertion force, and more importantly a high retention force. If the trocar unintentionally slips out of the patient, there would be a large amount of gas escape into the environment. Single-use valved trocars may provide more reproducible insertion and retention forces [16].
- The pressure used in the pneumoperitoneum should be as low as possible for the desired effect to minimize CO2 leakage from the trocar (e.g., 12 mmHg or less). Maintenance of pressure should be performed via a closed-circuit system [15].
- The entire peritoneal cavity should be aspirated before making an auxiliary incision [16].
- If the instrument and trocar are not matched in size, use trocar reducers when inserting 5 or 8-mm instruments through a 12-mm trocar [16].
- Skin incision length should be dependent upon the trocar diameter. If the incision length is suitable for the trocar, then there should be minimal leakage around the outside of the trocar. A common best practice is to press the trocar sleeve to the skin at the desired insertion point, prior to incision, to create a temporary marking on the skin; the surgeon can then use that marking as the boundaries for incision. Note that if a trocar is not well-designed, or functioning properly, there may be leakage through the trocar (Figure 5) [16].
- With the trocar valve closed, insert instruments as perpendicularly as possible. Instruments should also be inserted and removed as quickly as possible [14,15].
- Even with a well-designed trocar, there is some emission of gas during an instrument exchange (Figure 6). For this reason, exchanges should be minimized by using multifunctional devices, such as advanced energy devices that cut, coagulate and dissect. Propitiously, Harmonic devices have been demonstrated to allow significantly less gas leakage through the shaft and out of the handle in comparison with other devices [38]. When an instrument is fully inserted into a well-designed trocar, there should be minimal leakage around the shaft of the instrument (Figure 7) [16].
- Removal of the camera for cleaning should be minimized, for example, by using a trocar with a lens cleaning system [16].
- Use smoke evacuation through a closed system with an ULPA filter [14].
- At the end of the procedure, the trocars should remain in the patient until the pneumoperitoneum is fully desufflated through a smoke evacuator with an ULPA filter. Place the tip of the suction trocar away from the bowel, either above the liver or toward the abdominal wall. Do not remove a specimen until desufflation is complete [14].
|
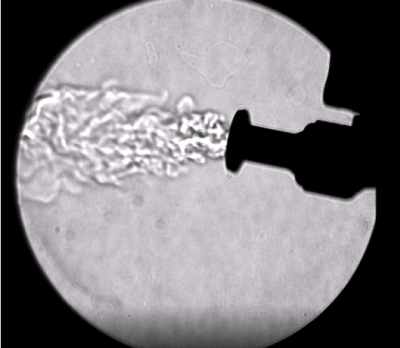
Figure 5. Leakage through an inadequately-sealed trocar prior to instrument insertion
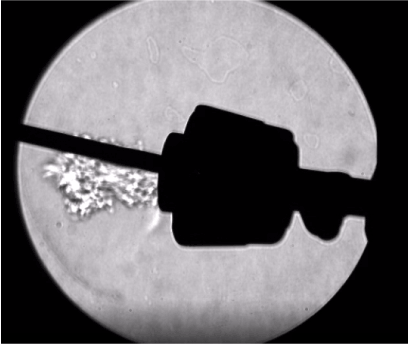
Figure 6. Emission of gas while instrument is being inserted
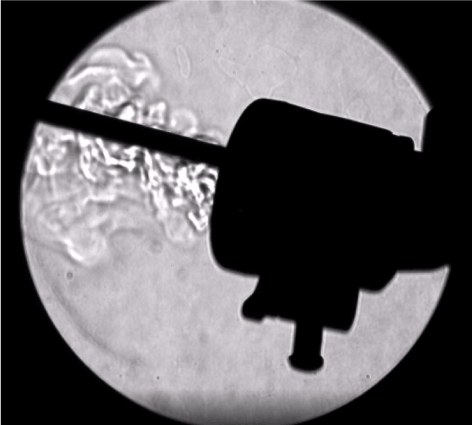
Figure 7. Leakage around the shaft after an instrument is fully inserted into the trocar
- World Health Organization (2014) Infection prevention and control of epidemic-and pandemic-prone acute respiratory infections in health care. World Health Organization.
- Li Q, Guan X, Wu P, (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med 382: 1199-1207. [Crossref]
- Liu J, Liao X, Qian S (2020) Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China 2020. Emerg Infect Dis 26:1320-1323. [Crossref]
- Chan JF-W, Yuan S, Kok K-H (2020) A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet 395: 514-523.
- Huang C, Wang Y, Li X (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet 395:497-506.
- Burke RM (2020) Active monitoring of persons exposed to patients with confirmed COVID-19—United States, January–February 2020. MMWR Morb Mortal Wkly Rep 69:245-246. [Crossref]
- Wang J, Zhou M, Liu F (2020) Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID-19) in China. J Hosp Infect 105: 100-101. [Crossref]
- Santarpia JL, Rivera DN, Herrera V, et al. (2020) Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxIV.
- Liu Y, Ning Z, Chen Y (2020) Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 582: 557-560.
- Ngaserin SHN, Koh FH, Ong BC, Chew MH (2020) COVID-19 not detected in peritoneal fluid: A case of laparoscopic appendicectomy for acute appendicitis in a COVID-19-infected patient. Langenbecks Arch Surg 405: 353-355. [Crossref]
- Coccolini F, Tartaglia D, Puglisi A (2020) SARS-CoV-2 is present in peritoneal fluid in COVID-19 patients. Ann Surg 272: e240-e242. [Crossref]
- Tommaselli GA, Ricketts CD, Clymer JW, Fryrear II RS (2020) Use of ultrasonic devices in laparoscopic surgery and risk of COVID-19 contamination: What does the evidence say? Glob Surg 6: 1-8.
- Soliman M (2020) Controversies in CO 2 Insufflation and COVID-19. Tech Coloproctol 24: 667-670. [Crossref]
- Francis N, Dort J, Cho E (2020) SAGES and EAES recommendations for minimally invasive surgery during COVID-19 pandemic. Surg Endosc 34: 2327-2331. [Crossref]
- European Society of Gynecological Endoscopists (2020) ESGE Recommendations on Gynaecological Laparoscopic Surgery during Covid-19 Outbreak. 2020; https://esge.org/wp-content/uploads/2020/03/Covid19StatementESGE.pdf. Accessed May 13, 2020.
- American Association of Gynecologic Laparoscopists (2020) Joint Statement on Minimally Invasive Gynecologic Surgery During the COVID-19 Pandemic. 2020; https://www.aagl.org/news/covid-19-joint-statement-on-minimally-invasive-gynecologic-surgery/. Accessed May 7, 2020.
- Pickett SD, Rodewald KJ, Billow MR, Giannios NM, Hurd WW (2010) Avoiding major vessel injury during laparoscopic instrument insertion. Obstet Gynecol Clin North Am 37: 387-397. [Crossref]
- Vrentas V, Herrmann A, Cezar C, Tchartchian G, Diesfeld P, et al. (2013) Reducing trocar movement in operative laparoscopy through use of a fixator. J Minim Invasive Gynecol 20: 842-847. [Crossref]
- Mues AC, Haramis G, Casazza C, Okhunov Z, Badani KK, et al. (2010) Prospective randomized single-blinded in vitro and ex vivo evaluation of new and reprocessed laparoscopic trocars. J Am Coll Surg 211: 738-743. [Crossref]
- Horstmann M, Horton K, Kurz M, Padevit C, John H (2013) Prospective comparison between the AirSeal® System valve-less Trocar and a standard Versaport™ Plus V2 Trocar in robotic-assisted radical prostatectomy. J Endourol 27: 579-582. [Crossref]
- Herati AS, Andonian S, Rais-Bahrami S (2011) Use of the valveless trocar system reduces carbon dioxide absorption during laparoscopy when compared with standard trocars. Urology 77: 1126-1132. [Crossref]
- Cahill R, Dalli J, Khan M, Flood M, Nolan K (2020) Solving the problems of gas leakage at laparoscopy. BJS 10: 1-10.
- Vigneswaran Y, Prachand VN, Posner MC, Matthews JB, Hussain M (2020) What is the appropriate use of laparoscopy over open procedures in the current COVID-19 climate? J Gastrointest Surg 24: 1686-1691. [Crossref]
- Towfigh S, Chen F, Mason R, Katkhouda N, Chan L, et al. (2006) Laparoscopic appendectomy significantly reduces length of stay for perforated appendicitis. Surg Endosc 20: 495-499.
- Diettrich NA, Kaplan G (1991) Laparoscopic surgery for HIV-infected patients: minimizing dangers for all concerned. J Laparoendosc Surg 1: 295-298. [Crossref]
- Kim JM, Chung YS, Jo HJ (2020) Identification of Coronavirus Isolated from a Patient in Korea with COVID-19. Osong Public Health Res Perspect 11: 3-7. [Crossref]
- Pritchett MA, Oberg CL, Belanger A (2020) Society for Advanced Bronchoscopy Consensus Statement and Guidelines for bronchoscopy and airway management amid the COVID-19 pandemic. J Thorac Dis 12: 1781-1798. [Crossref]
- Kwak HD, Kim SH, Seo YS, Song KJ (2016) Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med 73: 857-863. [Crossref]
- Weyandt GH, Tollmann F, Kristen P, Weissbrich B (2011) Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO 2 laser ablation. Arc Dermatol Res 303: 141-144. [Crossref]
- Ferenczy A, Bergeron C, Richart RM (1990) Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol 75: 114-118. [Crossref]
- Manson LT, Damrose EJ (2013) Does exposure to laser plume place the surgeon at high risk for acquiring clinical human papillomavirus infection? Laryngoscope 123: 1319-1320. [Crossref]
- Tuech JJ, Gangloff A, Di Fiore F (2020) Strategy for the practice of digestive and oncological surgery during the Covid-19 epidemic. J Visc Surg 157: S7-S12. [Crossref]
- Schwarz L, Tuech JJ (2020) Is the use of laparoscopy in a COVID‐19 epidemic free of risk? Br J Surg 107: e188. [Crossref]
- Mowbray N, Ansell J, Horwood J (2020) Safe management of surgical smoke in the age of COVID‐19. Br J Surg 3: 10. [Crossref]
- Khan MF, Dalli J, Cahill RA (2020) Gas Aerosol Jetstreams from Trocars during Laparoscopic Surgery‐A Video Vignette. Colorectal Dis 15215. [Crossref]
- Dalli J, Khan MF, Nolan K, Cahill RA (2020) Laparoscopic Pneumoperitoneum Escape and Contamination during Surgery using the Airseal Insufflation System: Video Vignette. Colorectal Dis 15255. [Crossref]
- Khan M, Cahill R (2020) Carbon dioxide gas leaks during transanal minimally invasive surgery. Tech Coloproctol 7: 1-2. [Crossref]
- Dalli J, Faraz Khan M, Nolan K, Cahill RA (2020) Gas Leaks Through Laparoscopic Energy Devices and Robotic Instrumentation‐Video Vignette. Colorectal Dis 15278. [Crossref]
Editorial Information
Editor-in-Chief
Article Type
Research Article
Publication history
Received date: September 30, 2020
Accepted date: October 08, 2020
Published date: October 15, 2020
Copyright
©2020 Cepress JM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Cepress JM (2020) Comparison of trocar performance in consideration of the COVID-19 pandemic. Med Devices Diagn Eng 5 DOI: 10.15761/MDDE.1000129







