Abstract
Study Objectives: Obstructive Sleep Apnea (OSA) is a sleep disorder primarily of the upper airway with a significant impact not only on the quality of life but is also associated with various systemic diseases. Several ophthalmological diseases are also associated with OSA, especially glaucoma. The purpose of this review is to take a closer look at the causality and mutual influence.
Methods: A systematic literature search was conducted using PubMed. A total of 19 studies with 316178 participants were included.
Results: Eleven of the sixteen studies concentrating on the prevalence of glaucoma in patients with OSA showed an association of both entities. One paper found a higher risk for progression of glaucoma in OSA patients. Five of the sixteen included studies failed to show a correlation between OSA and glaucoma. One study out of three surveying specific ophthalmological parameters did show an influence of OSA therapy on RNFL thinning and vision. One study showed a rise of IOP when two other studies showed no rising under CPAP.
Conclusions: Our findings suggest an association between OSA and glaucoma and especially between OSA and thinning of retinal nerve fibre layer (RNFL). CPAP therapy (continuous positive airway pressure) appears to be also suitable for patients with comorbid glaucoma.
Keywords: Obstructive sleep apnea, glaucoma, sleep-disordered breathing, RNFL, AHI
Brief summary: Still, the relationship between OSA and glaucoma is not yet fully understood. The most recent published review discussing this topic is from 2015 and showed that patients with severe OSA had a significantly higher risk to develop glaucoma than patients with mild or moderate OSA. Nowadays, patients with OSA are not tested systematically for glaucomatous changes and vice versa. With this review, we want to focus again on the question, whether an optical coherence tomography imaging should be performed in patients with OSA and also monitored under CPAP therapy.
Introduction
Obstructive sleep apnea (OSA) is a chronic condition characterized by phases of partial or even complete upper airway obstruction during the sleep usually accompanied by snoring. These episodes lead to oxygen desaturation, hypercapnia and recurrent arousals with concomitant activation of the sympathetic nervous system resulting in sleep fragmentation [1-3]. The severity of respiratory distress in OSA is described by the AHI (apnoe hypopnoea index) which is the total number of apneas and hypopneas per hour: AHI < 5/h is no OSA, AHI of 5/h to 15/h is a mild OSA, AHI of 15/h to 30/h is a moderate OSA and AHI > 30/h is a severe OSA [4]. Polysomnography is currently the gold standard for sleep monitoring. Apart from snoring, excessive daytime sleepiness, attention deficits, morning headaches and impaired daytime performance are frequent symptoms of OSA. Besides mentioned effects on sleep quality, arousals also lead to critical increases in blood pressure, oxidative stress and promotion of systemic inflammation [2].
Previous studies showed an association of OSA with an increased risk of cardiovascular and cerebrovascular diseases, stroke, coronary artery disease, diabetes, hypercholesterolemia and hypertension [5-8]. Furthermore, OSA has been shown to be a major risk factor concerning optic nerve disorders such as glaucoma, non-arteritic anterior ischemic optic neuropathy, central serous chorioretinopathy, papilledema and retinal vein occlusion [9-11]. The term glaucoma describes a collection of progressive, chronic optic neuropathies due to a degeneration of retinal ganglion cells and leading to visual impairment. On funduscopic examination, a cupping of the optic disc is found. Glaucoma can be divided into different clinical subgroups: primary open angle glaucoma (POAG), angle-closure glaucoma (ACG) and normal tension glaucoma (NTG) [12,13]. The pathophysiology of glaucomatous changes is not completely known. The most frequent subgroup is POAG which is associated to a high intraocular pressure (IOP). Hence, IOP is the main clinical endpoint accessible to therapeutic intervention [13].
The relationship between OSA and glaucoma is not yet fully understood. Several studies indicate that a change in the intraocular pressure during apnoe phases lead to glaucomatous changes in OSA patients. In these studies, contact lens sensors were used for continuous IOP-monitoring. Shinmei, et al. found a statistically significant decline of the IOP during apneas compared to non-apnoic phases in four out of seven patients with suspected OSA. In association to apnoic phases, the CLS-based IOP values dropped by 23.1 +/- 16.4 mV eq on average [14]. Carnero, et al. examined 20 participants with suspected OSA. The patient cohort was split into severe and mild/moderate OSA. Contrasting to Shinmei, et al. patients with severe OSA showed a longer IOP elevation compared to patients with a mild/moderate disease (P = 0.032/P = 0.028) [15]. Fang, et al. investigated the differences of the IOP values during and after varying body positions in OSA and non-OSA patients. The IOP was measured ten minutes after sitting, directly and 30 minutes after changing to supine position and again after resuming sitting position. OSA patients showed an increase of the IOP when changing from sitting to supine position (right eye p = 0.033, left eye p = 0.044) and a further increase after 30 minutes in supine position (right eye p = 0.001, left eye p = 0.246). A higher IOP in supine position was associated with a higher IOP in sitting position post-supine and the authors conclude that the IOP rises more in OSA patients in prolonged supine position [16].
This review concentrates on the causality and mutual influence of glaucoma and OSA and also the mutual influence of the respective therapies. The most recent published systematic review by Wu, et al. [17] discussing the connection between OSA and glaucoma included papers until 2014.
Materials and methods
This review was conducted in accordance to the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).
Literature search
PubMed was searched using the Medline terms: [(obstructive sleep apnea) OR (OSA) AND (glaucoma) AND (“2015/01/01”[PDat]: “2020/31/12” [PDat])]. A title/abstract screening was followed by a full text screening. Only articles written in English or German were screened. Eligible studies were original articles such as case-control studies, cohort studies, cross sectional studies and randomized controlled trials. Animal studies were not included. Only studies were included defining OSA by AHI via polysomnography or home sleep-apnea testing (HSAT/polygraphy). Studies were included using at least one of the following ophthalmic examination methods regarding glaucoma: optical coherence tomography imaging (OCT), measurement of intraocular pressure (IOP), sonography and perimetry for measurement of the visual field (VF). Literature published from 2015 until the end of 2020 was reviewed to exclude already published data of the last systematic review by Wu, et al. [17] and therefore avoid publication bias.
Results
Prevalence / Progression of glaucoma in OSA
A complete list of the epidemiological studies that have evaluated the prevalence and progression of glaucoma in OSA patients is provided on Table 1.
Table 1. Prevalence/Progression of glaucoma in OSA
Reference |
Population |
Eligibility |
Intervention |
Control group |
Study design |
Follow-up |
findings |
Correlation found |
Abdullayev et al. [18] |
N = 59 OSA, N = 28 with CPAP |
PSG, OCT, IOP, VF |
- |
N = 19 without OSA |
Prospective study |
- |
Average GCC thickness was significantly lower in mild OSA than in controls (left eye, p = 0.013). The GCC was significantly thinner in the inferior and inferonasal sectors of both eyes in OSA compared to controls (p = 0.029, p = 0.022, p = 0.037, and p = 0.019). Minimum GCC thickness in the left eyes of all OSA groups was significantly lower than in the control group (p < 0.05). |
yes |
Bagabas et al. [19] |
N = 83 N = 44 OSA |
PSG, VF, OCT, IOP |
- |
N = 39 no OSA |
Cross- sectional case series study |
- |
Glaucoma prevalence was higher among individuals with OSA (16%) than among non-OSA individuals (8%, p = 0.267). A consistent trend towards more glaucomatous changes was observed in OSA subjects. |
yes |
Bahr et al. [20] |
N = 101 Glaucoma/OH |
PG, IOP, VF |
- |
N = 14 no glaucoma/ OH |
Prospective study |
- |
There was a strong correlation between POAG and OH clinical glaucoma phenotypes and the AHI. LTG patients had a significantly lower rate of OSA compared to other glaucoma types and controls. |
yes |
Chuang et al. [21] |
N = 53 OSAS |
PSG, OCT(A), VF |
- |
- |
Retrospective cross-sectional study |
- |
There was significantly higher AHI in the NTG group (n = 27) than in the control group (n = 26; p < 0.001). Superficial and deep-layer peripapillary and macular area VD significantly decreased in the NTG group. |
yes |
Fan et al. [22] |
N = 32 POAG/ NTG/ suspect |
PSG, IOP, VF, OCT |
N = 7 CPAP, N = 2 upper airway surgery |
|
N = 5 no OSA, N = 7 glaucoma suspect |
Comparative cohort study |
> 3 years |
A more severe OSA was associated with a higher percentage of progression of glaucoma (P = 0.017). |
yes |
Findik et al. [23] |
N = 60 N = 44 OSA |
PSG, OCT, sonography, VF |
- |
N = 16 no OSA |
Prospective randomized study |
- |
Superior and inferior RNFL thickness values were significantly lower than those in the control group (P < 0.046). Glaucoma prevalence of OSA patients in this study was 6.8% (only in the severe OSA group). |
yes |
Friedlander et al. [24] |
N = 225 OSA |
PSG/ alio loco, VF, IOP |
- |
N = 312,494 no OSA |
Retrospective case review |
- |
The POAG prevalence rate among the OSA group (20.9 %) was significantly higher than among the medical center’s general population (2.5%, P < 0.00001). Severity of OSA (AHI) failed to demonstrate a significant correlation to any POAG subtype (P > 0.05). |
yes |
Gross et al. [25] |
N = 100, (N = 22 CPAP) |
PSG, IOP, VF |
- |
- |
Prospective study |
- |
There was no higher prevalence of glaucoma in OSA. No statistically significant correlation (ANOVA) was found between RDI, IOP, MD and cup-disc ratio. |
no |
Lee et al. [26] |
N = 848, N = 178 OSA |
OCT and after 2 years PSG |
- |
N = 670 no OSA |
Cross-sectional cohort study |
planned |
Participants with OSA showed thinner peripapillary RNFL inferotemporally (P = 0.026) and superotemporally (P = 0.008) compared with those without. A higher AHI was associated with thinner RNFL superotemporally (P = 0.007). There were no significant differences in optic disc measures between groups of OSA severity. |
yes |
Lee et al. [27] |
N = 865, N= 411 OSA |
PG, OCT |
- |
N = 454 no OSA |
Participants with severe OSA had thinner RNFL superotemporally than those without or with mild OSA (P < 0.001 and 0.001). Superotemporal RNFL was inversely associated with AHI (P = 0.004) and T90% (P = 0.005). |
6 years |
Participants with severe OSA had thinner RNFL superotemporally than those without or with mild OSA (P < 0.001 and 0.001). |
yes |
Morsy et al. [28] |
N = 100, N = 80 OSA |
PSG, IOP, VF, OCT |
- |
N = 20 no OSA |
Cross-sectional case control study |
- |
Glaucoma was diagnosed in 24 out of 80 (30.0 %) patients. There is a higher risk to develop glaucoma among OSA and the lowest oxygen saturation was significantly associated with vision threatening disorders (NTG, senile cataract and retinal ischemia, p = 0.001). |
yes |
Moyal et al. [29] |
N = 53 OSA |
PSG, OCT(A), VF, IOP |
- |
N = 28 no OSA |
Retrospective observational study |
- |
OCTA did not detect reduced ONH, RPC or macular blood vessel density in eyes with OSAS. RNFL thickness, Cup/Disc ratio, rim area, and GCC were not significantly modified. |
no |
Pedrotti et al. [30] |
N = 296 OSA |
PSG, IOP, OCT, VF |
- |
- |
Cross-sectional cohort study |
- |
Severe OSA was significantly associated with glaucoma (OR, 95% CI 1.05 to 5.93, p = 0.037). 11.1 % (N = 33) of OSA patients had glaucoma. |
yes |
Swaminathan et al. [31] |
N = 25 OSA+ glaucoma, N = 13 CPAP |
PSG, VF, IOP |
CPAP |
- |
Retrospective cross sectional study |
> 2 years, (PSG within 12 months of final VF) |
Progressors and nonprogressors had nonsignificantly different IOP (13.1±2.8 vs. 14.9±2.5 mm Hg), mean ocular perfusion pressure (49.7±5.5 vs. 48.8±9.0 mm Hg) and AHI (31.3±18.6 vs. 26.4±24.0). AHI was not correlated with slopes of VF mean deviation (P = 0.190) or pattern standard deviation (P = 0.312), and no substantial increase in risk of progression was found with increase in AHI (independently of CPAP) |
no |
Teberik et al. [32] |
N = 103 OSA |
PSG, IOP, sonography (CCT), OCT |
- |
N = 37 without OSA |
Prospective case-control study |
- |
The mean values of RNFL thickness in all quadrants were not different in OSA and control group (p = 0.274). The IOP and CCT measurement averages in OSA were lower than the control group (no statistical significance). |
no |
Wozniak et al. [33] |
N = 235 POAG |
PG, OCT, VF, CCT |
- |
N = 160 no POAG |
Case control study |
- |
There was no significant difference in OSA prevalence between the matched groups (P = 0.91 for AHI≥5 and P = 0.66 for AHI≥15). The AHI was not associated with the severity of visual field defect or RNFL thinning after adjustment for confounders. |
no |
AHI = Apnoe Hypopnoe Index; CCT = central corneal thickness; CPAP = continuous positive airway pressure; GCC = ganglion cell complex; IOP= intraocular pressure; LTG = low tension glaucoma; MD = mean deviation; NTG = normal tension glaucoma; OCT (A) = optical coherence tomography angiography; OH = ocular hypertension; ONH = optic nerve head; OSA = obstructive sleep apnea; PG = polygraphy; PSG = polysomnography; POAG = primary open angle glaucoma; RDI = respiratory disturbance index; RNFL = retinal nerve fibre layer; RPC = radial peripapillary capillary; T90% = sleep time with oxygen saturation level <90% ; VD = vessel density; VF = visual field. |
Abdullayev, et al. [18] conducted a prospective study including 59 patients with OSA (N = 28 with CPAP) and 19 controls to evaluate the prevalence of glaucoma in OSA patients using and also not-using CPAP compared to controls. Based on the AHI, all patients with OSA were divided into three groups: 19 patients with mild OSA (32.2%), 16 patients with moderate OSA (27.1%) and 24 patients with severe OSA (40.67%). Average Retinal nerve fiber layer (RNFL) thickness values in right and left eyes were 91.0 ± 10.3 µm and 89.7 ± 10.3 µm (mild OSA = group one), 93.2 ± 7.01 µm and 89.6 ± 8.5 µm (moderate OSA = group two), 95.5 ± 10.4 and 93.1 ± 8.7 (severe OSA = group three) and 95.2 ± 9.8 and 95 ± 8.6 (control group). No statistical significance was found (p > 0.05). Only in group two a statistically significant correlation between AHI and average RNFL thickness of the left eye was found (p = 0.010). Also the ganglion cell complex (GCC) thickness was examined. In comparison to the control group, group one showed significant lower values in the inferior and inferonasal sectors of both eyes (p = 0.029, p = 0.022, p = 0.037 and p = 0.019). The average GCC and minimum GCC of the left eyes were significant lower in all OSA groups compared to the control group (p = 0.013, p = 0.010, p = 0.019, p = 0.004). The authors conclude that a periodic evaluation of RNFL and GCC thickness could have diagnostic value in early identification of glaucoma in OSA patients.
In a cross-sectional case series study including 83 participants, Bagabas, et al. [19] compared the prevalence of glaucoma in OSA patients with that of the standard population. The measured IOP of both eyes was higher in individuals with OSA than in the control group (left eye 14.8 ± 3.3 vs. 13.1 ± 3.3, p = 0.023, right eye 14.5 ± 3.4 vs. 13 ± 3.7, p = 0.052). The mean cup/disk ratio was similar in both groups (left eye 0.24 ± 0.13 vs. 0.25 ± 0.22, p = 0.760, right eye 0.25 ± 0.15 vs. 0.24 ± 0.20, p = 0.892). Only one patient had an IOP > 21 mmHg (OSA group). Sixteen percent of patients with OSA presented with comorbid glaucoma and 8 % of the participants without OSA presented with glaucoma (p = 0.267) which can only be seen as a trend.
Bahr, et al. [20] carried out a prospective study to assess the risk for glaucoma and ocular hypertension in patients with OSA. A total of 101 patients with suspected glaucoma were screened for OSA. The diagnosis of glaucoma was confirmed in 87 patients, the control group comprised 14 participants without glaucoma. OSA was significantly more prevalent in patients with a high IOP: the median AHI in POAG was 22.7 events per hour and 21.9 events per hour in the OH group. In regard to the AHI, highly significant differences were found between the four groups (Chi2 = 22, df = 3, p < 0.0001) with lower values in the LTG group compared to the POAG group (Hodges-Lehmann = − 13.8, 95% CI (− 18.6 – − 8.8; p < 0.0001) and the control group (Hodges-Lehmann = 12.1; 95% CI -19.9 – − 2.4; p < 0.02). Of the 53 POAG patients, 48 presented with OSA (11 = mild, 23 = moderate, 14 = severe). In conclusion, the study supports the hypothesis that OSA leads to a rising IOP and therefore the risk of POAG and OH rises.
In the retrospective study of Chuang, et al. [21], the prevalence of normal tension glaucoma in 53 OSA patients was evaluated. RNFL thickness decreased in moderate/severe OSA compared to mild OSA (88.72 ± 13.58 vs. 96.64 ± 10.64, p = 0.05) without statistical significance. The GCC thickness decreased significantly comparing both groups (88.13 ± 11.89 vs. 96.28 ± 6.34, p = 0.003). There was no difference of IOP in both groups (15.32 ± 2.50 vs. 15.67 ± 3.14, p = 0.68). The AHI was significantly higher in patients with NTG than in patients without NTG (45.39 ± 18.34 vs. 31.55 ± 25.15, p < 0.001). In conclusion, the study showed a high AHI to be a risk factor for NTG in OSA population. The authors also conclude that OCTA is a good monitor for ophthalmic microcirculation in OSA, because the vascular changes highly correlated with the visual field defects.
Fan, et al. [22] investigated the correlation between severity of OSA and glaucoma progression. Fifty-three patients with POAG, NTG or suspected glaucoma were included. A high severity of OSA was non-significantly correlated to a higher percentage of RNFL thinning progression (p = 0.096). When combining groups into no/mild OSA and moderate/severe OSA the difference of RNFL thinning progression between both groups were statistically significant (64.7% vs 26.7%, p = 0.042). A higher severity of OSA was non-significantly correlated with a higher percentage of progression in visual field (VF, p = 0.219). The patients in the moderate/severe OSA group showed a higher percentage of progression in VF compared to the no/mild OSA group, without statistical significance (17.6% vs 0.0%, p = 0.229). After adjustment for different cofactors (age, sex, diabetes mellitus, hypertension, hyperlipidemia, and BMI) the multivariate Cox regression analysis showed that severe OSA had an 8.448-fold higher risk of RNFL thickness progression than no or mild OSA did (95% CI 1.464–48.752, p = 0.017). According to the authors, an undiagnosed severe OSA should be taken into consideration in patients with glaucoma progression despite adequate ophthalmological treatment.
A prospective, randomized study by Findik, et al. [23] included 60 patients, examined via spectral-domain optical coherence tomography, investigated whether an association between OSA and glaucomatous optic neuropathy exists. Sixteen patients formed the control group and 44 were diagnosed with OSA (14 mild, 15 moderate and 15 severe OSA). Also, the ophthalmic, retinal and posterior ciliary artery pulsatile index (PI) and resistive index (RI) were measured by coloured Doppler sonography. In the severe OSA group RNFL thickness values decreased significantly in mean values, superior and inferior quadrants compared to the other groups (p = 0.026, p = 0.046, and p = 0.024). No significant differences were observed between all four groups in RNFL thickness values of the nasal and temporal quadrants (p > 0.05). There was a negative correlation between AHI and mean values, superior and inferior RNFL thickness values (r = - 0.313, p = 0.015; r = - 0.3, p = 0.02; r = - 0.278, p = 0.032), while there was a positive correlation with IOP values (r = 0.472, p < 0.01). The PI and RI in the posterior ciliary artery were statistically significant higher in the severe OSA group (p < 0.05). Because of its smaller diameter, the PCA is more susceptible to stenosis and this can lead to optic nerve ischemia and the following decrease in RNFL thickness.
Friedlander, et al. [24] conducted a retrospective case review including 225 OSA patients and comparing them to 312,494 patients without OSA in terms of POAG/NTG prevalence. Forty-seven of the 225 participants were diagnosed with POAG (20.9 %) and 7,975 patients without OSA presented with POAG (2.5 %, p < 0.00001). No statistically significant differences of the prevalence in the different OSA groups defined by AHI were found using logistic regression models. The authors conclude that patients with a recently diagnosed sleep breathing disorder who are to undergo surgery should be seen by an ophthalmologist perioperative to avoid potential vision loss.
A prospective study conducted by Gross, et al. [25] could not find a higher prevalence of glaucoma in OSA patients. Of the 100 included patients, 78 were diagnosed with OSA (14 mild, 23 moderate, 41 severe). The prevalence of glaucoma in their OSA cohort was 2%. There was no statistically significant correlation between OSA severity and IOD, VF and cup/disk ratio. Nevertheless, the authors plead for PSG in glaucoma patients presenting with OSA symptoms and also an ophthalmological examination in OSA patients.
In 2019, Lee, et al. [26] found that young OSA patients showed a thinner RNFL at the inferotemporal and superotemporal segments compared with those without OSA (p = 0.026 and p = 0.008). Patients with mild OSA showed significantly lower RNFL values at the inferotemporal and superotemporal segments (p = 0.033, p = 0.028) compared with those without OSA. The moderate or severe OSA group did not differ significantly from the mild/no OSA group. Also no significant difference was found between no/mild OSA and moderate/severe OSA. An increase of the AHI by 5 events per hour was significantly associated with a thinner RNFL by 1.5 µm superotemporally (p = 0.007). In another study from 2020 Lee, et al. [27] investigated a middle-aged/older patient collective. There was no significant difference in RNFL thickness in patients with and without OSA (p > 0.13), but patients with severe OSA had significantly thinner RNFL superotemporally compared with patients without OSA (p < 0.001) and with mild OSA (p = 0.001). There was no significant difference in superotemporal RNFL thickness in no, mild and moderate OSA or between the moderate and severe OSA group (p = 0.021). The superotemporal RNFL was thinner by about 0.9 μm with every increase in AHI of 5 events per hour (p = 0.004), and by 34 μm with every increase of one percent in T90% (p = 0.005).
Morsy, et al. [28] found a higher risk to develop glaucoma in OSA patients. Twenty-four of the 80 OSA patients presented with glaucoma (30%) with a 7-times greater risk to develop vision-threatening disorders such as glaucoma, senile cataract and retinal ischemia. AHI, basal oxygen level, lowest oxygen saturation index, desaturation index, total severity index and arousal index were significantly associated with the occurrence of glaucoma (p = 0.001). OSA patients with NTG showed a significantly thinner RNFL and a higher cup/disk ratio (both p = 0.001).
In a study conducted by Moyal, et al. [29] OCT scans did not detect significant modifications in OSA patients. There were no statistically significant differences between all groups (no, mild, moderate, severe OSA) concerning IOP and CCT (p = 0.55, p = 0.83). Also there were no statistically significant differences of RNFL thinning and cup/disk ratio (p > 0.156, p = 0.333). Patients with severe OSA had an altered mean defect in VF (p = 0.014).
Pedrotti, et al. [30] found a higher prevalence of glaucoma in OSA patients (11.1%) in a cohort of 296 patients. Severe OSA was significantly associated to occurrence of glaucoma (p = 0.037). Therefore, patients with previous diagnosed or suspected OSA should be thoroughly assessed by an ophthalmologist.
In 2018, Swaminathan, et al. [31] did not find a higher risk for glaucoma progression in OSA patients. Of the 25 enrolled patients with OSA, 11 were classified as progressors and 14 as non-progressors. Progressors and non-progressors had similar AHI values (31.3 ± 18.6 vs. 26.4 ± 24.0, p = 0.58) and the OSA severity was not significantly associated with glaucoma progression (p = 0.41). No statistically significant correlations were identified between AHI and MD, pattern standard deviation (PSD), or visual field index (VFI, p = 0.190, p = 0.312, p = 0.228). Compared to non-progressors, the odds ratio of being a progressor with an increase in AHI of 5 events per hour was 1.06 (95% CI: 0.09–1.3).
Teberik, et al. [32] conducted a prospective case-control study including 103 patients with OSA. The mean IOP values of patients with OSA were non-significantly lower than those of the control group. It was also found that the differences between the mean values of RNFL thickness in all quadrants were similar in both OSA and control groups (p = 0.274). When splitting the OSA group in mild, moderate and severe OSA, no statistically significant differences in RNFL and IOP was found.
Also, no higher prevalence of glaucoma in OSA patients was found by Wozniak, et al. [33]. OSA was diagnosed in 58% of POAG patients and in 54% of control patients (p = 0.44). The AHI was no significant predictor of the glaucoma severity stages measured by visual field loss (p = 0.40). There was also no significant association between AHI and RNFL thinning (p = 0.97 adjusted) and between OSA and non-OSA groups (p > 0.2). The authors therefore do not recommend a general screening for OSA in POAG patients.
In summary, 11 of the 16 reviewed studies showed an association of OSA and glaucomatous changes. Four of the studies showed a correlation between OSA severity (AHI) and occurrence of glaucoma and one study correlated the lowest oxygen saturation to the prevalence of glaucoma. One paper found a higher risk for progression of glaucoma in OSA patients. Of the above mentioned studies, one showed alterations in GCC and three in RNFL thinning in OSA patients. Five of the sixteen included studies failed to show a correlation between OSA and glaucoma.
Influences of OSA therapy on glaucoma
Gold standard therapy for OSA-treatment is CPAP. Several studies investigated the influence of CPAP on IOP. Another treatment option for OSA is upper airway surgery to prevent upper airway collapse. The following studies compared IOP values of OSA patients treated with one of those therapy options (Table 2).
Table 2. Influence of OSA therapy on glaucoma
Reference |
Population |
Eligibility |
Intervention |
Control group |
Study design |
Follow-up |
findings |
influence |
Abdullayev et al. [18] |
N = 59 OSA, N = 28 with CPAP |
PSG, OCT, IOP, VF |
CPAP |
N = 19 without OSA |
Prospective study |
- |
There was no statistically significant difference in CCT and RNFL values between OSA with and without CPAP and the control group. The mean deviation value in left eyes in non-CPAP was significantly higher than that of the control group (p = 0.054). Mean PSD values in the right eyes of CPAP and non-CPAP were significantly higher than those of the control group (p = 0.016 and p = 0.014). |
no |
Fan et al. [22] |
N = 32 POAG/ NTG/ suspect |
PSG, IOP, VF, OCT |
N = 7 CPAP, N = 2 upper airway surgery |
N = 5 no OSA, N = 7 glaucoma suspect |
Comparative cohort study |
> 3 years |
There was no statistically significant difference of progression, RNFL thickness, MD and VFI in patients treated with CPAP/surgery in comparison to the no-treatment group. |
no |
Hirunpatravong et al. [34] |
N = 6 POAG and OSA |
PSG, VF, IOP, sonography |
CPAP |
- |
Prospective study |
IOP every 3 months, VF at baseline and 12 months |
POAG and OSA patients demonstrated significant IOP rising after CPAP therapy (p = 0.006) but did not show progression of glaucomatous damage. MD, PSD, and VFI were not significantly different after CPAP therapy. |
yes but no progression |
Lin et al. [35] |
N = 108 OSA |
PSG, OCT |
Upper airway surgery |
- |
Prospective single-blind study |
Baseline, 6 months after surgery |
The visual sensitivities on SAP, ML thickness on OCT, and oxygenation status on PSG significantly improved 6 months after upper airway surgery in patients with severe OSA. |
yes |
Swaminathan et al. [31] |
N = 25 OSA, N = 13 CPAP |
PSG, VF, IOP |
CPAP |
- |
Retrospective cross sectional study |
> 2 years |
The mean IOP after initiation of CPAP therapy in progressors (14.2±3.3 mmHg) and non-progressors (13.9±2.7 mmHg) was similar (p = 0.85) during the course of follow-up (mean 1.3 years after CPAP initiation). |
no |
Ulusoy et al. [36] |
N = 106 N = 38 CPAP |
PSG, IOP, cup/disk ratio |
CPAP |
N = 32 OSA without CPAP, N = 36 no OSA |
Cross-sectional cohort study |
IOP and fundus C/D ratio were higher when no CPAP was used (p = 0.000), glaucoma incidence was lower in patients using CPAP (5.2 %) in comparison to non-users (12.5 %). |
yes |
CCT = central corneal thickness; C/D ratio = cup disk ratio; CPAP = continuous positive airway pressure; IOP = intraocular pressure; ML = macular layer; NTG = normal tension glaucoma; OCT = optical coherence tomography; OSA = obstructive sleep apnea; POAG = primary open angle glaucoma; PSD = pattern standard deviation; PSG = polysomnography; RNFL = retinal nerve fibre layer; SAP = standard automated perimetry; VF = visual field; VFI = visual field index. |
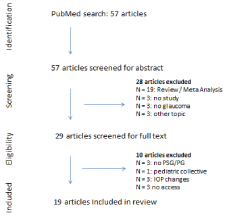
Figure 1. Literature selection process
Abdullayev, et al. [18] compared ophthalmological parameters of CPAP-users, OSA patients without CPAP and controls. There were no statistically significant differences in IOP, CCT and cup/disk ratio in the three groups. The MD value of left eyes in the non-CPAP group was significantly higher than that of the control group (p = 0.054). The RNFL in the nasal quadrant of left eyes was significantly thinner in the non-CPAP group than in the control group (p = 0.047). The average RNFL thickness values did not differ significantly (CPAP 93.38 ± 8.54, non-CPAP 94.10 ± 10.09, no OSA 95.21 ± 9.80, p = 0.817).
In 2019, Fan, et al. [22] investigated the influence of CPAP and upper airway surgery on ophthalmological parameters. Of 27 OSA patients, seven were treated with CPAP and two patients had surgery. A progression of RNFL values was seen in 66.7% of patients with treatment and in 44.4% of patients without treatment (p = 0.420). Also, no statistically significant difference in progression of MD and VF of OSA patients treated and not treated were found. The authors are conducting another study to investigate the neuroprotective effects of OSA treatment in glaucoma patients.
Hirunpatravong et al. [34] included 6 patients in a prospective study to evaluate the long-term effect of CPAP on IOP. The average AHI was 37.42 +/- 15.62/h before treatment and improved to 2.53 +/- 1.55/h after 12 months of CPAP. Also twelve months after initiation of CPAP therapy, the mean IOP significantly increased compared with that at the baseline (19.08 +/- 3.47 vs. 17.83 +/- 2.89 mmHg, p = 0.006). There were no statistically significant differences in MD and VFI before and after 12 of months of CPAP therapy. In conclusion, a risk of glaucoma progression in CPAP users could not be verified or falsified and the authors recommend IOP monitoring and a regular IOP screening in CPAP users.
In a prospective, single blinded study from 2019, Lin, et al. [35] examined the effect of upper airway surgery on ophthalmological values in 108 patients. In the mild/moderate group, VFI, IOP and RFNL thickness values did not change significantly before and after treatment. MD values in VF decreased significantly after treatment (−2.17 +/- 1.54 vs. −1.24 +/- 1.19, p = 0.0001). In the severe OSA group, MD and VFI changed significantly after upper airway surgery (−2.15 +/- 1.75 vs. −1.17 +/- 1.08, p < 0.0001, 97.9 +/- 3.1 vs. 99.0 +/- 1.3, p = 0.0009). No statistically significant differences were observed in IOP and RNFL thickness values.
Swaminathan, et al. [31] also investigated the influence of CPAP on their patient collective. During the course of follow-up (mean 1.3 years after initiation of CPAP) the mean IOP after initiation of CPAP therapy was similar in glaucoma progressors (N = 6, IOP 14.2 +/-3.3 mmHg) and non-progressors (N = 7, IOP 13.9 +/- 2.7 mmHg, p = 0.85). The authors discuss that a final statement on the impact of CPAP on glaucoma progression cannot be made due to the short using time of CPAP.
A study from 2015 compared ophthalmological parameters of patients without OSA to OSA patients using a CPAP device and non-users [36]. The IOP was significantly higher in patients not using CPAP compared to those using CPAP, the IOP in the CPAP was group was similar to the OSA-free group (mean IOP 15.1 +/- 3.5, median 14.0, 16.7 +/- 3.1, 17.0, 14.1 +/- 2.4, 14.0, p = 0.000). The same results were seen in terms of cup/disk ratio (mean ratio 0.4 +/- 0.1, median 0.3, 0.4 +/- 0.2, 0.5, 0.3 +/- 0.1, 0.3, p = 0.000). Glaucoma prevalence was 5.2 % in the CPAP group, 12.5 % in the non-CPAP group and 0 % in the OSA-free group. Ulusoy, et al. conclude that CPAP has positive and healing effects on glaucoma.
In conclusion, one study out of three surveying specific ophthalmological parameters could show an influence of OSA therapy on RNFL thinning and vision. One study showed a rise of IOP when two other studies showed no rising under CPAP (in progressors and non-progressors).
Discussion
This narrative review aimed to summarize the current findings on the association of glaucoma and obstructive sleep apnea. Studies investigating the association and prevalence of both entities as well as studies that focused on the evolution of glaucoma parameters under OSA treatment were analyzed.
Overall, a total of 19 studies with 316178 participants were included in this review [18-36]. The diagnosis of OSA was based on the AHI measured in polysomnography or home sleep-apnea testing. The main features of all studies are presented on Table 1 and 2.
The results of the included studies are often contradictory. Therefore, an association between OSA and glaucoma remains controversial. Out of the 16 studies investigating the prevalence of comorbid glaucoma and OSA, an association was found in eleven of them. There appears to be a higher risk of glaucoma prevalence among OSA patients.
Especially the thinning on RNFL seen via OCT correlated with the occurrence of OSA. Five of the above mentioned studies showed a statistically significant association of thinning of RNFL, or specific quadrants, with OSA. Glaucomatous optic neuropathy is associated with thinning of RNFL and this is one of the first visible signs on OCT scans followed by progression of optic disc excavation and leading to changes in visual field [37,38]. Only two of the above discussed studies showed statistically significant visual field defects in patients with OSA. Therefore, according to current evidence, an ophthalmological examination using OCT appears to be the best option for early detection of glaucomatous changes in OSA patients.
The only therapeutic approach to treat glaucoma is lowering the IOP. In a preceding prospective study, CPAP therapy was associated with IOP rising [39]. Six recent studies were included investigating the effect of OSA therapy on different ophthalmological outcomes. Abdullayev, et al. [18] and Swaminathan, et al. [31] found no statistically significant differences in IOP before and after initiation of CPAP therapy.
Hirunpatravong, et al. [34] conducted a prospective study including 6 POAG patients with newly diagnosed OSA. After initiation of CPAP therapy the patients developed significant IOP rising in comparison to the baseline IOP, but did not show progression of glaucoma. On the other hand, Ulusoy, et al. [36] included 38 patients using CPAP device, 32 OSA patients not using CPAP device and 36 healthy controls. The IOP was significantly lower in the CPAP group than in the non-CPAP group, but similar to the control group. No effect on IOP was seen in the patient cohort of Lin, et al. [35]. They included 108 patients who underwent upper airway surgery as OSA treatment. But visual sensitivities on SAP, ML thickness on OCT, and oxygenation status on PSG significantly improved 6 months after upper airway surgery in severe OSA patients. Four of those studies have a relatively small patient collective (6 – 28 patients) and therefore the evidence is not conclusive. The two larger studies showed promising ophthalmological data after initiation of CPAP therapy and also after upper airway surgery in severe OSA.
Another interesting link between OSA and glaucoma is hypoxia with upregulation of HIF-1α. The occurrence of intermittent hypoxia, as in OSA patients, leads to upregulation of HIF-1α protein (which activates the transcription of genes coding for erythropoietin and endothelial vascular growth factor) [40]. This upregulation is dependent on the severity of hypoxemia [41]. HIF-1α expression was also found in the retina and optic nerve of glaucomatous eyes [42,43]. It is possible that HIF-1α is not only helpful in classifying and monitoring OSA but also can apply as a possible therapeutic approach to treat glaucoma.
Hitherto, the severity of OSA is classified using the AHI (and not serum proteins like HIF-1α). The AHI on the other hand, as a continuous variable, did not always correlate with glaucoma specific parameters. Since using the AHI as the only OSA-defining parameter is controversial among sleep medicine experts, it would not be surprising if it was not able to adequately depict a possible interaction with glaucoma-specific parameters. Most of the included studies examined a correlation between AHI and glaucoma. Some studies also investigated a possible correlation between median or minimum nocturnal oxygen saturation, oxygen desaturation index (ODI) or sleep time with oxygen saturation level below 90% (T90%).
Bahr, et al. [20] found a higher ODI in POAG patients than in LTG patients or healthy controls. Chuang, et al. [21] detected a higher ODI in NTG patients compared with controls and Morsy, et al. [28] showed the lowest oxygen desaturation index to be a significant predictor of vision-threatening disorders. Lee, et al. [27] found that the superotemporal RNFL was inversely associated with T90% and that it was thinner by 34 μm with every increase of one percent in T90%. In another study of Lee, et al. [26] a correlation between a lower oxygen saturation and RNFL thickness could not be found. In their cohort comprising only young adults the range of the SpO2 Nadir was narrow (87 – 93 %), as expected in young adults and therefore the authors conclude, that a meaningful analysis was limited. This depicted cohort will be followed up through the decades to document any further optic disc changes in relationship to OSA parameters.
Our findings suggest an association of OSA and glaucoma. This hypothesis is congruent with a meta-analysis from the year 2015 by Wu, et al. [17], who included 12 studies with 36909 subjects on the association between OSA and glaucoma risk (OR = 1.65; 95% CI). Patients with severe OSA had significantly higher risk to develop glaucoma (OR = 5.49; 95% CI) than patients with mild or moderate OSA and OSA patients showed an increased risk to develop POAG (OR = 1.87; 95% CI) but not NTG (OR = 3.57; 95% CI).
The present study is not a meta-analysis, so the direct comparability of the included study is not given, which is the main limitation. We included studies from January 2015 until March 2021 and thus the studies analysed by Wu, et al. did not overlap with ours.
Conclusion
In conclusion, our findings suggest an association between OSA and glaucoma, and especially OSA and RNFL thinning as an early detector of glaucoma. Since there is controversy regarding an increase of IOP by the use of CPAP in the included studies, we suggest that further studies are needed to investigate this relationship. On the basis of the recent findings, CPAP and upper airway surgery can be recommended also to OSA patients with comorbid glaucoma. Not only AHI but also other polysomnographic measurements should be taken into account when screening for OSA in glaucoma patients.
All authors have read and approved to the manuscript.
Authorship and contributorship
BL, HG and KB have made substantial contributions to conception and design as well as acquisition of data, analysis and interpretation of data. KB and BL drafted the article and HG revised it critically for important intellectual content. All authors gave final approval of the version to be published and agreed to act as guarantor of the work (ensuring that questions related to any part of the work are appropriately investigated and resolved).
Funding
No funding was received for conducting this study. All authors declare the absence of any financial support for creating this review.
Competing interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Wenner JB, Cheema R, Ayas NT (2009) Clinical manifestations and consequences of obstructive sleep apnea. J cardiopulm rehabil prev 29: 76-83. [Crossref]
- Golbidi S, Badran M, Ayas N, Laher I (2012) Cardiovascular consequences of sleep apnea. Lung 190: 113-132. [Crossref]
- Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP (2010) Pathophysiology of sleep apnea. Physiol Rev 90: 47-112. [Crossref]
- Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, et al. (2012) Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events: deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J clin sleep med 8: 597-619. [Crossref]
- Veasey SC, Rosen IM (2019) Obstructive Sleep Apnea in Adults. N Engl J Med 380: 1442-1449.
- Urbano F, Roux F, Schindler J, Mohsenin V (2008) Impaired cerebral autoregulation in obstructive sleep apnea. J Appl Physiol (1985) 105: 1852-1857. [Crossref]
- Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342: 1378-1384. [Crossref]
- Arzt M, Young T, Finn L, Skatrud JB, Bradley TD (2005) Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med 172: 1447-1451. [Crossref]
- Huon L-K, Liu SY-C, Camacho M, Guilleminault C (2016) The association between ophthalmologic diseases and obstructive sleep apnea: A systematic review and meta-analysis. Sleep Breath 20: 1145-1154. [Crossref]
- Farahvash A, Micieli JA (2020) Neuro-Ophthalmological Manifestations of Obstructive Sleep Apnea: Current Perspectives. Eye Brain 2: 61. [Crossref]
- Mentek M, Aptel F, Godin-Ribuot D, Tamisier R, Pepin J-L, et al. (2018) Diseases of the retina and the optic nerve associated with obstructive sleep apnea. Sleep med rev 38: 113-130. [Crossref]
- Liu S, Lin Y, Liu X (2016) Meta-analysis of association of obstructive sleep apnea with glaucoma. J glaucoma 25: 1-7. [Crossref]
- Weinreb RN, Khaw PT (2004) Primary open-angle glaucoma. Lancet 363: 1711-1720. [Crossref]
- Shinmei Y, Nitta T, Saito H, Ohguchi T, Kijima R, et al. (2016) Continuous Intraocular Pressure Monitoring During Nocturnal Sleep in Patients with Obstructive Sleep Apnea Syndrome. Invest Ophthalmol Vis Sci 57: 2824-2830. [Crossref]
- Carnero E, Bragard J, Urrestarazu E, Rivas E, Polo V, et al. (2020) Continuous intraocular pressure monitoring in patients with obstructive sleep apnea syndrome using a contact lens sensor. PLoS One 15: e0229856. [Crossref]
- Fang SY, Wan Abdul Halim WH, Mat Baki M, Din NM (2018) Effect of prolonged supine position on the intraocular pressure in patients with obstructive sleep apnea syndrome. Graefes Arch Clin Exp Ophthalmol 256: 783-790. [Crossref]
- Wu X, Liu H (2015) Obstructive sleep apnea/hypopnea syndrome increases glaucoma risk: Evidence from a meta-analysis. Int J Clin Exp Med 8: 297. [Crossref]
- Abdullayev A, Tekeli O, Yanık Ö, Acıcan T, Gülbay B (2019) Investigation of the Presence of Glaucoma in Patients with Obstructive Sleep Apnea Syndrome Using and Not Using Continuous Positive Airway Pressure Treatment. Turk J Ophthalmol 49: 134-141. [Crossref]
- Bagabas N, Ghazali W, Mukhtar M, Alqassas I, Merdad R, et al. (2019) Prevalence of Glaucoma in Patients with Obstructive Sleep Apnea. J Epidemiol Glob Health 9: 198-203. [Crossref]
- Bahr K, Bopp M, Kewader W, Dootz H, Doge J, et al. (2020) Obstructive sleep apnea as a risk factor for primary open angle glaucoma and ocular hypertension in a monocentric pilot study. Respir Res 21: 258.
- Chuang L-H, Koh Y-Y, Chen HSL, Lo YL, Yu CC, et al. (2020) Normal tension glaucoma in obstructive sleep apnea syndrome: A structural and functional study. Medicine (Baltimore) 99: e19468. [Crossref]
- Fan Y-Y, Su W-W, Liu C-H, Chen SHL, Wu SC, et al. (2019) Correlation between structural progression in glaucoma and obstructive sleep apnea. Eye (Lond) 33: 1459-1465. [Crossref]
- Fındık H, Çeliker M, Aslan MG, Celiker FB, Inecikil MF, et al. (2019) The relation between retrobulbar blood flow and posterior ocular changes measured using spectral-domain optical coherence tomography in patients with obstructive sleep apnea syndrome. Int Ophthalmol 39: 1013-1025. [Crossref]
- Friedlander AH, Graves LL, Chang TI, Kawakami KK, Lee UK, et al. (2018) Prevalence of primary open-angle glaucoma among patients with obstructive sleep apnea. Oral Surg Oral Med Oral Pathol Oral Radiol 126: 226-230. [Crossref]
- Gross NJ, Funk J, Pache M, van der List M, Laubmann-Volz A, et al. (2015) Prevalence of glaucoma in obstructive sleep apnea. Ophthalmologe 112: 580-584. [Crossref]
- Lee SSY, McArdle N, Sanfilippo PG, Yazar S, Eastwood PR, et al. (2019) Associations between Optic Disc Measures and Obstructive Sleep Apnea in Young Adults. Ophthalmology 126: 1372-1384. [Crossref]
- Lee SS-Y, Sanfilippo PG, Hunter M, Yazar S, James A, et al. (2020) Optic Disc Measures in Obstructive Sleep Apnea: A Community-based Study of Middle-aged and Older Adults. J glaucoma 29: 337-343. [Crossref]
- Morsy NE, Amani BE, Magda AA, Nabil AJ, Pandi-perumal SR, et al. (2019) Prevalence and Predictors of Ocular Complications in Obstructive Sleep Apnea Patients: A Cross-sectional Case-control Study. Open Respir Med J 13: 19-30. [Crossref]
- Moyal L, Blumen-Ohana E, Blumen M, Blatrix C, Chabolle F, et al. (2018) Parafoveal and optic disc vessel density in patients with obstructive sleep apnea syndrome: An optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol 256: 1235-1243. [Crossref]
- Pedrotti E, Demasi CL, Bruni E, Bosello F, Plinio Di Sarro P, et al. (2017) Prevalence and risk factors of eye diseases in adult patients with obstructive sleep apnoea: Results from the SLE.E.P.Y cohort study. BMJ Open 7: e016142. [Crossref]
- Swaminathan SS, Bhakta AS, Shi W, Feuer WJ, Abreu AR, et al. (2018) Is Obstructive Sleep Apnea Associated With Progressive Glaucomatous Optic Neuropathy? J glaucoma 27: 1-6. [Crossref]
- Teberik K, Eski MT, Balbay EG, Kaya M (2018) Evaluation of Intraocular pressure, Corneal thickness, and Retinal nerve fiber layer thickness in patients with Obstructive Sleep Apnea Syndrome. Pak J Med Sci 34: 817-822. [Crossref]
- Wozniak D, Bourne R, Peretz G, Kean J, Willshire C, et al. (2019) Obstructive Sleep Apnea in Patients with Primary-open Angle Glaucoma: No Role for a Screening Program. J glaucoma 28: 68-675. [Crossref]
- Hirunpatravong P, Kasemsup T, Ayudhya WN, Apiwattanasawee P (2019) Long-term Effect of Continuous Positive Air Pressure Therapy on Intraocular Pressure in Patients with Primary Open-angle Glaucoma with Obstructive Sleep Apnea. J Curr Glaucoma Pract 13: 94-98. [Crossref]
- Lin P-W, Lin H-C, Friedman M, Chang HW, Salapatas AM, et al. (2019) Effects of OSA Surgery on Ophthalmological Microstructures. Ann Otol Rhinol Laryngol 128: 938-948. [Crossref]
- Ulusoy S, Erden M, Dinc ME, Yavuz N, Caglar E, et al. (2015) Effects of Use of a Continuous Positive Airway Pressure Device on Glaucoma. Med Sci Monit 21: 3415-3419. [Crossref]
- Lee VW, Mok KH (1999) Retinal nerve fiber layer measurement by nerve fiber analyzer in normal subjects and patients with glaucoma. Ophthalmology 106: 1006-1008. [Crossref]
- Kremmer S, Ayertey HD, Selbach JM, Steuhl KP (2000) Scanning laser polarimetry, retinal nerve fiber layer photography, and perimetry in the diagnosis of glaucomatous nerve fiber defects. Graefes Arch Clin Exp Ophthalmol 238: 922-926. [Crossref]
- Kiekens S, Veva De G, Coeckelbergh T, Tassignon J, Van de Heyning P, et al. (2008) Continuous positive airway pressure therapy is associated with an increase in intraocular pressure in obstructive sleep apnea. Invest Ophthalmol Vis Sci 49: 934-940. [Crossref]
- Gabryelska A, Szmyd B, Szemraj J, Stawski R, Sochal M, et al. (2020) Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1α protein. J clin sleep med 16: 1761-1768. [Crossref]
- Kaczmarek E, Bakker JP, Clarke DN, Csizmadia E, Kocher O, et al. (2013) Molecular biomarkers of vascular dysfunction in obstructive sleep apnea. PLoS One 8: e70559. [Crossref]
- Tezel G, Wax MB (2004) Hypoxia-inducible factor 1α in the glaucomatous retina and OpticNerve head. Arch ophthalmology 122: 1348-1356. [Crossref]
- Reszeć J, Zalewska R, Bernaczyk P, Chyczewski L (2012) HIF-1 expression in retinal ganglion cells and optic nerve axons in glaucoma. Folia histochemica et cytobiologica 50: 456-459. [Crossref]

