Abstract
Hydrocortisone may be administered intravenously, orally, or topically and hydrocortisone is well absorbed after oral or topical administration. In infants, hydrocortisone has been used to treat hypotension, to prevent and treat bronchopulmonary dysplasia, to treat congenital adrenal hypertension, adrenal hypoplasia, and Addisonian crisis. In children, hydrocortisone has been used to treat acute adrenal insufficiency (Addison crisis), congenital adrenal hyperplasia, adrenal hypoplasia, inflammatory bowel disease, ulcerative colitis, proctosigmoiditis, acute hypersensitivity reactions, angioedema, hypotension, severe acute asthma, and life-threatening acute asthma. The efficacy and safely of hydrocortisone have been reviewed in infants and children. Hydrocortisone has been found an effective and safe agent to prevent and treat bronchopulmonary dysplasia. Hydrocortisone is metabolised into phase I and phase II metabolites and phase II metabolites are prevalent over phase I metabolites. The pharmacokinetics of hydrocortisone have been studied and the total body clearance of hydrocortisone is higher in children, infants, and newbornsthan in newborns and infants. The prophylaxis with hydrocortisone has been reviewedin infants. Hydrocortisone prevents bronchopulmonary dysplasia, decreases the mortality-rate in infants with bronchopulmonary dysplasia, increases the systolic blood pressure, improves oxygenation, treats adrenal insufficiency, and treats hypotension in infants. The treatment with hydrocortisone has been reviewed in infants and children. Hydrocortisone treats bronchopulmonary dysplasia, chronic lung disease, dermatitis, and eczema. Hydrocortisone migrates into the breast-milk in significant amounts. The aim of this study is to review hydrocortisone dosing, pharmacokinetics, prophylaxis, and treatment in infants and children, and hydrocortisone metabolism and migration into the breast-milk.
Keywords
breast-milk, children, dosing, efficacy-safely, hydrocortisone, infants, metabolism, pharmacokinetics, prophylaxis, treatment
Introduction
Structure-activity of corticoids
Chemical modifications of cortisol molecule have generated derivatives with greater separation of glucocorticoid and mineralocorticoid activity; for a number of synthetic glucocorticoid, the effects on electrolytes are minimal even at highest doses used. In addition, these modifications have led to derivatives with greater potencies and with longer duration of action. A vast array of steroid preparations is available for oral, parenteral, and topical use. None of these currently available derivatives effectively separates anti-inflammatory effects from effects on carbohydrate, protein, and fat metabolism or from suppressive effects on the hypothalamic-pituitary-adrenal axis. Some steroids that are classified predominantly as glucocorticoids (e.g., cortisol) also possess modest but significant mineralocorticoid activity and thus may affect fluid and electrolyte handling in the clinical setting. At doses used for replacement therapy in patients with primary adrenal insufficiency, the mineralocorticoid effects of these glucocorticoids are insufficient to replace that of aldosterone, and concurred therapy with a more potent mineralocorticoid generally is needed. In contrast, aldosterone is exceedingly potent with respect to Na+ retention but has only minimal effects on carbohydrate metabolism. Even at levels that maximally affect electrolyte balance, aldosterone has no significant glucocorticoid activity and thus acts as a pure mineralocorticoid [1].
Administration, distribution, metabolism and elimination of corticoids
Hydrocortisone and numerous congeners, including the synthetic analogues, are orally effective. Certain water-soluble esters of hydrocortisone and its synthetic congeners are administered intravenously to achieve high concentrations of drug rapidly in systemic or targeted body fluids. More prolonged effects are obtained by intramuscular injection of suspension ofhydrocortisone, its esters, and congeners. Minor changes in chemical structure may markedly alter the rate of absorption, time of onset of effect, and duration of action. Glucocorticoids also are absorbed systemically from sites of local administration, such as synovial spaces, the conjunctival sac, skin, and respiratory tract. When administration is prolonged, when the site of application is covered with an occlusive dressing, or when large areas of skin are involved, absorption may be sufficient to cause systemic effects, including suppression of the hypothalamic-pituitary-adrenal axis. After absorption, 90% or more of cortisol in plasma is reversibly bound to protein under normal circumstances. In most tissues, only the fraction of corticosteroid that is unbound is active and can enter cells. Two plasma proteins account for almost all of the steroid-binding capacity; corticosteroid-binding globulin (also called transcortin) and albumin. Corticosteroid-binding globulin is an α globulin secreted by the liver that has high affinity for steroids (dissociation constant of about 1 nM) but relatively low total binding capacity, whereas albumin, also produced by the liver, has relatively large binding capacity but low affinity (estimated dissociation constant of about 1 mM). In tissues with prolonged capillary transit time (e.g., liver, spleen), steroid dissociates from albumin. At high steroid concentrations, the capacity of corticosteroid-binding globulin binding is exceeded, and a slightly greater fraction of the steroid exists in the free state. Corticosteroid-binding globulin has relatively high affinity for cortisol and some of its synthetic congeners and low affinity for aldosterone and glucuronide-conjugated steroid metabolites; thus, greater percentages of these last steroids are found in the free form. A special state of physiological hypercortisolism occurs during pregnancy. The elevated circulating oestrogen levels include corticosteroid-binding globulin production and corticosteroid-binding globulin and total plasma cortisol increases several-folds; the physiological significance of these changes remains to be stablished. The aldosterone levels also rise 3- to 10-fold in pregnancy, reflecting the activity of the elevated progesterone plasma levels as a mineralocorticoid receptor antagonist. Because progesterone is also a glucocorticoid receptor antagonist, it may contribute to the elevated levels of cortisol. As a general rule, the metabolism of steroid hormones involves sequential additions of O or H atoms, followed by conjugation to form water-soluble derivatives. Reduction of the 4,5 double bond occurs at both hepatic and extra-hepatic sites, yielding inactive compounds. Subsequent reduction of the 3-ketone substituent to the 3-hydroxy derivative, forming tetrahydrocortisol, occurs only in the liver. Most of these a ring-reduced steroids are conjugated through the 3-hydroxyl group with sulfate or glucuronide by enzymatic reactions that take place in the liver and, to a lesser extent, in the kidney. The resultant sulfate esters and glucuronides are water soluble and are excreted in urine. Neither biliary nor faecal excretion is of quantitative importance in humans. Synthetic steroids with an 11-keto group such as cortisone and prednisone must be enzymatically reduced to the corresponding 11β-hydroxy derivative before they are biologically active. The type 1 isozyme of 11β-HSD (11β-hydroxysteroid dehydrogenase type 1) catalyses the reduction, predominantly in the liver but also in specialized sites as adipocytes, bone, eye, and skin. In settings in which this enzymatic activity is impaired, it is prudent to use steroids that do not require enzymatic activation (e.g., hydrocortisone or prednisolone rather than cortisone or prednisone). Such settings include individuals with severe hepatic failure and patients with the very rare condition of cortisone reductase deficiency [1].
Clinical use of hydrocortisone in infants and children
Hydrocortisone has been used to manage congenital adrenal abnormality and adrenal insufficiency due to hypopituitarism. Hypotension in the preterm infant often responds to low-dose intravenous hydrocortisone [2]. Hydrocortisone is used to treat cortisol deficiency and pressor-resistant hypotension in infants and children. Adjuvant therapy with hydrocortisone is used to treat persistent hypoglycaemia. Hydrocortisone is the main adrenal corticosteroid with primarily glucocorticoid effects. Hydrocortisone increases the expression of adrenergic receptors in the vascular wall, there by enhancing vascular reactivity to other vasoactive substances, such as norepinephrine and angiotensin II. Infants and children who are cortisol deficient (< 15 µg/dl) likely respond to hydrocortisone and the blood pressure increases within 2 hours after the first dose. Hydrocortisone also stimulates the liver to form glucose from amino acids and glycerol, and stimulates the deposition of glucose as glycogen. Peripheral glucose utilization is diminished, protein breakdown is decreased, and lipolysis is activated. The net result is an increase in blood glucose levels. The renal effects include increased calcium elimination. The elimination half-life of hydrocortisone is 9 hours in preterm infants. Hydrocortisone is incompatible with midazolam, nafcillin, pentobarbital, phenobarbital, and phenytoin [3].
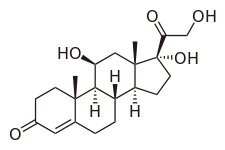
Hydrocortisone molecular structure (molecular weight = 362.46 grams/mole)
Literature search
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “hydrocortisone dosing infants, children“, “hydrocortisone efficacy, safely, infants, children”, “hydrocortisone metabolism”, “hydrocortisone pharmacokinetics infants, children”, “hydrocortisone prophylaxis infants, children”, “hydrocortisone treatment infants, children”, and “hydrocortisone breast-milk”. In addition, the books: The Pharmacological Basis of Therapeutics [1], Neonatal Formulary [2], “NEOFAX”® by Young and Mangum, [3]”, and The British National Formulary for Children [4] have been consulted.
Results
Administration schedules of hydrocortisone to infants and children
Administration to infants [2].
Treatment of neonatal hypotension: Hydrocortisone often increases blood pressure as effectively as dopamine and may work when a catecholamine does not. Give: 2.5 mg/kg intravenously 4 times-daily. This treatment is usually enough to reduce the need to use other vasopressor drugs. Try and withdraw treatment within 2 to 4 days, because steroid use increases the risk of fungal infection, and also seems to increase the risk of focal gut perforation, especially if the infant is also given ibuprofen or indomethacin.
Prevention of bronchopulmonary dysplasia: Low-dose trials (0.5 mg/kg intravenously twice-daily for 12 days and half this for 3 days) delivered no benefit except to a sub group with chorioamnionitis. Later development was not affected by such treatment. The results from different trials, however, are somewhat varied; the recently complete STOP-BRONCOPULMONARY DYSPLASIA study group examined the effects of hydrocortisone (or placebo) in very-low-birth-weight infants who were ventilated between 7 and 14 days of age. Whist they reported no statistical significantly differences in their primary outcome (a composite of death or bronchopulmonary at 36 weeks postmenstrual age) they did nonetheless have a significant difference in mortality-rate at that time that favoured treatment (15.5% versus 23.7%; odds ratio = 0.59 [95% confidence interval = 0.35 to 0.995]).
Treatment of bronchopulmonary dysplasia: Give: 2.5 mg/kg twice-daily for 7 days, and a reducing dose for further 2 weeks, was an effective as dexamethasone in one study, and did not appear to have the latter’s detrimental effect on development.
Treatment of congenital adrenal hyperplasia: Give: 3 to 5 mg/m2 of hydrocortisone thrice-daily, plus at least 200 µg of fludrocortisone once-daily, provide a good starting point for neonatal care. Infants with 21-hydrolase deficiency usually need an additional 2 to 4 mmol/kg of sodium daily.
Treatment of adrenal hypoplasia: Production of cortisol normally averages to 6 to 9 mg/m2 daily and, making allowance for absorption, 8 to 10 mg/m2 of hydrocortisone daily in 3 divided doses given orally will meet normal replacement needs (although need may rise several-fold during any acute illness). Give a higher dose in the morning than at other times.
Treatment of Addisonian crisis: This requires intravenous glucose and 10 mg bolus followed by a continuing 100 mg/m2 daily by infusion of hydrocortisone. Rapid fluid replacement may be necessary with 0.9% sodium chloride. The high serum potassium almost always correct itself, but 2 ml/kg of 10% calcium gluconate and/or an infusion of glucose and insulin may be needed if a cardiac arrhythmia develops. In infants, aged 1 to 12 months, give glucose intravenously and an initial 2 to 4 mg/kg slow intravenous bolus injection then 2 to 4 mg/kg 4 times-daily.
Administration to children [4].
Administration of hydrocortisone for treatment of acute adrenocortical insufficiency (Addison crisis) by slow intravenous injection or by intravenous infusion.
Children aged 1 month to 11 years: Give initially 2 to 4 mg/kg of hydrocortisone, and then 2 to 4 mg/kg 4 times-daily, adjust the dose according to the response, when stable reduce the dose over 4 to 5 days to oral maintenance dose.
Children aged 12 to 17 years: Give: 100 mg of hydrocortisone 4 times-daily or thrice-daily.
Oral treatment of congenital adrenal hyperplasia
Children: Give: 9 to 15 mg/m2of hydrocortisone in 3 divided doses, and then adjust the dose according to the response.
Oral treatment of (1) adrenal hypoplasia, (2) Addison disease, (3) chronic maintenance or replacement therapy
Children: Give: 8 to 10 mg/m2 daily of hydrocortisone daily in 3 divided doses, the larger dose to be given in the morning and the smaller dose in the evening, higher doses may be needed.
Intravenous treatment of inflammatory bowel disease-induction of remission
Children aged 2 to 17 years: Give: 2.5 mg/kg of hydrocortisone 4 times-daily (maximum per dose = 100 mg).
Continuous intravenous infusion of inflammatory bowel disease-induction of remission
Children aged 2 to 17 years: Give: 10 mg/kg of hydrocortisone daily (maximum = 400 mg daily).Rectal treatment of (1) ulcerative colitis and (2) proctosigmoiditis
Children aged 2 to 17 years: Give initially 1 metered application of cortisone 1 to 2 times-daily for 2 to 3 weeks, and then reduce the dose to 1 metered application once-daily on alternative days, to be inserted in the rectum.
Intravenous treatment of (1) acute hypersensitivity reactions and (2) angioedema
Children aged 1 to 5 months: Give initially 25 mg of hydrocortisone thrice-daily, and then adjust the dose according the response.
Children aged 6 months to 5 years: Give initially 50 mg of hydrocortisone thrice-daily, and then adjust the dose according to the response.
Children aged 6 to 11 years: Give initially 100 mg of hydrocortisone thrice-daily, and then adjust the dose according to the response.
Children aged 12 to 17 years: Give initially 200 mg of hydrocortisone thrice-daily, and then adjust the dose according to the response.
Intravenous treatment of hypotension resistant to inotropic treatment and volume replacement (limited evidence)
Children: Give: 1 mg/kg of hydrocortisone 4 times-daily (maximum per dose = 100 mg).
Intravenous treatment of (1) severe acute asthma and (2) life-threatening acute asthma
Children aged 1 month to 1 year: Give: 4 mg/kg of hydrocortisone 4 times-daily (maximum per dose = 100 mg), alternatively 25 mg 4 times-daily until conversion to oral prednisolone is possible, the dose given preferentially as sodium succinate.
Children aged 5 to 11 years: Give: 4 mg/kg of hydrocortisone 4 times-daily (maximum per dose = 100 mg), alternatively 50 mg 4 times-daily until conversion to oral prednisolone is possible, the dose given, preferentially, as sodium succinate.
Children aged 12 to 17 years: Give: 4 mg/kg of hydrocortisone 4 times-daily (maximum per dose = 100 mg), alternatively 100 mg 4 times-daily until conversion to oral prednisolone is possible, the dose, preferentially, as sodium succinate.
Efficacy and safely of hydrocortisone in infants and children
Early treatment with low-dose of hydrocortisone effectively and safely prevents bronchopulmonary dysplasia or death in preterm infants exposed to chorioamnionitis [5]. Hydrocortisone efficaciously and safely treats hypotension associated with impaired adrenal function among critically ill newborns [6]. Early systemic hydrocortisone is an effective therapy for the prevention of bronchopulmonary dysplasia in preterm infants [7]. Hydrocortisone is efficacy, safe, and easy to administer to newborns, infants, and children and it is rapidly absorbed following oral dosing [8]. Hydrocortisone is an effective and appropriate treatment for diaper dermatitis in infants and children [9]. Hydrocortisone normalizes the cardiovascular status and decreases press or requirement in preterm infants [10]. Hydrocortisone effectively and safely treats hypotension in children undergoing cardiac surgery [11], and hydrocortisone butyrate 0.1% in lipocream effectively and safely treats dermatitis in a paediatric population [12].
Metabolism of hydrocortisone
Sarkar, et al. [13] studied the metabolism of hydrocortisone using human liver perfusion. Phase I metabolites include tetrahydrocortisone and dihydrocortisol which accounts from 8% to 10% of the total metabolites and phase II metabolites are tetrahydrocortisol and tetrahydrocortisone glucuronides and hydrocortisone sulfate which account from 45% to 52% of total metabolites.

Tetrahydrocortisone molecular structure (molecular weight = 364.4758 grams/mole)
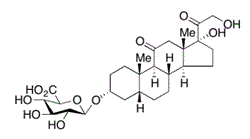
Tetrahydrocortisone 3-glucuronide molecular structure (molecular weight = 540.606 grams/mole)
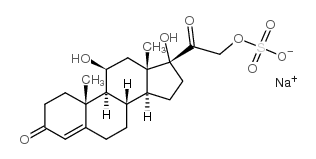
Hydrocortisone sulfate molecular structure (molecular weight = 464.505 grams/mole)
Bailey, et al.[14] investigated the metabolism of hydrocortisone in human liver microsomes and the metabolites which are formed are cortisone, dihydrocortisol, dihydrocortisone and 6β-hydroxycortisol.
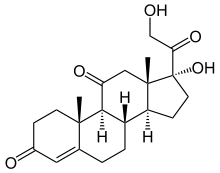
Cortisone molecular structure (molecular weight = 360.4 grams/mole)
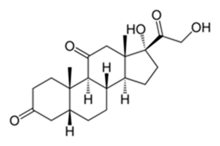
Dihydrocortisone molecular structure (molecular weight = 362.46 grams/mole)
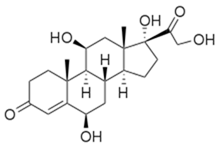
6β-Hydroxycortisol molecular structure (molecular weight = 378.46 grams/mole)
Pharmacokinetics of hydrocortisone in newborns and infants
Vezina, et al. [15] studied the pharmacokinetics of hydrocortisone in 62 newborns and infants with median postmenstrual age, postnatal age, and body-weight of 27 weeks (range, 23 to 41), 0.7 weeks (range, 0.1 to 9.3), and 1.2 kg (range, 0.5 to 4.4). Thirty-three (53.2%) subjects were males and 29 (46.8%) were females and hydrocortisone was intravenously infused at a dose of 45 mg/m2 daily divided in 4 doses.
This table shows that the distribution volume of hydrocortisone is larger than the water volume. The most important biological covariates for predicting the total body clearance and the distribution volume of hydrocortisone are the body-weight and the postmenstrual age. There is a remarkable interindividual variability in the pharmacokinetic parameters and this variability is accounted by the wide variation of subject’s demographic characteristics and disease of the subjects included in the study.
Pharmacokinetics of hydrocortisone in children, infants, and newborns
Michelet, et al. [16] investigated the pharmacokinetics of hydrocortisone in 12 children aged 39.9 months (range, 24.8 to 59.6) with a body-weight of 16.2 kg (range, 12.2 to 21.0), in 6 infants aged 16.6 months (range, 4.1 to 22.1) with a body-weight of 11.1 kg (range, 6.7 to 12.5), and in 6 newborns aged 0.77 months (range, 0.53 to 0.87) with a body-weight of 3.65 kg (range, 2.80 to 4.90). Twenty-three subjects had congenital adrenal hyperplasia and 1 subject had hypopituitarism and hydrocortisone was administered orally at a dose of 2.5 mg (range, 2.0 to 4.0) to children, at a dose of 2.0 mg (range, 2.0 to 2.5) to infants, and at a dose of 0.5 mg (range, 0.4 to 0.7) to newborns.
This table shows that hydrocortisone is rabidly absorbed following oral dosing, the central distribution volume is larger than the water volume, the central distribution volume is smaller than the peripheral distribution volume, and there is a remarkable inter individual variability in the pharmacokinetic parameters. This variability is accounted by the wide variation of subject’s demographic characteristics and disease. This study shows that newborns have a lower and more variable total body clearance than infants and children which can be explained by the lower metabolism of hydrocortisone and by a lower elimination-rate in infants than in children. The total body clearance is greater in children in infants and in newborns than in infants and newborns. For comparison of the total body clearance obtained in infants and newborns see table 1. Hydrocortisone is cleared from the body by metabolism and by renal route and both elimination pathways increase with infant maturation and child development table 2.
|
Parameter
|
Final estimate
|
%RSE
|
95% CI
|
Bootstrap (median)
|
Bootstrap 95% CI
|
|
TBC (L/h)
|
20.2
|
10.8
|
15.9 – 24.5
|
20.4
|
16.1 – 25.6
|
|
PMA effect
|
0.11
|
17.6
|
0.075 – 0.145
|
0.11
|
0.076 – 0.154
|
|
DV (L)
|
244
|
21.5
|
160 – 328
|
270
|
170 – 592
|
|
Baseline cortisol (ng/ml)
|
1.37
|
16.1
|
0.792 – 1.95
|
1.39
|
0.882 – 2.04
|
|
Variability of TBC (CV%)
|
0.293 (54.1%)
|
22.6
|
0.163 – 0.423
|
0.293
|
0.157 – 0.484
|
|
Variability of cortisol (CV%)
|
2.41 (155%)
|
11.9
|
1.85 – 2.97
|
2.37
|
1.78 – 2.90
|
|
Variability of RV by proportional error model (CV%)
|
0.328 (57.3%)
|
13.2
|
0.243 – 0.413
|
0.326
|
0.246 – 0.413
|
|
RV by additive error model+SD
|
0.049+0.220
|
15.8
|
0.034 – 0.064
|
0.048
|
0.033 – 0.065
|
Table 1. Pharmacokinetic parameter estimates of unbound hydrocortisone which are obtained in 62 newborns and infants. Hydrocortisone was intravenously infused at a dose of 45 mg/m2 daily divided in 4 doses. Values are by Vezina, et al. [15]
TBC = total body clearance, PMA = postmenstrual age, DV = distribution volume, CV% = %coefficient of variance, b%RSE = %standard error estimate, CI = confidence interval, RV = residual variability, SD = standard deviation.
Table 2. Pharmacokinetic parameters of hydrocortisone which have been obtained in 12 children, 6 infants, and 6 newborns. Hydrocortisone was administered orally at a dose of 2.5 mg (range, 2.0 to 4.0) to children, at a dose of 2.0 mg (range, 2.0 to 2.5) to infants, and at a dose of 0.5 mg (range, 0.4 to 0.7) to newborns. Values are by Michelet, et al. [16]
|
Disposition model
|
Parameter estimate (RSE, CV%)
|
Bootstrap median (95% CI)
|
LLP (95% CI)
|
|
Maximum absorption rate (nmol)
|
4,810 (19%)
|
5,053 (2,880 – 7,530)
|
3,589 – 8,550
|
|
Vmabs (nmol/h)
|
21,600 (10%)
|
21,900 (17,600 – 26,500)
|
18,500 (18,500 – 26,000)
|
|
TBC/F (L/h)
|
400 (7%)
|
412 (353 – 468)
|
255 – 473
|
|
DVo/F (L)
|
10.6 (11%)
|
10.6 (8.58 – 12.6)
|
9.31 – 12.0
|
|
Q/F (L/h)
|
160 (18%)
|
159 (104 – 237)
|
123 – 210
|
|
DVp/F (L)
|
124 (14%)
|
124 (84.6 – 163)
|
102 – 150
|
|
BASE (nM)
|
13.3 (6%)
|
13.3 (12.7 – 13.8)
|
11.8 – 15.0
|
|
Interindividual variability
|
|
ωKabs (CV%)
|
48.1% (35%)
|
45.5% (12.3 – 117)
|
14.1 – 81.8
|
|
ωVabs (CV%)
|
46.0% (17%)
|
45.5% (30.2 – 62.6)
|
35.1 – 61.8
|
|
ωTBC (CV%)
|
19.3% (17%)
|
18.4% (10.6 – 26.2)
|
13.5 – 26.5
|
|
ωBASE (CV%)
|
34.4% (22%)
|
32.9% (20.0 – 48.0)
|
26.3 – 47.2
|
|
ωF (CV%)
|
36.0% (22%)
|
35.1 % (18.3 – 51.6)
|
25.9 – 49.3
|
|
Residual variability
|
|
oexpb (CV%)
|
14.5% (8%)
|
14.4% (12.5 – 16.9)
|
14.0 – 15.1
|
TBC = Total Body Clearance, F = bioavailability, LLP = log-likelihood profiling,Vmabs = maximum absorption rate, DVo/F = apparent central distribution volume, Q/F = apparent intercompartmental total body clearance, DVp/F = apparent peripheral distribution volume, BASE = cortisol baseline of dexamethasone suppressed healthy adults,ω = interindividual variability, Kabs = absorption rate, Vabs = distribution volume, oexpb = residual variability, CV% = %coefficient of variation.
Prophylaxis with hydrocortisone in infants
Early low-dose of hydrocortisone prevents bronchopulmonary dysplasia in preterm infants [17]. Prophylactic hydrocortisone increases the survival-rate in preterm infants with bronchopulmonary dysplasia [18]. Prophylaxis with hydrocortisone significantly decreases the mortality-rate and improves the survival-rate in infants with bronchopulmonary dysplasia [19]. Prophylactic hydrocortisone increases the systolic blood pressure and improves oxygenation in infants with persistent pulmonary hypertension [20]. Early prophylaxis with low-dose hydrocortisone treats adrenal insufficiency in preterm infants [21]. A prophylactic dose of hydrocortisone effectively treats refractory hypotension in preterm infants [22].
Treatment of infants and children with hydrocortisone
Early therapy with low-dose of hydrocortisone increases the survival-rate in preterm infants with bronchopulmonary dysplasia [23]. Early low-dose of hydrocortisone confers benefits in preterm infants with bronchopulmonary dysplasia [24]. Early low-dose of hydrocortisone successfully treats bronchopulmonary dysplasia in preterm infants [25]. Early low-dose of hydrocortisone reduces moderate or severe bronchopulmonary dysplasia in preterm infants [26]. Administration of post natal hydrocortisone treats bronchopulmonary dysplasia in preterm infants [27]. Early low-dose of hydrocortisone treats bronchopulmonary dysplasia and does not induce adverse-effects in children [28]. Hydrocortisone should replace dexamethasone in the treatment of infants with chronic lung disease [29]. Administration of hydrocortisone to infants treats early bronchopulmonary dysplasia and has no long-term effects on neuro development in children [30]. Perinatal treatment with hydrocortisone has no long-term effects on either neuro-structural brain development and neurocognitive outcomes in children [31]. Hydrocortisone 0.1% lotion effectively and safely treats mild to moderate atopic dermatitis in children aged 3 months to 18 years [32], and hydrocortisone ointment is a valuable treatment of eczema in infants and children [33].
Migration of hydrocortisone into the breast-milk
The concentration of hydrocortisone was measured in breast-milk samples obtained from 23 lactating women. The mean concentration of hydrocortisone in milk is 1.6 ng/ml shortly after dosing and is higher in the morning than in the evening [34]. The concentration of hydrocortisone was measured in the breast-milk samples obtained from 13 lactating women and ranges from 1.45 to 8.34 ng/ml [35]. The concentration of hydrocortisone was measured in the breast-milk samples obtained from lactating 7 women who delivered spontaneously and in 6 lactating women who underwent elective Caesarean section. In women who delivered spontaneously the concentration of hydrocortisone in the breast-milk is 17.2, 16.8, and 7.4 ng/ml on days 1, 2, and 3, respectively, after delivery. In women who underwent Caesarean section the breast-milk concentration of hydrocortisone is 26.5, 15.1, and 14.1 ng/ml on days 3, 4 and 6, respectively, after delivery [36]. The concentration of hydrocortisone was measured in the breast-milk samples obtained from 11 lactating women monthly up to 12 months of lactation. The concentration of hydrocortisone in the breast-milk averages to 7.2 ng/ml but varies with the time after delivery and ranges from 0.2 to 32 ng/ml [37]. These results indicate that hydrocortisone migrates into the breast-milk in significant amounts.
Discussion
Hydrocortisone and numerous congeners, including the synthetic analogues, are orally effective. Certain water-soluble esters of hydrocortisone and its synthetic congeners are administered intravenously to achieve high concentrations of drug rapidly in systemic or targeted body fluids. More prolonged effects are obtained by intramuscular injection of suspension of hydrocortisone, its ester, and congeners. Glucocorticoids are absorbed systemically from sites of local administration, such as synovial spaces, the conjunctival sac, skin, and respiratory tract. Two plasma proteins account for almost all of the steroid-binding capacity; corticosteroid-binding globulin (also called transcortin) and albumin. Corticosteroid-binding globulin is an α globulin secreted by the liver that has high affinity for steroids (dissociation constant of about 1 nM) but relatively low total binding capacity, whereas albumin, also produced by the liver, has relatively large binding capacity but low affinity (estimated dissociation constant of about 1 mM). The metabolism of steroid hormones involves sequential addition of O or H atoms, followed by conjugation to form water-soluble derivatives. Reduction of the 4,5 double bond occurs at both hepatic and extra-hepatic sites, yielding inactive compounds. Subsequent reduction of the 3-ketone substituent to the 3-hydroxy derivative, forming tetrahydrocortisol, occurs only in the liver. Most of these a ring-reduced steroid is conjugated through the 3-hydroxyl group with sulfate or glucuronide by enzymatic reactions that take place in the liver and, to a lesser extent, in the kidney. The resultant sulfate ester and glucuronides are water soluble and are excreted in urine [1]. Hydrocortisone has been used to manage congenital adrenal abnormality and adrenal insufficiency due to hypopituitarism. Hypotension in the preterm infant often responds to low-dose of intravenous hydrocortisone. Hydrocortisone may be administered intravenously, orally, or topically and following oral and topical administration hydrocortisone is well absorbed. In infants, hydrocortisone has been used to treat hypotension, to prevent and to treat bronchopulmonary dysplasia, to treat congenital adrenal hypoplasia, to treat adrenal hypoplasia, and to treat Addisonian crisis [2]. Hydrocortisone is used to treat cortisol deficiency and pressor-resistant hypotension in infants and children. Adjuvant therapy with hydrocortisone is used to treat persistent hypoglycaemia. Hydrocortisone is the main adrenal corticosteroid with primarily glucocorticoid effects. Hydrocortisone increases the expression of adrenergic receptors in the vascular wall, thereby enhancing vascular reactivity to other vasoactive substances, such as norepinephrine and angiotensin II. Infants and children who are cortisol deficient (< 15 µg/dl) likely respond to hydrocortisone and the blood pressure increases within 2 hours after the first dose. Hydrocortisone also stimulates the liver to form glucose from amino acids and glycerol, and stimulates the deposition of glucose as glycogen. Peripheral glucose utilization is diminished, protein breakdown is decreased, and lipolysis is activated. The net resultant is an increase in blood glucose level. The renal effects include increased calcium elimination. The elimination half-life of hydrocortisone is 9 hours in preterm infants [3]. In children, hydrocortisone has been used to treat acute adrenocortical insufficiency (Addison crisis), congenital adrenal hyperplasia, adrenal hypoplasia, inflammatory bowel disease, ulcerative colitis, proctosigmoiditis, acute hypersensitivity reactions, angioedema, hypotension, severe acute asthma, and life-threatening acute asthma [4]. Hydrocortisone has been found efficacy and safe in infants and children. Early treatment with low-dose of hydrocortisone effectively and safely prevents bronchopulmonary dysplasia or death in preterm infants exposed to chorioamnionitis [5], hydrocortisone effectively and safely treats hypotension associated with impaired adrenal function in critically ill newborns [6], early systemic hydrocortisone prevents bronchopulmonary dysplasia in preterm infants [7], hydrocortisone is efficacy, safe, and easy to administer to newborns, infants, and children and is rapidly absorbed [8], effectively treats diaper dermatitis in infants and children [9], normalizes cardiovascular status and decreases the pressor requirement in preterm infants [10], effectively and safely treats hypotension in children undergoing cardiac surgery [11], and hydrocortisone butyrate 0.1% in lipocream effectively and safely treats dermatitis in a paediatric population [12]. The metabolism of hydrocortisone has been studied using human liver perfusion. Phase I metabolites of hydrocortisone are tetrahydrocortisone and dihydrocortisol which account from 8% to 10% of total metabolites and phase II metabolites are tetrahydrocortisol and tetrahydrocortisone glucuronides and hydrocortisone sulfate which account from 45% to 52% of the total metabolites [13]. The metabolism of hydrocortisone has been studied in human liver microsomes and the metabolites generated are cortisone, dihydrocortisol, dihydrocortisone, and 6β-hydroxycortisol [14]. Vezina, et al. [15] studied the pharmacokinetics of hydrocortisone in 62 newborns and infants, the total body clearance of hydrocortisone is 20.2 L/h, and the 95% coefficient interval is 15.9 and 24.5 L/h indicating that the total body clearance varies among the newborns and infants [15]. Michelet, et al. [16] studied the pharmacokinetics of hydrocortisone in 12 children, 6 infants, and 6 newborns, the total body clearance of hydrocortisone is 400 L/h, and the 95% coefficient interval is 353 and 468 L/h indicating that the total body clearance of hydrocortisone varies in children, infants, and newborns [16]. Hydrocortisone is cleared from the body by metabolism and by renal route and both elimination pathways increase with infant maturation and child development. Thus the total body clearance of hydrocortisone is higher in children, infants, and newborns than in newborns and infants. The prophylaxis with hydrocortisone has been reviewed in infants. Early low-dose of hydrocortisone prevents bronchopulmonary dysplasia in preterm infants [17], prophylactic hydrocortisone increases the survival-rate in preterm infants with bronchopulmonary dysplasia [18], decreases the mortality-rate and improves the survival-rate in preterm infants with bronchopulmonary dysplasia [19], and increases systolic blood pressure and improves oxygenation in infants with persistent pulmonary hypertension [20]. Early prophylaxis with low-dose hydrocortisone treats adrenal insufficiency in preterm infants [21], and a prophylactic dose of hydrocortisone effectively treats refractory hypotension in preterm infants [22]. The treatment of infants and children with hydrocortisone has been extensively reviewed. Early therapy with low-dose of hydrocortisone increases the survival-rate in preterm infants with bronchopulmonary dysplasia [23-26]. Administration of postnatal hydrocortisone treats bronchopulmonary dysplasia in preterm infants [27]. Early low-dose hydrocortisone treats bronchopulmonary dysplasia and does not induce adverse-effects in children [28]. Hydrocortisone should replace dexamethasone in treatment of infants with chronic lung disease [29]. The administration of hydrocortisone to infants treats early bronchopulmonary dysplasia without inducing long-term effects on either neuro-structural brain neurodevelopment and neurocognitive outcomes in children [30]. Perinatal treatment with hydrocortisone has no long-term effects on either neuro-structural brain development and neurocognitive outcomes in children [31]. Hydrocortisone 0.1% lotion effectively and safely treats mild to moderate atopic dermatitis in children [32] and hydrocortisone ointment effectively treats eczema in infants and children [33].The migration of hydrocortisone in breast-milk has been reviewed. The concentration of hydrocortisone was measured in breast-milk samples obtained from 23 lactating women, averaged to 1.6 ng/ml shortly after dosing, and is higher in the morning than in the evening [34]. The concentration of hydrocortisone was measured in breast-milk samples of 13 lactating women and ranges from 1.45 to 8.34 ng/ml [35]. The concentration of hydrocortisone was measured in breast-milk samples obtained from 7 women who delivered spontaneously and is 17.2, 16.8, and 7.4 ng/ml on day 1, 2, and 3, respectively, after delivery. In 6 women who underwent elective Caesarean section the concentration of hydrocortisone in the breast-milk is 26.5, 15.1, and 14.1 ng/ml on day 3, 4, and 6, respectively, after delivery [36]. The concentration of hydrocortisone was measured in the breast-milk samples obtained from 11 lactating women monthly up to 12 months of lactation and averages to 7.2 ng/ml but varies with the time after delivery and ranges from 0.2 to 32 ng/ml [37]. These results indicate that hydrocortisone migrates into the breast-milk in significant amounts.
In conclusion, hydrocortisone may be administered intravenously, orally, or topically and after oral and topic administration is well absorbed. In infants, hydrocortisone has been used to treat neonatal hypotension, to prevent and treat bronchopulmonary dysplasia, to treat congenital adrenal hypertension, to treat adrenal hypoplasia, and to treat Addisonian crisis. In children, hydrocortisone is used to treat adrenocortical insufficiency (Addison crisis), to treat congenital adrenal hyperplasia, adrenal hypoplasia, inflammatory bowel disease, ulcerative colitis, proctosigmoiditis, acute hypersensitivity reactions, angioedema, hypotension, severe acute asthma, and life-threatening acute asthma. The efficacy and safely of hydrocortisone have been reviewed in infants and children. Hydrocortisone is extensively metabolised into phase I and phase II metabolites and the latter metabolites are prevalent over the former metabolites. The pharmacokinetics of hydrocortisone have been studied in newborns and infants and in children, infants and newborns. The total body clearance is 20.2 L/h in newborns and infants and is 400 L/h in children, infants, and newborns. The prophylaxis with hydrocortisone has been reviewed in infants and the treatment with hydrocortisone has been reviewed in infants and children. Hydrocortisone migrates into the breast-milk in significant amounts. The aim of this study is to review the clinical pharmacology of hydrocortisone in infants and children.
Conflict of Interests
The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria.
This article is a review and drugs have not been administered to men or animals.
Acknowledgments
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.
References
- Schimmer BP, Funder JW (2018) Adrenocorticotropic Hormone, Adrenal Steroids, and the Adrenal Cortex. In The Goodman & Gilman’s. The Pharmacological Basis of the Therapeutics, BruntonHilal-dandan LL, Knollmann BC, (Eds) Mc Graw Hill, 13thEdition, USA, New York, pp. 845-861.
- Neonatal Formulary (2020) Hydrocortisone. Oxford University Press. (8thEdn), Great Clarendon Street, Oxford, OX2, 6DP UK, pp. 384-386.
- Young TE, Mangum B. NEOFAX® (2010) Hydrocortisone. Thomas Reuters Clinical Editorial Staff, (23rdEdn), Montvale, USA, pp. 296-297.
- The British National Formulary for Children (2020) Hydrocortisone. Macmillan, (78thEdn) Hampshire International Business Park, Hampshire, Lime Three Way, Basingstoke, Hampshire UK,pp.456-458.
- Zhou J, Yu Z, Chen C (2021) Hydrocortisone for Preventing Mortality and Bronchopulmonary Dysplasia in Preterm Infants with or without Chorioamnionitis Exposure: A Meta-Analysis of Randomized Trials.Am J Perinatol 38:662-668. [Crossref]
- Sushko K, Al-Rawahi N, Watterberg K, Van Den Anker J, Litalien C, et al. (2021) Efficacy and safely of low-dose versus high-dose hydrocortisone to treat hypotension in neonates: a protocol for a systematic review and meta-analysis. BMJPaedr Open5: e001200. [Crossref]
- Morris IP,Goel N, Chakrabort M (2019) Efficacy and safely of systemic hydrocortisone for the prevention of bronchopulmonary dysplasia in preterm infants: a systematic review and meta-analysis. Eur J Pediatr 178: 1171-1184. [Crossref]
- Neumann U, Whitaker MJ, Wiegand S, Krude H, Porter J, et al. (2018) Absorption and tolerability of taste-masked hydrocortisone granules in neonates, infants and children under 6 years of age with adrenal insufficiency. ClinEndocrinol (Oxf) 88:21-29. [Crossref]
- Keshavarz A, ZeinalooAA, Mahram M, Mohammadi N, Sadeghpour O, et al. (2016) Efficacy of Traditional Medicine Product Henna and Hydrocortisone on Diaper Dermatitis in Infants. Iran Red Crescent Med J18:24809. [Crossref]
- Seri I, Tan R, Evans J (2001) Cardiovascular effects of hydrocortisone in preterm infants with pressor-resistant hypotension. Pediatrics 107:1070-1074. [Crossref]
- Neunhoeffer F, Renk H, Hofbeck M, Grenz C, Haller C, et al. (2015) Safely, efficacy and response to a hydrocortisone rescue therapy protocol in children with refractory hypotension after cardiopulmonal bypass. PediatrCardiol 36:640-645. [Crossref]
- Abramovits W, Oquendo M (2010) Hydrocortisone butyrate 0.1% lipocream in pediatric patients with atopic dermatitis. Skinmed 8:72-79. [Crossref]
- Sarkar U, Rivera-Burgos D, Large EM, Hughes DJ, Ravindra KC, et al. (2015) Metabolite profiling and pharmacokinetic evaluation of hydrocortisone in a perfused three-dimensional human liver bioreactor. Drug MetabDispos43:1091-1099. [Crossref]
- Bailey E, Greaves MS, Murphy D, West HF (1966) Corticosteroid metabolism and rheumatoid arthritis.Ann Rheum Dis 25: 516-524. [Crossref]
- Vezina HE, Ng CM, Vazquez DM, Barks JD, Bhatt-Mehta V (2014) Population pharmacokinetics of unbound hydrocortisone in critically ill neonates and infants with vasopressor-resistant hypotension. PediatrCrit Care Med 15:546-553. [Crossref]
- Michelet R, Melin J, Parra-Guillen ZP, Neumann U, Whitaker JM, et al. (2020) Paediatric population pharmacokinetic modelling to assess hydrocortisone replacement dosing regimens in young children. Eur J Endocrinol 183: 357-368. [Crossref]
- Shaffer NL, Baud O, Lacaze-Masmonteil T, Peltoniemi OM, Bonsante F, et al. (2019) Effect of Prophylaxis for Early Adrenal Insufficiency Using Low-Dose Hydrocortisone in Very Preterm Infants: An Individual Patient Data Meta-Analysis. J Pediatr207:136-142. [Crossref]
- Baud O, Maury L, Lebail F, Ramful D, El Moussawi F, et al. (2016) Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet 387:1827-1836. [Crossref]
- Watterberg KL,Gerdes JS, Cole CH, Aucott SW, Thilo EH, et al. (2004) Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics 114: 1649-1657. [Crossref]
- AlsaleemM, Malik A, Lakshminrusimha S, Kumar VH (2019) Hydrocortisone Improves Oxygenation Index and Systolic Blood Pressure in Term Infants With Persistent Pulmonary Hypertension. Clin Med Insights Pediatr 13: 1179556519888918. [Crossref]
- Watterberg KL, Shaffer ML, Mishefske MJ,Leach CL, Mammel MC, et al. (2007) Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics 120:40-48. [Crossref]
- Ng PC, Lee CH, Bnur FL, Chan IHS, Lee AWY, et al. (2006) A double-blind, randomized, controlled study of a "stress dose" of hydrocortisone for rescue treatment of refractory hypotension in preterm infants. Pediatrics 117: 367-375. [Crossref]
- Shaffer ML, Baud OB, Lacaze-Masmonteil T, Peltoniemi OM, Bonsante F, et al. (2019) Effect of Prophylaxis for Early Adrenal Insufficiency Using Low-Dose Hydrocortisone in Very Preterm Infants: An Individual Patient Data Meta-Analysis. J Pediatr207:136-142. [Crossref]
- HéneauA, Guimiot F, Mohamed D, Novais ARB, Alberti C, et al. (2018) Placental Findings and Effect of Prophylactic Hydrocortisone in Extremely Preterm Infants. Pediatrics 141: 20171788. [Crossref]
- Baud O, Watterberg KL (2019) Prophylactic postnatal corticosteroids: Early hydrocortisone. SeminFetal Neonatal Med 24: 202-206. [Crossref]
- He Y, Zhang Y, Gao S, Wang X, He N, et al. (2020) Hydrocortisone to treat early bronchopulmonary dysplasia in very preterm infants: study protocol for a randomized controlled trial. Trials 21: 762. [Crossref]
- Onland W, Offringa M, Cools F, De Jaegere AP, Rademaker K, et al. (2011) Systemic Hydrocortisone To Prevent Bronchopulmonary Dysplasia in preterm infants (the SToP-BPD study); a multicenter randomized placebo controlled trial. BMC Pediatr 11: 102. [Crossref]
- Peltoniemi OM, Lano A, Puosi R, Yliherva A, Bonsante F, et al. (2009) Trial of early neonatal hydrocortisone: two-year follow-up. Neonatology 95:240-247. [Crossref]
- Rademaker KJ, de Vries LS, Uiterwaal CSPM, Groenendaal F, Grobbee DE, et al. (2008) Postnatal hydrocortisone treatment for chronic lung disease in the preterm newborn and long-term neurodevelopmental follow-up. Arch Dis Child Fetal Neonatal Ed 93:F58-F63. [Crossref]
- Rademaker KJ, Uiterwaal CSPM, Groenendaal F, Venema MMATU, van Bel F, et al. (2007) Neonatal hydrocortisone treatment: neurodevelopmental outcome and MRI at school age in preterm-born children. J Pediatr 150:351-357. [Crossref]
- Lodygensky GA, Rademaker K, Zimine S, Gex-Fabry M, Lieftink AF, et al. (2005) Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease.Pediatrics 116:1-7. [Crossref]
- Pundir S, Wall CR, Mitchell CJ, Thorstensen EB, Lai CT, et al. (2017) Variation of Human Milk Glucocorticoids over 24 hour Period. J Mammary Gland Biol Neoplasia 22: 85-92. [Crossref]
- Matheson R, Kempers S, Breneman D, Draelos Z, Johnson CE, et al. (2008) Hydrocortisone butyrate 0.1% lotion in the treatment of atopic dermatitis in pediatric subjects. J Drugs Dermatol 7:266-271. [Crossref]
- Hill LW (1955) Hydrocortisone ointment in the treatment of infantile eczema.N Engl J Med 252:1038-1039. [Crossref]
- van der Voorn B, de Waard M, Dijkstra LR, Heijboer AC, Rotteveel J, et al. (2017) Stability of Cortisol and Cortisone in Human Breast Milk During Holder Pasteurization. J PediatrGastroenterolNutr 65: 658-660. [Crossref]
- Patacchioli FR, Cigliana G, Cilumbriello A, Perrone G, Capri O, et al. (1992) Maternal plasma and milk free cortisol during the first 3 days of breast-feeding following spontaneous delivery or elective cesarean section. GynecolObstet Invest 34:159-163. [Crossref]
- Kulski JK, Hartmann PE (1981) Changes in the concentration of cortisol in milk during different stages of human lactation. Aust J ExpBiol Med Sci 59:769-778. [Crossref]







