Thyroid nodules are very frequent in the general population, with a very low occurrence of malignancy among them, so that it is a challenge to identify nodules suspected of malignancy and to make the necessary decision of conduct. A study was conducted on 517 patients of both sexes seen at the Endocrinology Outpatient Clinic of UNAERP, a secondary health service. The sample consisted of 464 women (80.97%) and 53 men (20.03%) ranging in age from 15 to 89 years (mean: 52.3 years, 51.8 years for women and 57.1 years for men). All patients had clinically diagnosed thyroid nodules, later confirmed by specific examination. A total of 1808 nodules were studied (1468 in women and 340 in men). The study was approved by the “Plataforma Brasil” Protocol 96398818.0.0000.5498 Ethics Committee and all patients gave written informed free consent to participate. We are aware of the fact that the diagnosis of malignancy for a thyroid nodule is a puzzle and that each investigative exam performed corresponds to one of its parts. The more exams we can perform, the easier it will be to reach a correct diagnosis. However, if the number of exams should be limited for economic reasons, in our opinion, the option should be for ultrasound-guided thyroid FNB associated with TI-RADS classification, despite its limitations.
thyroid nodules, thyroid benign nodules, thyroid malignant nodules, thyroid ultrasound, thyroid elastography, thyroid FNAB
The thyroid, a word whose name originates from the Greek word (tiros) which means “shield”, is the first endocrine gland to form in the human embryo, developing during the third week of intrauterine life in the direction of the caudal region, accompanying the migration of the aortic arch [1].
The embryogenesis of the thyroid is complete at about the third month of pregnancy, with the rudimentary thyroid being bilobulated and linked by a ventral isthmus located between the second and third ring of the trachea and growing laterally even after birth (Table 1). Distribution of the mean values of the laboatory parameters of thyroid function.
The thyroid has a highly organized structure that can synthesize and store large amounts of its secretion products, i.e., the thyroid hormones triiodothyronine (T3) and thyroxine (T4). The gland consists of follicles, spherical structures of 200 to 900 µm consisting of a single layer of epithelial cells – follicular cells – that limit a central lumen filled with colloid. These cells are cuboid (15 mm high in the normal status), polarized and with the apex turned towards the follicular lumen [2].
The colloid is viscous and mainly consists of one glycoprotein – thyroglobulin (Tg) – which anchors in its peptides (tyrosine) the precursors of all thyroid hormones. The colloid of different follicles may have different colors, being basophilic or acidophilic according to its composition and reflecting the activity of each follicle. Colloid reabsorption occurs in the apical portion of the cytoplasm in the form of periodic acid Schiff (PAS)-positive droplets. This region is rich in lysosomes that can process the colloidal material, disaggregating T4 and T3 from the thyroglobulin molecule [2].
The height of the follicular cells, the dimensions of the follicles and the quantity of colloid vary according to the functional activity of the gland, which is predominantly controlled by pituitary thyrotropin hormone (TSH) according to the levels of circulating thyroid hormones (negative feedback). Imbalance of the feedback axis changes the volume of the gland and the morphology of the thyroid tissue [2].
The adult thyroid contains about 3 million follicles of varying size, 300 µm on average, which form the parenchyma of the gland, and about 20 to 40 follicles form the lobules. The stroma consists of connective tissue [2].
The thyroid gland contains a second type of cells, which are globose and clear with argyrophilic granules in the cytoplasm – the so-called parafollicular cells (C cells). These cells are rich in mitochondria, do not reach the cell lumen and are responsible for the production of calcitonin, a hypocalcemia hormone that acts on the homeostasis of the calcium of the organism without feedback control by TSH [3].
About one hundred years ago, the importance of thyroid hormones was first determined for the development, growth and maintenance of quality of life of human beings. The thyroid, even more than other endocrine glands, undergoes morphological changes that accompany physiological modifications such as pregnancy and senility, and may also show alterations that do not reflect physiological variations.
The thyroid gland has a high growth potential that causes its enlargement - goiter – due to continued or repeated hyperplasia, with this condition being diffuse or nodular [4]. Multinodular colloid goiter is the type most frequently detected, being due to insufficient or excessive hormone production caused by autoimmune hyperthyroidism – Graves’ Disease –, by endemic goiter (iodine deficiency), by enzymatic defects – congenital goiter -, by inflammatory or autoimmune processes, or by the presence of neoplasia – benign or malignant nodules. This increase in volume may be asymptomatic or may provoke symptoms related to the compression of neighboring organs and, less frequently, due to gland hyperfunction [5].
Thyroid nodules are very frequent in the general population, with a very low occurrence of malignancy among them, so that it is a challenge to identify nodules suspected of malignancy and to make the necessary decision of conduct [6-9].
A study was conducted on 517 patients of both sexes seen at the Endocrinology Outpatient Clinic of UNAERP, a secondary health service. The sample consisted of 464 women (80.97%) and 53 men (20.03%) ranging in age from 15 to 89 years (mean: 52.3 years, 51.8 years for women and 57.1 years for men). All patients had clinically diagnosed thyroid nodules, later confirmed by specific examination. A total of 1808 nodules were studied (1468 in women and 340 in men). The study was approved by the “Plataforma Brasil” Protocol 96398818.0.0000.5498 Ethics Committee and all patients gave written informed free consent to participate.
The clinical exam was reviewed by a single examiner who evaluated the aspect of the cervical region, the number, size and consistency of palpable nodules, nodule adherence to other cervical structures, palpation of locoregional adenomegaly, and hoarseness [10]. All subjects were submitted to determination of TSH, Free T4, Free T3, Thyroglobulin (Tg), Anti-Tg- Antithyroglobulin, Anti TPO –antithyroperoxidase- and TRAB- Anti-TSH receptor antibody and to electrochemiluminescence, following normal reference values for adults: TSH = 0.27 to 4.5 µIU/ml; Free T4 = 0.8 to 1.9 ng/dl; Free T3 = 2.0 to 4.4 ng/ml; Thyroglobulin = 1.4 to 78 ng/ml; Anti TPO < 34 IU/ml; Anti Tg ≤ 116 IU/ml; TRAB ≤ 1.75 IU/L [11]. The association of hypothyroidism, hyperthyroidism and autoimmune thyroiditis was determined, as well as their association with thyroid nodules (TN) and their characteristics [11].
Ultrasound (US) examination was performed in patients with palpable TN or TN diagnosed during an echography exam of the cervical region due to other diseases, using a Samsung- Madison 8000 EX apparatus (Samsung Madison Co, Seoul, Korea) and the lesions were classified as solid, cystic or mixed; single or multiple, size, number, with malignancy; hypoechogenic, hyperechogenic, isoechogenic or anechoic. Their size and number were also determined. Solid and hypoechogenic TN with height greater than width and with the presence of calcifications were considered to be suspected of malignancy. Patients with 1 to 12 nodules were identified, with size ranging from 3 to about 80 mm. In patients with several TN, the dominant or suspected one was punctured. Cervical ganglia were suspected when rounded in shape, with the presence of a hilus (central hypoechogenicity) and less defined limits [8].
Calcifications inside or around or both inside and around the nodule were assessed and classified as present or absent and in terms of type [12]. Nodule vascularization was assessed and classified as absent or present and, if present, if it was at the periphery or central or in both regions. The presence of predominantly or exclusively central vascularization was considered suspected of malignancy [13-16].
The resistance elastography exam was applied to some nodules (only in 20 patients) and the results were classified as: suspected of malignancy (R ≥ 0.7) and not suspected of malignancy (R ≤ 0.4). R values between 0.5 and 0.6 were considered to be inconclusive [17,18].
US examination also permitted us to classify the nodules by the TIRADS classification, which considers as suspicious a markedly hypoechogenic nodule of imprecise limits and containing calcifications [19,20].
Ultrasound examination was used to guide FNAB of nodules larger than 10 mm, suspected of malignancy. FNAB was performed on an ambulatory basis and the material obtained was examined after staining with hematoxylin-eosin and classified according to the BETHESDA criteria. TN classified as BETHESDA 3 were submitted to another FNAB and, if necessary, to a third FNAB. TN nodules with persistency of BETHESDA 3 classification and suspicion of malignancy in other exams, and TN classified as BETHESDA 4, 5 and 6 were submitted to surgery because a molecular study was not available for them [21-27].
The excised specimens were analyzed in a pathology service using hematoxylin-eosin staining and the malignant cases detected continued to be investigated and to receive specific treatment.
Statistical analysis
Data were analyzed in terms of means and standard deviation, and graphs were obtained with the Microsoft Excel 2016 software [27-30].
We analyzed 517 patients (464 women and 53 men) with 1808 nodules, 1468 (81.20 %) detected in women and 340 (18.80 %) in men. The number of nodules ranged from 1 to 12 per patient, with a more frequent presence of 1 to 2 nodules. The mean prevalence of nodules was 3.2 nodules each for women and 6.4 nodules each for men.
Patient age ranged from 15 to 89 years (15 to 89 years among women and 20 to 89 years among men) with means of 51.8 and 57.1 for women and men, respectively (Figure 1).
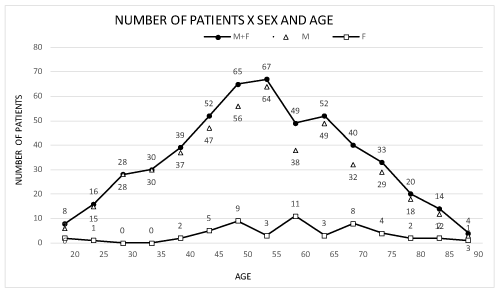
Figure 1. Patient distribution by age and sex. M: male; F: female.
Thyroid volume ranged from 3 to 276 cm3 (mean: 15 cm3, standard deviation 24.8 cm3. The number of nodules per participant increased with age from 1 to 12 (R2 = 0.44) and their mean volume ranged from 1.47 to 2.82 cm3. The older patients had more voluminous thyroid and nodules, as well as a higher mean number of nodules per gland (Figures 2-4).
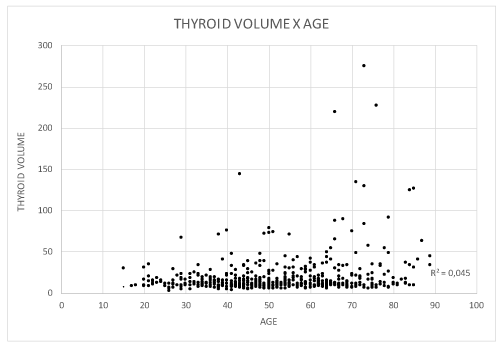
Figure 2. Patient distribution according to thyroid volume and age (years).
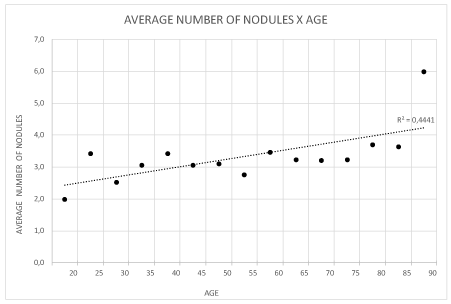
Figure 3. Patient distribution according to number of nodules and age (years).
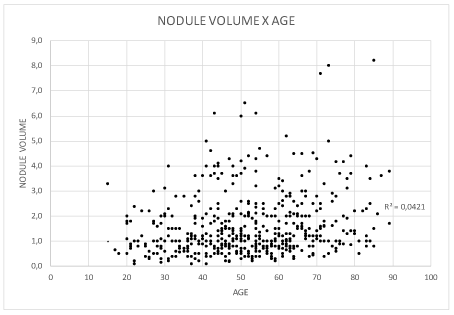
Figure 4. Patient distribution according to nodule volume and age (years).
Table 1 shows the mean values of TSH (µIU/ml), T4 (ng/ml), Anti-Thyroperoxidase Antibody (AntiTPO = IU/ml), Thyroglobulin (Tg = ng/ml), Anti-thyroglobulin antibody (Anti Tg = IU/ml) compared to the results of FNB cytology and analyzed by the BETHESDA criteria, all of them determined before the execution of any other investigative procedure (Table 1).
The highest TSH values were detected in the BETHESDA classes 6 (papilliferous carcinoma, mean TSH = 6.38 µIU/ml) and 4 (follicular neoplasia follicular, mean TSH = 5.88 µIU/ml). The lowest TSH value was detected in class 3 (inconclusive result, TSH = 0.56 µIU/ml). Classes 2 (benign) and 5 (possible papilliferous carcinoma) showed an intermediate TSH value of 2.00 µIU/ml /ml (Table 1 and Figures 4-7).
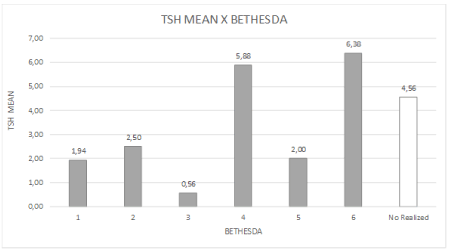
Figure 5. Distribution of mean TSH values and BETHESDA classification.
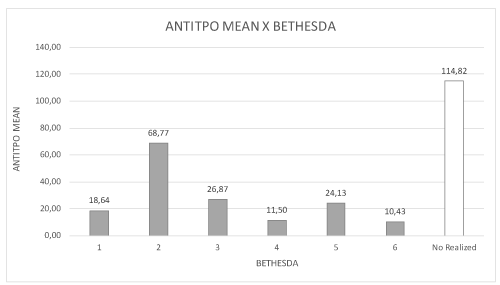
Figure 6. Distribution of mean antithyroperoxidase (Anti-TPO) values and BETHESDA classification.
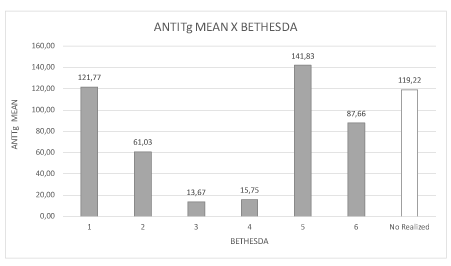
Figure 7. Distribution of mean antithyroglobulin (anti-TG) levels and BETHESDA classification.
Traditional US was applied to 517 patients with 1808 nodules, with the single or dominant nodule being classified as follows: 354 isoechoic, 151 hypoechoic, and 12 hyperechoic.
Macrocalcification was detected in 25 patient with 1 to 11 nodules ranging in volume from 1.58 to 1.74 cm3; microcalcification was detected in 24 patients with up to 8 nodules ranging in volume from1.58 to 1.78 cm3; ring-like or eggshell peripheral calcification was detected in 17 patients with up to 11 nodules ranging in volume from 1.57 to 2.15 cm3, and 5 cases of fully calcified nodules or nodules with mixed calcification (macro/micro and peripheral) were also detected. Nine nodules with microcalcification, 2 with macrocalcification, 2 with peripheral calcifications of the ring type or eggshell type and one with partial peripheral calcification were diagnosed as malignant (classical type of papilliferous carcinoma).
The power DOPPLER exam showed a predominance of CHAMMAS 3 (predominantly peripheral vascularization) and CHAMMAS 2 (exclusively peripheral vascularization) classification, both with vascular signs suggestive of benignity. Fifty-six patients were classified as CHAMMAS 1 (absence of vascularization), 93 as CHAMMAS 2 (only peripheral vascularization), 343 as CHAMMAS 3 (peripheral and central vascularization with a predominance of the former), 15 como CHAMMAS 4 (peripheral and central vascularization with the possibility of malignancy), and 6 as CHAMMAS 5 (only central vascularization suggestive of malignancy).
Fourteen patients with papilliferous carcinoma were detected and confirmed, 10 women and 4 men with a total of 19 malignant nodules and 4 patients with more than one malignant focal point. Of these patients, 3 were diagnosed as CHAMMAS 1, 2 as CHAMMAS 2, 9 as CHAMMAS 3, and 5 as CHAMMAS 4 (suggestive of malignancy).
TI-RADS classification mainly based on hypoecogenicity, undefined limits and presence of calcifications, was applied to 53 lesions with calcifications not associated with malignancy and to 14 malignant lesions. Among the nonmalignant lesions, one was classified as TR-RADS 3, 8 (15.09%) were classified as TI-RADS 4, 30 (56.60%) as TI-RADS 5, and 13 (24.54%) as TI-RADS 6. Among the 14 malignant lesions, one (7.2%) was classified as TI-RADS 3, 6 (42.8) were classified as TI-RADS 5, and 7 (50%) as TI-RADS 6. TI-RADS 5 and 6 classification indicated suspected malignancy and corresponded to 92.8% of the malignant lesions [18,19].
FNAB revealed that 13 of the 19 nodules diagnosed as malignant by anatomopathological examination were classified as BETHESDA 5 (suggestive of papilliferous carcinoma), and 6 as BETHESDA 6 (papilliferous carcinoma). Fourteen of these modules had calcifications: 8 of the micro type, 3 of the macro types and 3 of the ring types. The anatomopathological exam of the excised lesions confirmed the presence of papilliferous carcinoma in all of them. BETHESDA 2 was the predominant class in the present study (nodular hyperplasia with or without the presence of lymphocytic thyroiditis). The cytology of a female patient was repeatedly classified as BETHESDA 1 and the patient was monitored by US. Three patients were diagnosed as BETHESDA class 3 after re-puncture on three distinct occasions, with no diagnostic clarification being obtained. Due to lack of economic conditions for a molecular study, all of them opted for exeresis of the lesions.
Papilliferous carcinomas were diagnosed in 19 patients (3.69% prevalence), 14 of them women (2.97% prevalence) and 5 men (0.96% prevalence).
Thyroid nodules are very frequent in the general population, being detected in 4 to 7% of the adult population investigated by palpation, in about 30% investigated by US, and in about 60% investigated at autopsy [3]. Three to 28% of excised nodules are malignant, with carcinomas being detected in 5 to 7% of patients diagnosed with TN. The differentiated nodules originated from a follicular cell, being classified as papilliferous carcinoma, the most frequent and least aggressive, and as follicular carcinoma associated with genetic mutations and more aggressive. Anaplastic carcinoma is a considerably aggressive undifferentiated carcinoma occurring in about 1% of cases and is associated with the presence of various mutated genes. Medullary carcinoma originates from C or parafollicular cells and secretes calcitonin [1-3].
Thus, the major challenge is to determine which of these diagnosed nodules are malignant and require a specific conduct. Several investigative methods can be used to aid this clinical mission: measurement of hormones and antithyroid antibodies, standard US contemplating the echogenicity of the nodules in question, vascularization, and the presence of calcifications, the TI-RADS classification, and FNB with cytological examination of the excised material using the BETHESDA classification [6].
The mean volume of the nodules ranged from 1.47 to 2.82 cm3. The older patients had more voluminous thyroid glands and nodules, as well as a larger mean number of nodules per gland, suggesting that the increase in both thyroid volume and the number and volume of nodules is part of the process of thyroid aging (Figures 2-4).
TSH levels were higher in the BETHESDA classes 5 (probable papilliferous carcinoma) and 6 (papilliferous carcinoma; 5.88 and 6.38 µIU/ml), suggesting a possible participation of subclinical hypothyroidism in the development of thyroid malignancy. The lower gland activity may be due to changes/mutations in the synthesis chain of thyroid hormone that would favor the onset of dedifferentiation of follicular cells (Table 1).
Table 1. Distribution of the mean values of the laboatory parameters of thyroid function.
|
Mean values |
|
BETHESDA |
TSH
(uIU/ml) |
T4L
(ng/dl) |
ANTITPO
(IU/ml) |
Tg
(ng/ml) |
ANTITg |
1 |
1.94 |
1.14 |
18.64 |
39.01 |
121.77 |
2 |
2.50 |
1.23 |
68.77 |
57.69 |
61.03 |
3 |
0.56 |
1.07 |
26.87 |
207.33 |
13.67 |
4 |
5.88 |
1.04 |
11.50 |
0.40 |
15.75 |
5 |
2.00 |
1.37 |
24.13 |
42.14 |
141.83 |
6 |
6.38 |
3.49 |
10.43 |
163.00 |
87.66 |
Not done |
4.56 |
1.14 |
114.82 |
38.11 |
119.22 |
The presence of anti-TPO antibodies was more frequent in the BETHESDA 2 class (nodular hyperplasia) and the presence of antithyroglobulin was higher in classes 1, 5 and 6 (inconclusive, probable and/or papilliferous carcinoma). Hashimoto’s thyroiditis may have been present in the nodules that were not classified due to insufficient biopsy material and may also have been associated with the development of malignancy with increases anti-TG (Figure 6, Table 1).
Standard US examination of the nodules with calcifications revealed that 53 lesions were hypoechoic, 7 hyperechoic, and 7 isoechoic. Of the 14 malignant lesions with calcifications, 8 were hypoechoic (57%), 3 isoechoic (21.5%), and 3 hyperechoic (21.5%). Hypoechogenicity, as well as in definition of the limits or invasion of the capsule and/or neighboring tissues, are considered to be a signal suggestive of malignancy.
The higher vascularization of the nodule suggests a high level of cell metabolism and the possibility of malignancy. The same process is observed in thermography of the nodules whose red color refers to high temperature and possible malignancy [20-22]. When vascularization of the 55 nodules was evaluated by the Lagalla-Chammas classification, 343 were classified as Chammas 3, 93 as Chammas 2 and 21 as Chammas 4 and 5 as suspected. Of those with calcifications and malignancy, 1 was classified as Chammas 1, 1 as Chammas 2, 7 were classified as Chammas 3, 4 as Chammas 4 and 1 as Chammas 5. Vascularization did not show a correlation with signals of malignancy in this study [8,13,14].
TI-RADS classification mainly based on hypoechogenicity, undefined limits and presence of calcifications was applied to 53 lesions with calcifications not associated with malignancy and to 14 malignant lesions. Among the nonmalignant lesions, one was classified as TR-RADS 3, 8(15.09%) were classified as TI-RADS 4, 30(56.60%) as TI-RADS 5, and 13(24.54%) as TI-RADS 6. Among the 14 malignant lesions, one (7.2%) was classified as TI-RADS 3, 6(42.8) were classified as TI-RADS 5, and 7(50%) as TI-RADS 6. TI-RADS 5 and 6 classes indicated suspected malignancy and corresponded to 92.8% of the malignant lesions [18,19].
The BETHESDA classification of the lesion material obtained by FNB of the malignant nodules was B2 in 1 case, B3 in 2 cases, B4 in 1 case, B5 in five cases (35.72%) (suspected for malignancy, probable papilliferous carcinoma) , and B6 in 6 cases (42.72%, malignant, papilliferous carcinoma) [23-27].
All 12 patients were submitted to exeresis of their lesions and 3 of the 14 malignant lesions (21.42%) showed invasion of the capsule and/or neighboring tissues. None of the excised lesions showed adenomegaly containing metastases of the carcinoma.
We are aware of the fact that the diagnosis of malignancy for a thyroid nodule is a puzzle and that each investigative exam performed corresponds to one of its parts. The more exams we can perform, the easier it will be to reach a correct diagnosis. However, if the number of exams should be limited for economic reasons, in our opinion, the option should be for ultrasound-guided thyroid FNB associated with TI-RADS classification, despite its limitations.
- Bianco AC (2001) Physiology of the Thyroid Gland. (2nd edn), Thyroid Gland: Functions and Dysfunctions - Diagnosis and Treatment. Lemos, São Paulo, Brazil, pp: 33-46.
- Prates JC (2001) Anatomy. (2nd edn), Thyroid Gland: Functions and Dysfunctions - Diagnosis and Treatment. Second Edition. Lemos, São Paulo, Brazil, pp: 27-31.
- Borges MF, Abelin NMA, Toledo SPA (2001) Calcitonin - Physiology and Deficiency. (2nd edn), Thyroid: Thyroid Gland: Functions and Dysfunctions - Diagnosis and Treatment. Lemos, Sao Paulo, Brazil, pp: 47-66.
- Gidugli Neto J. (2001) Pathologic anatomy. (2nd edn), Thyroid Gland: Functions and Dysfunctions - Diagnosis and Treatment. Lemos, São Paulo, Brazil; pp: 67-79.
- Lamberg BA (1991) Endemic goiter--iodine deficiency disorders. Ann Med 23: 367-372. [Crossref]
- Alves MLD, Gabarra MHC (2017) Evaluation of Thyroid Function in a Group of recently Diagnosed Patients with Thyroid Diseases Followed up at the Endocrinology Outpatient Clinic of the University of Ribeirão Preto (UNAERP)- São Paulo, Brazil. J Endocrinol 1: 000102.
- Alves MLA, Maciel RMB, Valeri FV, Dias da Silva MR, Contrera JD, et al. (2002) Predictive Value of Clinical Examination, Ultrasound, Aspiration Cytology, and Thyroglobulin Serum in the Thyroid Node Single Nontoxic: Prospective Study of 110 Surgically Treated Patients. Bras Endocrinol Metab 46: 648-653.
- Alves MLD, Gabarra MHC (2017) Clinical, Laboratory and Ultrasound Evaluation and Aspirative Cytology of Benign and Malignant Thyroid Nodules. EC Endocrinol Metab Res 1: 119-127.
- Alves MLD, Gabarra MHC (2017) Clinical, Laboratory and Ultrasound Profile of patients with Solid and Cystic Thyroid Nodules. Open Acc J Thy Res Ther 1: 00001.
- Alves MLD, Maciel RMB, Valeri FV, Dias da Silva MR, Contrera JD, et al. (2002) Predictive Value of Clinical Examination, Scintigraphy, Ultrasonography, Aspirative Cytology, and Serum Thyroglobulin Serum in the Non-Toxic Thyroid Node: Prospective Study of 110 Surgically Treated Patients. Arq Bras Endocrinol Metab 46 (6):648-653.
- Alves MLD, Gabarra MHC (2017) Evaluation of Thyroid Function in a Group of Recently Diagnosed Patients with Thyroid Diseases Followed up at the Endocrinology Outpatient Clinic of the University of Ribeirão Preto (UNAERP) – São Paulo, Brazil. J Endocrinol 1: 000102.
- Leblowska UM, Dzieciol, Lemancecewicz D, Boguslowicz W, Lewswuk A (2004) The influence of the vascularization of the follicular thyroid nodules on the proliferative activity of the follicular cells. Folia Morphol (Wars) 63: 79-81. [Crossref]
- Foschini MP, Ragazzi M, Parmeggiani AL, Righi A, Flamminio F, et al. (2007) Comparison between echo-color Doppler sonography features and angioarchiteture of thyroid nodules. Int J Surg Pathol 15: 135-142. [Crossref]
- Lagalla R, Caruso G, Novara V, Cardinale AE (1993) Flowmetric analysis of thyroid diseases: hypothesis on integration with qualitative color-Doppler study. Radiol Med 85: 606-610. [Crossref]
- Chammas MC (2009) Thyroid nodules: evaluation with power-Doppler and duplex Doppler Ultrasound. Otolaryngol Head Neck Surg 132: 874-882. [Crossref]
- Cakal E, Schin M, Ünsal O, Güngünes A, A Kaymak E, et al. (2015) Elastography in the differential diagnosis of thyroid nodules. Ultrasound Imaging 37: 251-257. [Crossref]
- Bathia JCS, Rasaklkar DP, Lee YP, Wong KT, King AD, et al. (2011) Cystic change in thyroid nodules: a confound factor for real-time qualitative thyroid ultrasound elastography. Clin Radiol 66: 799-807. [Crossref]
- Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, et al. (2017) AT ACR Thyroid Imaging, Reporting and Data System (TI-RADAS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol 14: 587-595. [Crossref]
- Zhuang Y, Li C, Hua Z, Chen K, Lin JL (2018) A novel TIRADS of US classification. Biomed Eng Online 17: 82. [Crossref]
- Samuels BI (1972) Thermography: a valuable tool in the detection of thyroid diseases. Radiology 102: 59-62.
- Alves MLD, Andrade J, Cherri J, Takashi M, Piccinato CE, et al. (1988) Role of Thermography in the Selection of Thyroid Nodules for Surgical Indication. Arq Bras Endocrinol Metab 32: 97-99.
- Alves MLD, Gabarra MHC (2016) Comparison of power Doppler and thermography for the selection of thyroid nodules in which fine-needle aspiration biopsy is indicated. Radiol Bras 49: 311-315. [Crossref]
- Cibas ES, Ali SZ, NCI Thyroid FNA State of the Science Conference (2009) The Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol 132: 658-665. [Crossref]
- Hoang JK, Langer JE, Middleton WD, Wa CC, Hammers LW, et al. (2015) Managing incidental thyroid nodules detection imaging: ACR Incidental Thyroid Findings Committee. J Am Coll Radiol 12: 143-150. [Crossref]
- Gharib H, Papini E, Paschike R, Task AACE/AME/ETA, Task Force on Thyroid Nodules (2015) American Association of Clinical Endocrinologists, Associazone Medici Endocrinologi and European Thyroid Association Medical Guidelines for Clinical Pratice for the diagnosis and management of thyroid nodules. J Endocrinol Invest 33: 1-50. [Crossref]
- Park SY (2016) The diagnostic performance of thyroid US in each category of the Bethesda System for Reporting Thyroid Cytopathology. PLOS One 11: e0155898. [Crossref]
- Haugen BR, Alexander EK, Bible KC, Doberty CM, Mandel SJ, et al. (2016) 2015 American Thyroid Association Management Guidelines for Adults Patients with Thyroid Nodules and Diferentiated Thyroid Cancer. Thyroid 26: 1-133. [Crossref]
- Bennett BM (1976) On an approximate test for homogeneity of coefficients of variation. Contribution to applied statistics dedicated to A. Linder. Experientia 22: 169-171.
- Reed GF, Lynn F, Meade BD (2002) Use of coefficient of variation in assessing variability of quantitative assays. Clin Diagn Lab Immunol 9: 1235-1239. [Crossref]
- Moore DS, McCabe GP, Craig BA (2009) Introduction to the Practice of Statistics. (6th edn), W.H. Freeman, New York, NY.







