Drug-induced liver injury (DILI) is a rare adverse reaction to medications or herbal and dietary supplements (HDS). Several studies have shown an increase in incidence over the last decades with a disproportionate higher increase in cases connected to HDS. Even though DILI is very rare, it is one of the leading causes of acute liver failure (ALF). The pathogenesis is not entirely understood, and specific diagnostic markers are not available yet. This together with the vast number of etiologic agents and variable presentations makes diagnosing DILI challenging. The aim of this study was to determine the demographic and clinical features, the most common causal agents in Latvia, the resulting liver injury patterns, and their relationships to its severity, to improve the understanding of the disease. This analysis was a retrospective study on patients diagnosed with DILI at the hepatology department of the Latvian Infectiology Center from 2014 to 2017. Among the 128 included patients 58.6% were women, and the mean age was 54 years. In 52 cases a single drug was implicated (40.6%), in 28 cases HDS (21.9%), and in 48 cases multiple agents were suspected (37.5%). Antimicrobials were the most frequently implicated class of drugs, and the most frequent HDS were multivitamin and herbal combinations. The proportion of HDS-induced injury increased from 17.9% in 2014 to 25% in 2017; these patients had fewer comorbidities (p = 0.044), men were younger and had even fewer comorbidities than women. These findings call for more regulation and testing of freely available HDS. The main injury pattern was hepatocellular in 78 cases (66.7%), 19 cases showed a mixed pattern (16.25), and 20 cases were cholestatic (17.1%). The liver injury patterns of several etiologic agents differed from those described as their “signature” patterns found in the literature, questioning their validity. Risk factors for severe liver injury were a high number of comorbidities (p = 0.041), underlying chronic liver disease (p = 0.028), hypersensitivity reaction (p = 0.017), male gender (p = 0.050), and possibly diabetes mellitus (p = 0.389).
drug induced liver injury, liver failure, dietary supplements
Drug-induced liver injury (DILI) is a rare adverse reaction to a drug or an herbal and dietary supplement (HDS). The wide range of possible presentations, the lack of specific diagnostic markers, and a large number of potential etiologic agents make this condition very challenging to diagnose. All other etiologies of acute liver disease must be ruled out first. Therefore, it is still a diagnosis of exclusion. DILI is traditionally divided into intrinsic and idiosyncratic liver injury. Intrinsic DILI occurs in a predictable and dose-dependent fashion. The primary intrinsic agent is acetaminophen, every person exceeding a certain dose of intake will develop acute liver toxicity. Idiosyncratic DILI (iDILI) on the other hand is very rare and nearly impossible to predict. It has variable times to onset, from weeks to months, and less apparent dose dependency. This implies that susceptibility to iDILI is modified by environmental and genetic factors, but the pathogenesis is not entirely understood. Because of these difficulties, incidence rates are hard to establish. However, several studies have reported an increase in incidence in recent years with a disproportionate higher rise in DILI caused by HDS [1,2]. Even though DILI is overall a rare cause of acute liver disease, it is the leading cause of acute liver failure (ALF) in the USA [3]. Due to the relatively low level of knowledge about this disease, it is important to gather more information to find ways to reduce the incidence and to protect susceptible individuals.
This retrospective study, based on the analysis of clinical data of patients from 2014 to 2017, will provide information about DILI in Latvia, possible influences of age, sex, and comorbidities, and determine the most common causative agents as well as risk factors for severe liver injury.
The aim of this study was to analyze liver injury caused by different drugs and HDS in Latvia.
The study population included 135 patients diagnosed with DILI at the RAKUS Latvijas Infektoloģijas Centrs hepatology department from January 2014 until October 2017. The case files were examined and data on demographics, symptoms, comorbidities, blood parameters, liver biochemistry values, drugs and HDS taken, and implicated or suspected agents were collected using Microsoft Excel 2016 MSO.
The R-value was calculated in 117 patients who had both, serum ALT and ALP values available at the time of hospitalization (R = (ALT/ULN)/(ALP/ULN)) to determine the liver injury patterns. Different values of ULN were used according to gender. A hepatocellular pattern was defined as R ≥ 5, a mixed as 5 > R > 2, and a cholestatic as R ≤ 2.
The severity of liver injury was calculated for each patient according to the definitions of the International DILI Expert Working Group: “Mild” – elevated ALT or ALP levels reaching the DILI threshold but TB concentration below 2 x ULN; “Moderate” – DILI with TB concentration ≥ 2 x ULN, or symptomatic hepatitis; “Severe” – “moderate” DILI with INR ≥ 1.5.
Out of the 135 cases, seven were excluded due to insufficient data (n = 5) and intravenous drug abuse (n = 2).
The study protocol was approved by the University of Latvia Institute of Cardiology and Regenerative Medicine Scientific Research Ethics Committee and by the Science Division of RAKUS.
All variables were analyzed by descriptive statistics. The measurement of bivariate associations was done by t-tests for continuous variables and Pearson chi-squared tests for categorical variables. Analysis of variance (one-way ANOVA) was used for comparisons of groups. If variables did not follow a normal distribution, nonparametric analyses (Kruskal-Wallis-test) were performed. A two-tailed P value of < 0.05 was considered significant.
For the statistical analysis IBM SPSS Statistics version 22.0 was used.
Demographic and presenting features
The 128 patients included were treated at the RAKUS Latvijas Infektoloģijas Centrs hepatology department between January 2014 and October 2017.
One patient was 17 years old, the other patients were adults (≥ 18 years), the oldest patient was 85 years old, the overall mean age was 54 years, 44 (34.4%) patients were 65 years or older, and 75 (58.6%) were women. An independent-samples t-test was used to compare the mean age by gender. Female patients were shown to be statistically significantly older (M = 58.4, SD = 16.7) than male patients (M = 47.8, SD = 18.5); t (df = 126) = 3.40, p = 0.001.
When looking at the gender proportions within the age groups it was found that in the 20-29 years group there was a significantly higher proportion of male patients (male N = 12 (92.3%); female N = 1 (7.7%)) and the proportion of female patients was significantly higher in the 60-69 years group (male N = 6 (21.4%); female N = 22 (78.6%)), a Pearson chi-squared test was performed: χ² (df = 7, N = 128) = 25.07, p = 0.001.
Figure 1 shows two peaks of incidence in age groups 30-39 and 60-69 years in women. Men had three less pronounced peaks in age groups 20-29, 50-59, and 70-79 years. 2015 was the only year with more male than female patients, in the other years the ratio was stable (Table 1).
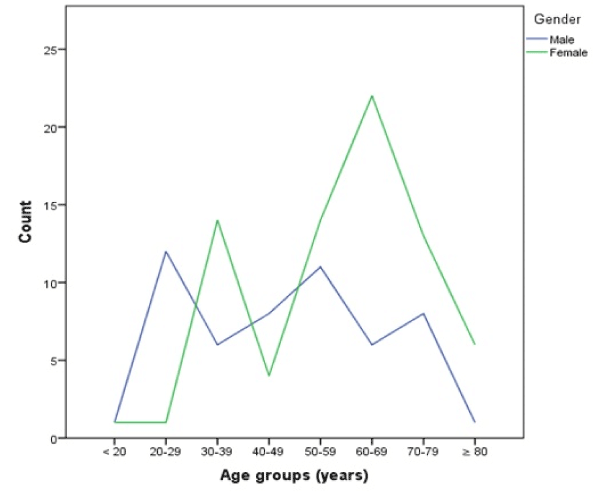
Figure 1. Case count per age group categorized by gender
Table 1. Case count and gender proportions per year.
|
2014 |
2015 |
2016 |
2017 |
Female, N (%) |
18 (64.3) |
18 (47.4) |
18 (60.0) |
21 (65.6) |
Male, N (%) |
10 (35.7) |
20 (52.6) |
12 (40.0) |
11 (34.4) |
Total, N |
28 |
38 |
30 |
32 |
HDS: Herbal and Dietary Supplement; N: Number of Cases.
Eleven (8.6%) patients had known underlying liver disease, 11 (8.6%) patients had diabetes mellitus, and two (1.6%) patients were pregnant.
Fifty-five (43%) patients had jaundice, 45 (35.2%) patients had right upper quadrant pain or discomfort, 42 (32.8%) patients had weakness, 39 (30.5%) patients had nausea, 32 (25%) patients had pruritus, 20 (15.6%) patients had hypersensitivity features (fever, rash, and/or eosinophilia), and 18 (14.1%) had no symptoms. None of the patients was HIV positive.
The serum biochemistry values (mean ± SD) first measured after hospitalization were as follows: ALT 895 ± 1031 U/L; alkaline phosphatase 261 ± 225 U/L.
Causative agents
A single drug was implicated in 52 (40.6%) cases, one or more HDS were implicated in 28 (21.9%) cases, and multiple medications or a combination of drugs and HDS were suspected in 48 (37.5%) cases. A total of 104 different drugs and 34 HDS were used by the study population. Out of these 59 drugs and 26 HDS were implicated in causing DILI.
The major classes of causative agents implicated in the “single drug” group were as follows: antimicrobials in 25.0% (n = 13), analgesics in 19.2% (n = 10), antihypertensive agents in 11.5% (n = 6), CNS agents in 9.6% (n = 5), lipid-lowering agents in 7.7% (n = 4), antineoplastic agents in 7.7% (n =4), acetaminophen in 3.8% (n = 2), and disease-modifying anti-rheumatic drugs (DMARDs) in 3.8% (n = 2). The most commonly implicated single drugs were: amoxicillin/clavulanate (n = 7), unspecified antibiotic (n = 4), ibuprofen (n = 4), atorvastatin (n = 4), alprazolam (n = 2), sulfasalazine (n =2), methimazole (n = 2), temozolomide (n = 2), and acetaminophen (n = 2). It can be noted that all patients with DILI due to amoxicillin/clavulanate, atorvastatin and methimazole were female.
The most commonly implicated HDS were as follows: multivitamin combinations (n = 7), herbal combinations (n = 7), anabolic steroids (n = 4), milk thistle (n = 3), protein powder (n = 2), and wobenzym (n = 2). Patients with DILI caused by anabolic steroids or protein powder were young (mean age 26 and 28 years respectively) and all male. Whereas all patients with DILI caused by milk thistle were female.
Overall, there was no statistically significant difference of the age of patients in the three groups of causative agents: one-way ANOVA: F (2, 125) = 0.70, p = 0.500. However, when analyzing each age group separately, it was found that a significantly higher proportion of the total of cases caused by HDS (25%) was present in the group of 20-29-year-olds when compared to cases caused by a single drug (1.9%, z-test p < 0.05). In the age groups < 20, and 40-49 years no cases were found to be caused by HDS only. Figure 2 shows two incidence peaks of HDS cases in 20-29- and 69-69-year-olds and two peaks of single drug cases in age groups 30-39 and 60-69 years. Cases due to multiple suspected agents were more equally distributed with a peak in the 50-59 years group.
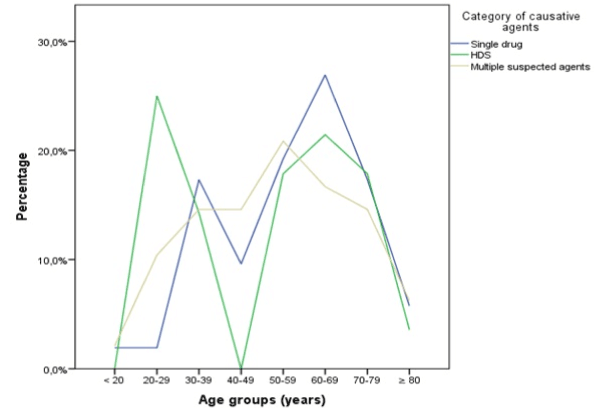
Figure 2. Proportions of causative agent categories per age group. Percentage is based on the total of each agent category.
HDS = herbal and dietary supplement
When calculating the mean age for both genders separately for each causative agent group, it can be observed that men were younger in the “HDS” and “multiple suspected agents” groups (Table 2).
Table 2. Mean age by gender and causative agent category.
|
Single drug |
HDS |
Multiple suspected agents |
Age, mean ± SD (y) |
Male |
54 ± 15 |
41 ± 20 |
47 ± 19 |
Female |
58 ± 17 |
61 ± 16 |
58 ± 16 |
Total |
56 ± 17 |
52 ± 21 |
53 ± 18 |
HDS: Herbal and Dietary Supplement; SD: Standard Deviation
It can be observed that women had a higher proportion of DILI caused by a single drug (45.3%; men 34.0%) and men a higher proportion caused by HDS (24.5%; women 20.0%) and by multiple suspected agents (41.5%; women 34.7%), but those differences were not statistically significant.
There were no significant differences in the percentages of causative agent categories implicated each year from 2014 until 2017. The proportions of causative agent categories per year are summarized in table 3. Fluctuations can be noted, but none of the three categories showed a clear upward or downward trend over the observed years.
Table 3. Causative agent categories per year.
Year |
Single drug |
HDS |
Multiple suspected agents |
2014 N (%) |
13 (46.4) |
5 (17.9) |
10 (35.7) |
2015 N (%) |
15 (39.5) |
11 (28.9) |
12 (31.6) |
2016 N (%) |
10 (33.3) |
4 (13.3) |
16 (53.3) |
2017 N (%) |
14 (43.8) |
8 (25.0) |
10 (31.3) |
Total N (%) |
52 (40.6) |
28 (21.9) |
48 (37.5) |
HDS: Herbal and Dietary Supplement; N: Number of Cases.
A statistically significant difference between the mean number of drugs patients took before the onset of DILI depending on the causative agent category was determined by one-way ANOVA: F (2, 125) = 15.92, p = 0.000. Confirmed by Kruskal-Wallis-test due to differences in SD: χ² (df = 2, N = 128) = 46.12, p = 0.000. A Tukey post hoc test showed that the number of drugs taken was significantly different between all three groups. The mean number of drugs taken also differed between women and men depending on the causative agent category (Figure 3).
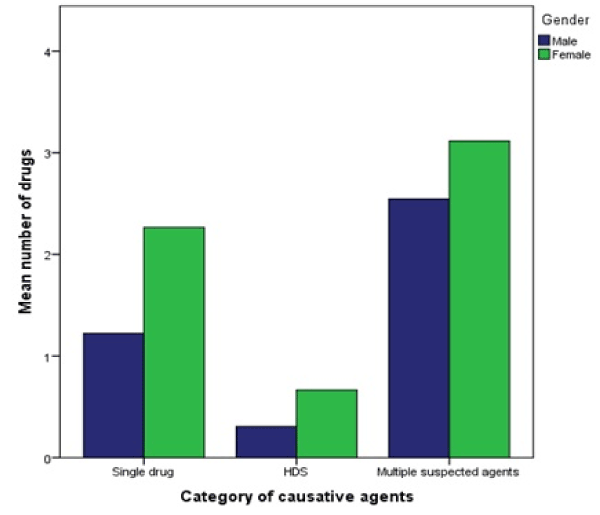
Figure 3. Mean number of drugs taken by patients depending on causative agent category and categorized by gender.
HDS = herbal and dietary supplement
The same test was used to show a significant difference between the mean number of HDS taken depending on the causative agent category: F (2, 125) = 36.27, p = 0.000. Confirmed by Kruskal-Wallis-test due to differences in SD: χ² (df = 2, N = 128) = 63.83, p = 0.000. A Tukey post hoc test confirmed the significant difference between all three groups. The mean number of HDS taken depending on gender and causative agent category differed as well.
The mean number of comorbidities differed statistically significantly between the causative agent categories – Kruskal-Wallis-test: χ² (df = 2, N = 128) = 6.24, p = 0.044, with the least comorbidities in the HDS group
(Figure 4). When looking at both genders separately, it should be noted that men with HDS-caused DILI had a mean of 0.38 comorbidities while women had 1.47. The findings of these tests are summarized in table 4.
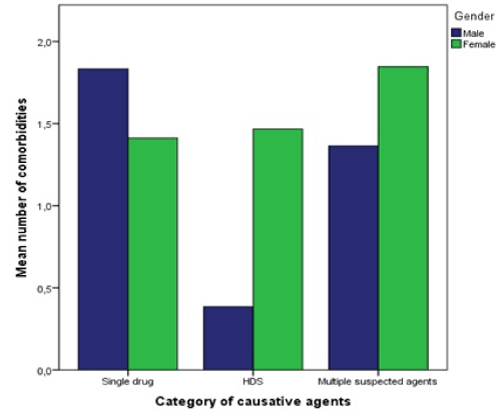
Figure 4. Mean number of comorbidities depending on causative agent category and categorized by gender. HDS = herbal and dietary supplement
Table 4. Number of drugs and HDS taken before DILI onset, number of comorbidities depending on causative agent category.
|
Single drug |
HDS |
Multiple agents |
P |
Nr. of drugs, mean (range)
Female
Male |
1.90 (1 – 10)
2.26
1.22 |
0.50 (0 – 3)
0.67
0.31 |
2.85 (1 – 10)
3.12
2.55 |
0.000 |
Nr. of HDS, mean (range)
Female
Male |
0.06 (0 – 1)
0.06
0.06 |
1.43 (1 – 3)
1.33
1.54 |
0.60 (0 – 3)
0.73
0.45 |
0.000 |
Nr. of comorbidities, mean (range)
Female
Male |
1.56 (0 – 4)
1.41
1.83 |
0.96 (0 – 4)
1.47
0.38 |
1.63 (0 – 6)
1.85
1.36 |
0.044 |
HDS: Herbal and Dietary Supplement.
An independent-samples t-test showed that women took statistically significantly more drugs than men (women M = 2.24, SD = 2.12; men M = 1.55, SD = 1.62), t (df = 126) = 2.00, p = 0.047.
There was no significant difference between the number of HDS women and men took (women M = 0.55, SD = 0.81; men M = 0.58, SD = 0.93): t (df = 126) = 0.25, p = 0.805.
Liver injury pattern
The R-value was calculated in 117 patients (91.4% of total) who had both, serum ALT and alkaline phosphatase values available at the time of hospitalization. Seventy-eight (66.7%) cases were classified as hepatocellular, 19 (16.2%) as mixed, and 20 (17.1%) as cholestatic. It can be noted that the proportion of women with a cholestatic injury pattern is higher, but with unclear statistical significance (women 22.1%, men 10.2%), χ² (df = 2, N = 117) = 3.34, p = 0.189. The mean age of patients with cholestatic injury pattern was higher than that of patients with mixed or hepatocellular injury (60, 53, 54 years, respectively), but these findings were not statistically significant: Kruskal-Wallis-test: χ² (df = 2, N = 117) = 2.20, p = 0.333 (Figure 5). The mean age of women was higher in all types of liver injury except for cholestatic injury, where both sexes had approximately the same mean age (Table 5).
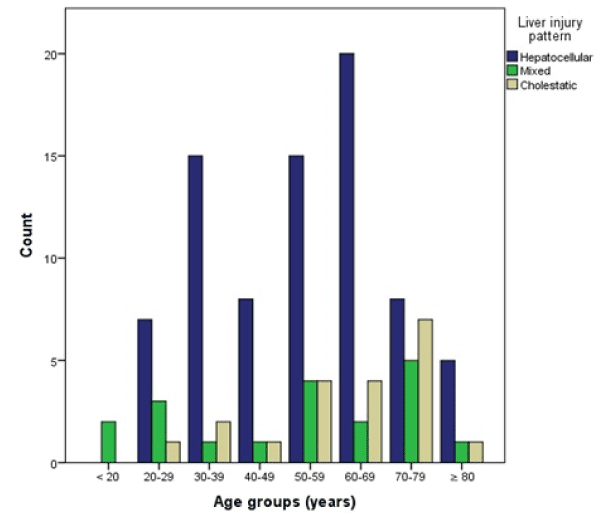
Figure 5. Case counts per age group categorized by liver injury pattern
Table 5. Mean age by gender and liver injury pattern
|
Liver injury pattern |
Hepatocellular |
Mixed |
Cholestatic |
Age, mean ± SD (y) |
Male |
49 ± 17 |
44 ± 20 |
59 ± 24 |
Female |
58 ± 16 |
62 ± 21 |
61 ± 16 |
Total |
54 ± 17 |
53 ± 22 |
60 ± 17 |
A significant relationship between liver injury pattern and severity could not be found: χ² (df = 4, N = 117) = 3.30, p = 0.510. But it can be observed that severe liver injury only occurred in hepatocellular (in 11.5%, N = 9) and cholestatic injury patterns (in 5%, N = 1).
Patients with mixed liver injury pattern had no preexisting chronic liver diseases, while 5% with cholestatic and 11.5% with hepatocellular injury patterns did.
There were no significant differences found between the injury patterns caused by the three agent categories: χ² (df = 4, N = 117) = 1.95, p = 0.746. However, HDS tended to cause less cholestatic injury (8.7%, N = 2) than single drugs (20.8%, N = 10) or multiple suspected agents (17.4%, N = 8).
When analyzing the most frequently implicated single drugs, it was found that amoxicillin/clavulanate (71.4%) and ibuprofen (75%) mainly caused hepatocellular injury, and the unspecified antibiotics all caused hepatocellular injury. Atorvastatin only caused cholestatic injury. Temozolomide only caused mixed injury and acetaminophen only hepatocellular injury.
The analysis of the most frequently implicated HDS showed that multivitamin combinations (80%), herbal combinations (71.4%), anabolic steroids (66.7%), milk thistle (100%), and protein powder (100%) mainly caused hepatocellular injury. Only some herbal combinations (28.6%) caused cholestatic injury.
Severity of liver injury
The peak serum biochemistry values (mean ± SD) were: ALT 940 ± 1093 U/L; alkaline phosphatase 266 ± 229 U/L; total bilirubin 114 ± 147 µmol/L; and INR 1.16 ± 0.29.
Twenty-four (18.8%) cases were classified as mild, 93 (72.7%) as moderate, and 11 (8.6%) as severe. The mean age did not differ significantly in the severity groups, but patients with mild DILI were younger (mean ± SD) 49 ± 18, moderate 55 ± 18, severe 54 ± 13 years. Severe DILI occurred only in the age groups from 30 to 79 years with 9 cases (81.8%) in patients between 40 to 69 years of age (Figure 6).
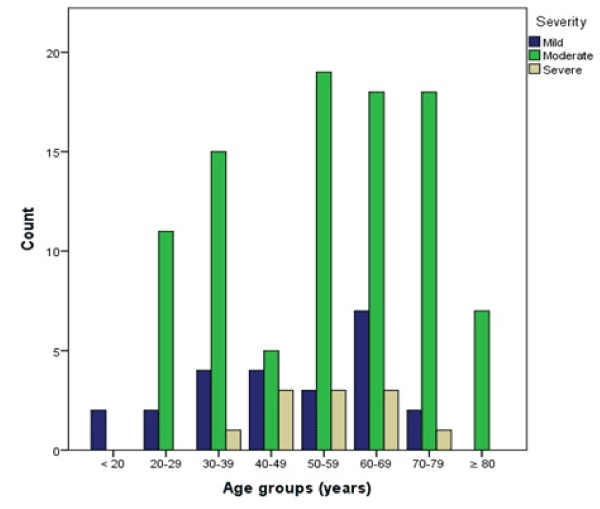
Figure 6. Case counts per age group categorized by severity
A Kruskal-Wallis-test showed that the duration of hospitalization was statistically significantly related to the severity of DILI: χ² (df = 2, N = 117) = 26.82, p = 0.000. The mean duration of hospitalization in days in patients with mild DILI was (mean ± SD) 3.83 ± 2.93, moderate 8.20 ± 6.18, severe 20.27 ± 13.29.
A Pearson chi-square test was performed, and a statistically significant relationship between gender and severity was found, χ² (df = 2, N = 128) = 5.94, p = 0.050, showing a higher severity in male patients (Figure 7). Of patients with severe DILI 72.7% (N = 8) were male and 27.3% (N = 3) were female.
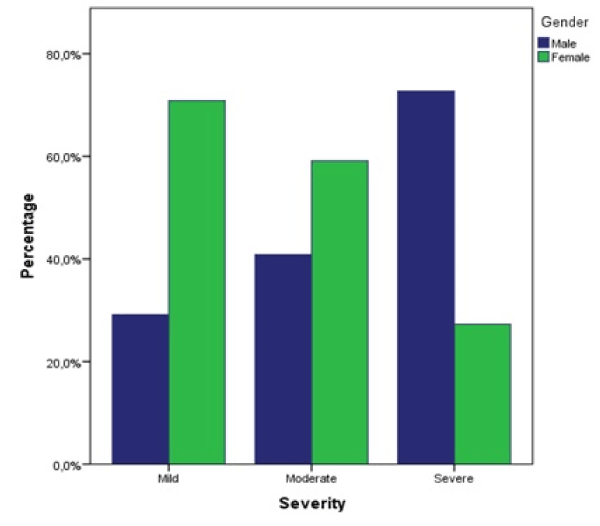
Figure 7. Proportions of severity categorized by gender. Percentage is based on total of each severity group
No significant differences in DILI severity depending on the category of causative agent were found, χ² (df = 4, N = 128) = 3.06, p = 0.549.
A significantly higher proportion of patients with severe DILI showed hypersensitivity features (45.5%) compared to mild (12.5%) and moderate injury (12.9%), χ² (df = 2, N = 128) = 8.13, p = 0.017 (Figure 8). There was a statistically significant relationship found between chronic liver disease (cirrhosis or chronic hepatitis C) and severity, χ² (df = 2, N = 128) = 7.14, p = 0.028. None of the patients with mild DILI had an underlying chronic liver disease. Of the patients with moderate DILI 8.6%, and with severe DILI 27.3% had a chronic liver disease.
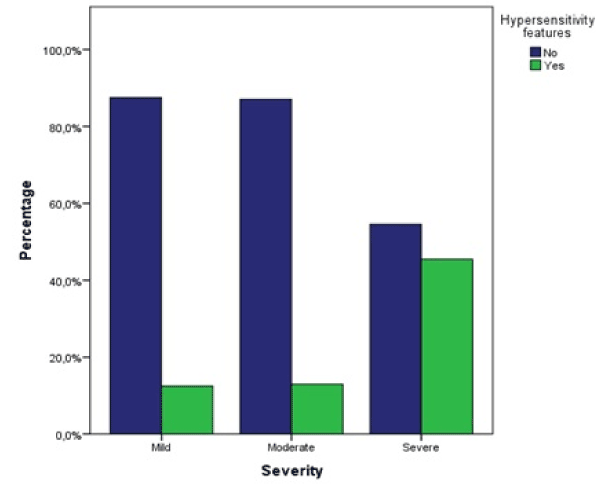
Figure 8. Proportions of hypersensitivity features (fever, rash, and/or eosinophilia) per severity groups
A statistically significant relationship between diabetes mellitus and the severity could not be shown, χ² (df = 2, N = 128) = 1.89, p = 0.389. However, there is a trend in diabetes patients towards more severe DILI. Of patients with mild DILI 4.2% had diabetes mellitus, with moderate DILI 8.6%, and with severe DILI 18.2% (Figure 9).
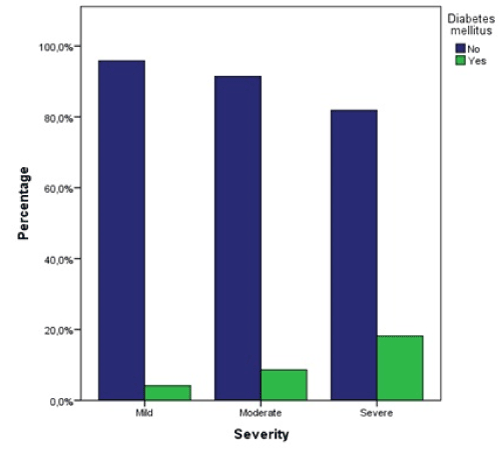
Figure 9. Proportions of diabetes mellitus patients per severity groups
A statistically significant difference between the mean number of comorbidities of patients categorized by the severity was determined by one-way ANOVA: F (2,125) = 4.56, p = 0.012. A Tukey post hoc test showed that the number of comorbidities was significantly higher in patients with severe DILI (M = 2.55, SD = 1.81) when compared to mild (M = 1.12, SD = 1.04, p = 0.010) and moderate (1.41 ± 1.32, p = 0.021). Confirmed by Kruskal-Wallis-test between moderate and severe DILI: χ² (df = 1, N = 104) = 4.19, p = 0.041. There was no statistically significant difference between mild and moderate severity (p = 0.617) (Figure 10).
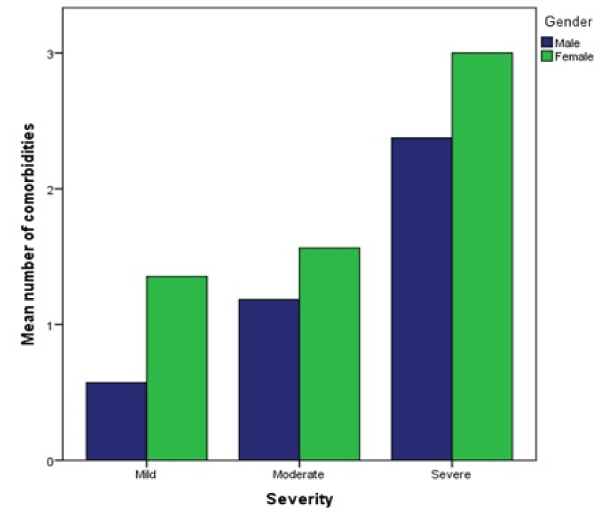
Figure 10. Mean number of comorbidities per severity group, categorized by gender
The mean number of drugs taken was highest in moderate severity and decreased in severe DILI. However, this finding was not statistically significant, Kruskal-Wallis-test: χ² (df = 2, N = 128) = 0.85, p = 0.654. It was found that the mean number of drugs in female patients was the lowest in severe DILI. Table 6 summarizes these findings.
Table 6. Mean numbers of comorbidities and mean numbers of drugs used by patients depending on severity.
|
Severity |
p |
Mild |
Moderate |
Severe |
Nr. of comorbidities, mean ± SD |
Male |
0.57 ± 0.53 |
1.18 ± 1.14 |
2.38 ± 1.92 |
|
Female |
1.35 ± 1.11 |
1.56 ± 1.42 |
3.00 ± 1.73 |
|
Total |
1.12 ± 1.03 |
1.41 ± 1.32 |
2.55 ± 1.81 |
0.041 |
Nr. of drugs, mean ± SD |
Male |
1.14 ± 0.90 |
1.66 ± 1.85 |
1.38 ± 0.74 |
|
Female |
2.06 ± 2.25 |
2.33 ± 2.11 |
1.67 ± 2.08 |
|
Total |
1.79 ± 1.98 |
2.05 ± 2.02 |
1.45 ± 1.13 |
0.654 |
SD: Standard Deviation.
Demographics
In this study, there was a clear predominance of female sex among patients (58.6%) and a higher mean age of women (58.4 years). The overall mean age was 54 years. These results correspond to the findings of most other DILI studies [1,4-7]. However, a Spanish study covering ten years from 1994 to 2004 showed no differences in gender distribution but a higher predominance of male patients at older ages, which are results opposite to those found in this study [8]. The reason why the number of male patients doubled in 2015 compared to 2014 and nearly halved again in the next year could not be determined.
Causative agents
The study results are consistent with other reports in implicating antimicrobials as the most frequent agent class, but with 25.0% of single-drug cases, the proportion was lower than the 45.5% found by Chalasani et al. [5]. This difference could be due to higher consumption of antibiotics in the USA. Amoxicillin-clavulanate was the most frequent single agent causing DILI as found by most other studies. However, all those patients were women. This result is surprising because other studies showed a clear male predominance with sex ratios of up to 4:1 (male:female) [9,10]. A possible reason for this could be a higher frequency of amoxicillin-clavulanate prescriptions for women in Latvia, but no data in the literature could be found confirming this hypothesis. Atorvastatin and methimazole caused DILI also occurred only in women in this study.
When comparing the next most frequent agent classes with other studies, it stands out that a higher proportion of DILI in this study was caused by analgesics, antihypertensive, lipid-lowering, and antineoplastic agents; CNS agents and DMARDs caused fewer cases than in reports from Spain, Iceland, and the USA [1,5,8]. These findings may indicate less use of CNS agents and DMARDs in Latvia.
The herbal supplement “milk thistle” (Silybum marianum) which was implicated in three cases must be considered most likely falsely implicated. There are no reported cases of it causing liver injury, on the contrary, it is used as a supplement for chronic liver diseases treatment. Still, if in future research more cases likely caused by milk thistle will be discovered this statement has to be reevaluated.
The proportion of cases caused by HDS in this study over the whole four years is on the same level as in other reports from European countries and the USA. There is no apparent yearly increase in HDS-caused cases as observed by other studies [3]. Nevertheless, the proportion of HDS cases changed from 17.9% in 2014 to 25% in 2017. It can be suspected that the low percentage of 2016 (13.3%) may not be the correct number as the proportion of multiple suspected agents was far higher (53.3%) than in the other years. So, an improved causality assessment might have shown a higher actual number of HDS-caused cases in 2016.
The most frequently implicated HDS were multivitamin and herbal combinations which is consistent with other reports [3,5]. Based on these findings, more regulations should be implemented regarding the development, testing, production, and supply of HDS, as well as public health education to decrease the number of unnecessarily consumed or dangerous HDS, to reduce the incidence of HDS-DILI. The identification of harmful substances in the mostly multi-ingredient HDS will be a difficult challenge for future research.
The reasons for taking HDS can be speculated on by looking at the results. Men with HDS-caused DILI were younger and healthier (fewer comorbidities and fewer medications were used) than women, and only men suffered from anabolic-steroid- and protein-powder-induced liver injury. Therefore, it can be suspected that men more frequently take HDS for bodybuilding and performance enhancement at a younger age, while women more frequently use HDS as an addition or substitution for medications to treat various diseases, or to increase their health at an older age.
Liver injury pattern
This study showed an approximately 10% higher proportion of hepatocellular liver injury (66.7%) than found by studies from Spain and the USA [4,8], but was consistent in cholestatic injury being the second largest and mixed injury the smallest proportion.
Patients with cholestatic injury were found to be older which is also consistent with the two mentioned studies. But, in this study, 75% of patients with cholestatic injury were women, while the other studies reported 50% or fewer women in the cholestatic groups [4,8]. Hypersensitivity features were most frequent in cholestatic injury which differs from the results of the Spanish study [8], where patients with mixed injury had the highest frequency of hypersensitivity features. Jaundice was surprisingly only registered in 50% of patients with cholestatic injury and in 57.9% of patients with mixed liver injury, while the peak TB measured was highest in cholestatic injury. This discrepancy may be due to mistakes during data collection from the handwritten case files, where jaundice may have been missed. The Spanish report showed the highest peak TB levels in cholestatic injury and also the highest frequency of jaundice in patients with cholestatic liver injury [8].
This study found like Chalasani et al. [4] that none of the patients with an underlying chronic liver disease developed mixed liver injury. However, these differences are not statistically significant and may be coincidental. The Spanish report showed a small proportion (3%) of patients with mixed injury having an underlying liver disease [8].
When comparing the patterns of liver injury caused by the most frequently implicated agents with the signature patterns listed on livertox.nlm.nih.gov some discrepancies can be noted.
Table 7 shows the different and similar findings. These differences indicate that not the causative agent alone is responsible for the presenting pattern of liver injury, but most probably a combination of individual host factors, environmental factors, and properties of the causative agent.
Table 7. Pattern of liver injury of most frequent causative agents compared with typical patterns provided by the livertox.nlm.nih.gov database. Colored patterns differ
Causative agent |
Typical pattern on “livertox” |
Pattern found in this study |
Amoxicillin/clavulanate |
Cholestatic |
Mainly hepatocellular |
Ibuprofen |
Any pattern |
Only hepatocellular |
Atorvastatin |
Any pattern |
Only cholestatic |
Alprazolam |
Cholestatic or mixed |
Hepatocellular and cholestatic |
Sulfasalazine |
Mixed, cholestatic or hepatocellular occurred |
Hepatocellular and mixed |
Methimazole |
Cholestatic or mixed |
Cholestatic and mixed |
Temozolomide |
Cholestatic or mixed |
Only mixed |
Multivitamin combinations |
Hepatocellular |
Mainly hepatocellular |
Herbal combinations |
Hepatocellular |
Mainly hepatocellular |
Anabolic steroids |
Cholestatic |
Hepatocellular and mixed |
Severity of liver injury
Compared to the American report smaller proportions of mild and severe DILI and a bigger proportion of moderate DILI occurred in this study [4].
The mean age did not differ significantly in the severity groups, a finding consistent with the Spanish study. But in this study patients with mild liver injury were younger, while the American study found a younger age of patients with severe DILI [4,8]. It should be noted that in this study 82% of severe DILI cases occurred in patients between 40 to 69 years of age.
A surprising finding is the high proportion of men in patients with severe DILI (72.7%). Both other mentioned studies found more women than men with severe injury.
In patients with severe DILI the mean ALT level was higher at the time of hospitalization (+ 740 U/L) and the peak (+ 621 U/L) when comparing this study with the American report; mean ALP levels were lower (- 148 U/L at hospitalization, - 249U/L at peak) [4]. These findings are due to the difference in injury patterns. Here 90% of patients with severe liver injury presented with a hepatocellular pattern and only 10% with a cholestatic pattern; in the American study it was 69% and 19% respectively, and 12.5% presented with a mixed injury pattern. Interestingly there were no patients with severe DILI due to a mixed injury pattern in this study.
Another difference is the high proportion (18.2%) of severe DILI caused by HDS in this study, only 2% HDS cases were found in the American report. It must be kept in mind, however, that the American study is from 2008 and the HDS consumption has increased in the USA since then, but not enough to explain the big difference found here [2]. Also, mild and moderate cases in the American study had an over three times higher proportion of HDS than severe cases. The proportions in this study were approximately equal in moderate and severe DILI but lower in mild cases [4].
The reasons behind these differences are not known, possibilities could be different habits of drug or HDS consumption in the two populations, different ethnicities, or different environmental factors.
It was found, like in the American report, that there is a relationship between underlying chronic liver disease or diabetes mellitus and severe liver injury. Chronic liver disease showed a statistically significant relation to severe DILI (p = 0.028). Diabetes mellitus did not show a significant association, but there was an apparent tendency towards more severe DILI (p = 0.389) [4].
The number of comorbidities was statistically significantly higher in patients with severe DILI (p = 0.041), and even higher in women. Interestingly the number of drugs taken by women was decreasing in severe liver injury. As mentioned in section 2.3.2 all the variables in this paragraph which led to severe liver injury in this study are known risk factors for the development of DILI. The fact that women took fewer drugs while having more comorbidities in the “severe” group implies less controlled diseases in those patients leading to more inflammatory or other pathologic processes which could have a negative impact on the severity of DILI [11]. However, these are only assumptions, and further research would be needed to confirm or disprove them.
The finding that hypersensitivity features are statistically significantly higher in severe DILI (p = 0.017) could not be compared with other studies because no similar tests were found in the literature. It can be hypothesized that a more pronounced hypersensitivity reaction (allergic or autoimmune) can lead to a more severe course of DILI.
Also, the statistically significantly higher rate of anemia in severe DILI (p = 0.006) could not be compared. There are two possible explanations for this finding: 1) The patient already had anemia before the onset of DILI, and it led to more severe injury; 2) The severe liver injury caused anemia. The fact that the INR was increased in patients with anemia suggests that at least in some of those patient’s severe liver injury led to anemia. But anemia patients also had a higher rate of underlying chronic liver disease which could be the cause of anemia as well. Without further investigation, there is no way of telling which of the two explanations is more likely or if both are true.
Limitations of this study include the retrospective study design. In the future, a prospective study could provide more specific anamneses of patients to acquire more information about duration, dosages, and precise names of used drugs and HDS. This way latencies from the start of exposure to onset of DILI could be calculated as well. Also, the reasons for HDS consumption could be evaluated. A follow up over several months would provide information about the outcome.
The large group of “multiple suspected agents” reduced the accuracy of the results of the correctly implicated agents. A standardized approach to causality assessment (e.g., RUCAM scale) could improve the confidence in finding the correct agent causing DILI in each patient. So, the group of “multiple suspected agents” would become as small as possible to gather more information about the implicated agents, the liver injury patterns they most frequently cause, and their outcome.
1) The 128 patients included in this study from 2014 until 2017 had a mean age of 54 years and a female predominance of 58.6%. Female patients were significantly older than male patients. The main symptoms were jaundice in 43%, right upper quadrant pain or discomfort in 35.2%, weakness in 32.8%, and nausea in 25%.
2) In 52 cases a single drug was implicated (40.6%), in 28 cases HDS (21.9%), and in 48 cases multiple agents were suspected (37.5%). Cases caused by HDS increased from 17.9% in 2014 to 25% in 2017.
3) Antimicrobials were the most frequently implicated class of drugs (25% of single drug cases), followed by analgesics (19.2%) and antihypertensives (11.5%). Amoxicillin/clavulanate was the most common causative single drug (n = 7). The most frequently implicated HDS were multivitamin (n = 7) and herbal combinations (n = 7).
4) The main injury pattern was hepatocellular in 78 cases (66.7%), 19 cases showed a mixed pattern (16.2%), and 20 cases were cholestatic (17.1%).
5) Patients with HDS-DILI had fewer comorbidities (p = 0.044), men were younger and had fewer comorbidities than women. All patients with DILI due to anabolic steroids or protein powder were male, and all due to amoxicillin/clavulanate, atorvastatin, and methimazole were female. The patterns of liver injury caused by each of the most frequently implicated agents differed from the literature in three out of ten. Patients with cholestatic injury were older than patients with other injury patterns, and 75% were women. Age did not have an impact on severity, but 82% of severe cases occurred at 40 to 69 years of age.
6) Risk factors for severe liver injury were a high number of comorbidities, especially uncontrolled (p = 0.041), underlying chronic liver disease (p = 0.028), hypersensitivity reaction (p = 0.017), male gender (p = 0.050), and possibly diabetes mellitus (p = 0.389).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S (2013) Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 144: 1419-1425, 1425 e1411-1413; quiz e1419-1420. [Crossref]
- Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, et al. (2017) Liver injury from herbal and dietary supplements. Hepatology 65: 363-373. [Crossref]
- Lee WM (2013) Drug-induced Acute Liver Failure. Clin Liver Dis 17: 10.1016/j.cld.2013.1007.1001. [Crossref]
- Bell LN, Chalasani N (2009) Epidemiology of idiosyncratic drug-induced liver injury. Semin Liver Dis 29: 337-347. [Crossref]
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, et al. (2008) Causes, Clinical Features, and Outcomes from a Prospective Study of Drug-Induced Liver Injury in the United States. Gastroenterology 135: 1924-1934.e1924. [Crossref]
- De Valle MB, Av Klinteberg V, Alem N, Olsson R, Björnsson E (2006) Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment Pharmacol Ther 24: 1187-1195. [Crossref]
- Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, et al. (2002) Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology 36: 451-455. [Crossref]
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, et al. (2005) Drug-Induced Liver Injury: An Analysis of 461 Incidences Submitted to the Spanish Registry Over a 10-Year Period. Gastroenterology 129: 512-521.
- Gresser U (2001) Amoxicillin-clavulanic acid therapy may be associated with severe side effects - Review of the literature. Eur J Med Res 6: 139-49. [Crossref]
- D Larrey, T Vial, A Micaleff, G Babany, M Morichau-Beauchant, et al. (1992) Hepatitis associated with amoxycillin-clavulanic acid combination report of 15 cases. Gut 33: 368-371. [Crossref]
- Lee SJ, Lee YJ, Park KK (2016) The pathogenesis of drug-induced liver injury. Expert Rev Gastroenterol Hepatol 10: 1175-1185. [Crossref]










