Background: The sacrum is an infrequent localization of tumours. Despite the modern spinal surgical techniques, sacral tumours still are an important challenge for surgeons. This study aimed to describe the clinical course and prognosis of surgically treated patients with sacral tumours.
Material and methods: A retrospective analysis from patients with sacral tumours seen from 2011 to 2017 at the National Cancer Institute, Mexico is presented. Multiple clinical variables were evaluated.
Results: A total of 13 patients were included in the study. The most common primary histologic type was chordoma, followed by chondrosarcoma and giant cell tumours.. The mean follow-up time was 51.4 months, mean progression-free survival was 31.6 months, 54% of the patients had complications related to surgery. At the end of the study, 54% of patients were still alive, with a mean overall survival of 51.3 months.
Conclusion: Sacral tumours are a complex disease with a high surgical complication rate, elevated recurrence, and frequent neurologic and functional sequelae. We present a case series of 13 patients surgically treated with a 54% complication rate.
sacral tumours, sacrectomy, spinal tumours
ST-Sacral Tumours; QT-Chemotherapy; RT-Radiotherapy; PFS-Progression-free survival; OS-Overall surveillance; FT-Following time; SE-Standard error; ILR-Iliolumbar reconstruction; OS-Overrall NA; WI-Wound infection; FT-Following time; SD-Standard deviation; CV-Coefficient of variation
Introduction
Sacral tumours (ST) are infrequent neoplasms with an incidence from 1–7 % of the spinal tumours [1]; ST may have benign or malignant behaviour, and the treatment is complex due to their elevated morbidity and mortality. Most of the time, the diagnosis is difficult and late by its vague and nonspecific course. Among the malignant ST, chordoma is the most frequent, followed by chondrosarcoma and giant cell tumour [2]. ST with metastatic origin mainly from breast, lung, or prostate tumours [3]. Due to the malignant behaviour of sacral tumours in its majority, it is well known that the natural history of sacral tumours is disease progression in a short time with the involvement of iliac bones, pelvis, abdominal viscera, and femur and all of this diminishes global survival, with a great impact in life quality during this survival period.
To relieve pain, improve nerve function, control local tumours, and improve quality of life for patients, surgery is widely performed, including minimally invasive surgery, palliative surgery, or radical surgery. Due to its poor response to chemotherapy (QT) and radiotherapy (RT), the treatment of choice for ST is total or partial sacral surgical resection [4-6]. However, despite modern spine surgery techniques, ST surgery still is an important challenge associated with high morbidity or severe neurological deficit, so the current challenge lies in the preservation of neurological function. Given the important role of radical resection in survival, reconstruction should be planned to achieve stability [6], without forgetting that quality of life must be one of the most important goals of treatment [7]. An essential aspect of surgical treatment is staging; there are two staging systems widely used for primary tumours of the spine, the Enneking staging system, and Weinstein-Boriani-Biagini classification. Both of them aid the surgeon to make an appropriate surgical decision. On the other hand, there are 3 important considerations in the surgical management of sacral tumours, the preservation and maximization of neurologic function, protection of ventral abdominal and pelvic structures, and lumbopelvic fixation [1]. In unresectable or recurrent tumours, non-surgical strategies have been applied in the management of select ST, like RT, cryoablation [8], or the use of nuclear medicine [9], each one with variable results. To date, surgical excision with wide resection margins offers the best long-term prognosis but it is associated with long-term functional compromise and mechanical instability [10]. Thus, it is important to identify other clinical or surgical characteristics in our patients to improve their outcomes. This study aimed to describe the clinical course and prognosis of surgically treated patients with sacral tumours.
A retrospective case series study was made from 2011-2017 in the National Cancer Institute, Mexico City, Mexico. Adult patients with a diagnosis of primary or metastatic ST were included, they were subjected to sacral surgery without previous treatment, incomplete expedients were excluded. The following variables were evaluated in our patients: Age, genre, histology of the tumour, clinical characteristics, surgical technique, surgically related complications, progression-free survival (PFS), overall surveillance (OS), and following time (FT). Finally, two representative cases were exposed.
Surgical Methods
Total sacrectomy can be performed by anterior or posterior approaches. The anterior procedure is an intraperitoneal approach used to expose the anterior surface to ligate the main tumour vessels, and to perform an anterior partial sacrectomy. After this, the rectus abdominis myocutaneous flap, based on the inferior epigastric vessel, is prepared, and a posterior sacrectomy is performed, dividing all sacral nerve roots in the thecal sac. After complete en bloc extirpation of the sacrum with the tumour, spinopelvic reconstruction and closure with a myocutaneous flap are performed. Spinopelvic reconstruction is undertaken using a modified Galveston technique or dual iliac screw fixation combined with posterior lumbar segmental fixation. These provide a long lever arm within the ilium to counteract the forces exerted by the lumbar spine. In the posterior approach, a spinolaminar and sacrococcygeal dissection is performed, the total or partial sacrectomy is achieved, and finally, iliac screw fixation combined with posterior lumbar segmental fixation is performed. The posterior approach is preferred by our group because complete resections can be yielded with fewer complications like bleeding, vascular injury, intestinal perforation, and postoperative recovery is better than the anterior approach.
Statistical analysis
Descriptive analysis was performed with means SE (standard error) or percentage as appropriate in each case.
A total of 13 patients were included in the study, of which 11 were primary tumours and 2 metastases. 8 cases were male, 5 female, and the mean age was 39.3 years. The most frequent tumour was chordoma (46%), followed by chondrosarcoma, fibrous solitary tumour, Ewing sarcoma, and giant cell tumour. The metastatic tumours were desmoplastic medulloblastoma and follicular thyroid carcinoma. 53% of the patients presented as first related symptom axial lumbosacral pain, two patients (15%) presented with new-onset constipation as the first symptom, in all the patients’ total resection of the tumour was achieved partial sacrectomy was performed in the 61.5% of the population, total sacrectomy in 38.5% of patients, and 15.3 % an iliolumbar fixation was required. Mean bleeding was 2292 mL (500-6000 mL). 84.6% of cases presented IIb stage according to the Enneking staging system and the presurgery Karnofsky scale was 90% or more in 61.5% of the patients. Post-Surgery Karnofsky at 6 months was 90% or more in 53.8% of the cases. The postsurgery ECOG score was the same or lesser than pre-surgery (except for the deceased patients). All surgeries were performed by posterior approach. Partial sacrectomy was performed in 61.5% of the population, and an iliolumbar fixation was conducted in 15.3%. The mean surgery time was 4.73 hours.
Surgery complications were present in 53.8% of patients, two patients (15.3%) died during the first week after surgery from bleeding, more precisely due to hypovolemic shock in the Intensive Care Unit, and three more patients presented bleeding with hypovolemic shock without long-term complications. The most common post-surgery complication was hypovolemic shock with 30.7%. 7.6% of patients presented cerebrospinal fluid leak, and 15.38% wound or soft tissue infection (WI) (Table 1). The mean follow-up time was 51.4 months, mean progression-free survival was 31.6 months.
Table 1. Sacrectomy cases. The cases are exposed in chronological order. ILR: Iliolumbar reconstruction; PFS: Progression free survival; OS: Overall NA (Does not apply); WI: wound infection
Representative cases
- Case 1 (Case 7 in table 1) In 2007 a 53-year-old male without relevant medical history, presented severe lumbosacral pain without irradiation, MRI was carried out. A tumor involving the inferior half of the sacrum was observed. A partial sacrectomy was performed, and the patient evolved without neurological deficit or complications. 70 months later, it was observed to recur with left sacroiliac joint involvement (Figure 1). A total sacrectomy with Galveston technique reconstruction was performed (Figure 1). The patient exhibited complications after surgery such as hypovolemic shock and an enterocutaneous fistula, which was resolved by colostomy. Two months after surgery, S2-S4 hypoesthesia and loss of sphincter´s control were evident. To date, there is no evidence of tumour recurrence.

Figure 1. Representative case 1. A, B, C: MRI of the sacrum in diverse sequences showing a solid tumour in the inferior half of the sacrum and left sacroiliac joint enhanced with gadolinium administration. D: Postsurgical pelvic anteroposterior radiography. It is evident the absence of sacrum and coccyx, Iliolumbar reconstruction with the Galveston technique can be appreciated
- Case 2 (Case 5 in table 1) A male of 18 years old with a history of desmoplastic medulloblastoma in the fourth ventricle and subtotal surgical resection (QT, and RT 36 + 20 Gy), was followed up. Two years after brain surgery, he started with paraesthesia, pain in the right L5 territory, saddle hypoesthesia, and constipation. Lumbosacral MRI was carried out and a solid tumour in the inferior sacrum was evident, fractionated RT 30 Gy to sacrum was done. Progression of the disease at sacrum occurred one year later. A partial sacrectomy was performed (Figure 2), no complications were observed, progression was evident three years later. Six months later the patient died due to systemic dissemination of the disease.
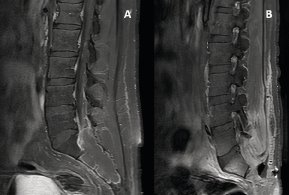
Figure 2. Representative case 2. A: Presurgery MRI showing a solid tumour in sacrum and coccyx enhanced by gadolinium administration. B: Postsurgical MRI showing no evidence of residual tumour
Partial or total sacrectomy is the procedure of choice to treat most sacral tumours. However, it’s a surgery with a high risk of complications and mortality, besides a local elevated recurrence rate [1,2,11,12]. The most frequent ST in our case series was chordoma, which correlates with previous studies reporting this one as the most frequent [13-15]. On the other hand, chondrosarcoma is one of the most common malignant tumours arising from the sacrum. Although chondrosarcoma is a low to intermediate-grade malignancy, it is invasive and has a high rate of local tumour recurrence [16]. In our study, just one patient had chondrosarcoma with a PFS of 79 months after a total sacrectomy. Giant cell tumours were found just in one case, although are reported as one of the most common benign sacral tumours and as highly recurrent in many studies [11,17,18]. Ewing sarcoma is not common but is diagnosed frequently at a younger age (in the first two decades). Our hospital provides care only for adult patients, and the youngest was 18 years old. He was treated with partial sacrectomy and showed recurrence 18 months later. On the other hand, we found one patient with PFS of 22 months and OS of 66 months derived from follicular thyroid metastasis carcinoma, which is rare by the few cases of this nature reported in the
literature with sacral lesions [18]. Pan, et al. reported that factors like an age > 60 years, distant metastases, and nonsurgical therapy were independent risk factors for survival reduction [19]. Locoregional recurrence affects > 50% of patients [20,21], and in dedifferentiated chordomas, characteristics such as tumour diameter >10 cm, marginal resection, and sacroiliac joint infiltration may be associated with increased risk of local recurrence and mortality. In this study, our patients were <60 years, predominantly men, with good presurgical Karnofsky, without identified metastases and all were treated surgically.
Patients who developed local recurrence had a PFS between 14 and 70 months, which is common on this type of tumour [22]. An important surgical goal in sacrectomy is to preserve the sacral nerves as much as possible, without compromising clear surgical margins, besides it is known that an adequate wide margin can improve the survival rate [10,23,24]. In parallel, maintaining the sphincter function gives satisfactory results from the patient’s perspective, it has been reported S3 nerve root is crucial to maintain the neurophysiology of bowel, bladder, and sexual function, and conservation of at least one S2 during surgery might benefit the patients [25,26]. Preoperative conditions of the bladder, bowel, motor functions, level of sacral tumour involvement, and corresponding level of sacrectomy predict their long-term functionality after surgery [27]. Higher resections have more pain and loss of physical function in comparison to patients with lower resections [26]. Two of the patients died due to hypovolemic shock. This is the greatest risk of the surgery, because of the high vascularity density of the region, composed by the iliac arteries and veins, presacral venous plexus, and arteries. A limitation of the posterior-only approach is inadequate visualization of these many vessels resulting in a possible unrecognized injury, an aggregate to this problem is the loss of normal anatomy and neovascularization of the tumour. Among the literature, the recommendation is previous arterial embolization of the tumour to prevent bleeding, due to the fact that it is an expensive procedure many of the patients refused to perform it. The main predictors in mortality and bleeding are the size and invasion of the tumour, the type of resection, and if it was treated previously with arterial embolization. On the other hand, there are few publications studying mortality and bleeding, the publications that report these adverse events had similar incidence rates (3.7-17.6%) [28-30]. As for the complexity and morbidity of this surgery, it is of most value to a highly trained multidisciplinary team to achieve the best outcome for these patients.
In our case series, case number 1 exposed above in representative cases, after total sacrectomy presented alterations in sensation, strength in lower limbs, and sphincters. Iliolumbar reconstruction was performed in two cases because they underwent type 2 resections and the instability of the spine was highly probable; according to the literature patients with type I resection do not require reconstruction [31]. None of our patients were reoperated for reconstruction. In most cases, in our study, the first ST-related symptom was axial lumbosacral pain, which is nonspecific and of late diagnosis. After surgery patients manifested bleeding frequently, even though it was lower than other mean bleeding volumes reported in the literature [27], however, it caused the death of two patients. WI was a common post-surgery problem, but it did not interfere with the hospital’s discharge. The PFS and OS were similar to previously described in the literature in each case depending on the tumour type [20].
ST is a challenging disease for the surgeon, most of the time they are recurrent, and the patient courses with neurological or mechanic sequelae. The vague symptoms and clinical course make the early diagnosis difficult. The PFS and OS depend on the biology of the tumor, and iliolumbar reconstruction is required in few cases with generally good mechanical results. More experience is needed to improve the outcomes of patients with ST.
- Stephens M, Gunasekaran A, Elswick C, Laryea JA, Pait TG, et al. (2018) Neurosurgical Management of Sacral Tumors: Review of the Literature and Operative Nuances. World Neurosurg 116: 362-369. [Crossref]
- Bederman SS, Shah KN, Hassan JM, Hoang BH, Kiester PD, et al. (2014) Surgical techniques for spinopelvic reconstruction following total sacrectomy: A systematic review. Eur Spine J 23: 305-319. [Crossref]
- Sciubba DM, Petteys RJ, Garces-Ambrossi GL, Noggle JC, McGirt MJ, et al. (2009) Diagnosis and management of sacral tumors. J Neurosurg Spine 10: 244-256. [Crossref]
- Yin X, Fan WL, Liu F, Zhu J, Liu P, et al. (2015) Technique and surgical outcome of total resection of lower sacral tumor. Int J Clin Exp Med 8: 2284-2288. [Crossref]
- Tarawneh AM, Sabou S, AlKalbani S, Pasku D, Quraishi NA (2020) Clinical outcomes of sacroplasty for metastatic sacral tumours: a systematic review and metaanalysis. Eur Spine J 29: 3116-3122. [Crossref]
- Zang J, Guo W, Yang Y, Wei R (2019) Surgical treatment of giant benign sacral neurogenic tumors using the posterior-only approach. Clin Neurol Neurosurg 185: 105483. [Crossref]
- van Wulfften Palthe ODR, Janssen SJ, Wunder JS, Ferguson PC, Wei G, et al. What questionnaires to use when measuring quality of life in sacral tumor patients: the updated sacral tumor survey. Spine J 17: 636-644. [Crossref]
- Kurup AN, Woodrum DA, Morris JM, Atwell TD, et al. (2012) Cryoablation of recurrent sacrococcygeal tumors. J Vasc Interv Radiol 23: 1070-1075. [Crossref]
- Costa RP, Cardile D, Murabito A, Tripoli V, Verderame F (2018) Ra223 in Bone Metastases with Osteolytic Activity. World J Nucl Med 17: 116-119. [Crossref]
- Kayani B, Hanna SA, Sewell MD, Saifuddin A, Molloy S, et al. (2014) A review of the surgical management of sacral chordoma. Eur J Surg Oncol 40: 1412-1420. [Crossref]
- Ramamurthy R, Bose JC, Muthusamy V, Natarajan M, Kunjithapatham D (2009) Staged sacrectomy - An adaptive approach: Clinical article. J Neurosurg Spine 11: 285-294. [Crossref]
- Stacchiotti S, Gronchi A, Fossati P, Akiyama T, Alapetite C, et al. (2017) Best practices for the management of local-regional recurrent chordoma: A position paper by the Chordoma Global Consensus Group. Ann Oncol 28: 1230–1242. [Crossref]
- Ong KO, Ritchie DA (2014) Pictorial essay: Tumours and pseudotumors of sacrum [Internet]. Vol. 65, Canadian Association of Radiologists Journal. Canadian Medical Association, pp: 113-120.
- Vanheule E, Huysse W, Herregods N, Verstraete K, Jans L (2019) Sacral tumours on MRI: A pictorial essay. J Belg Soc Radiol 103: 62. [Crossref]
- Brault N, Qassemyar Q, Bouthors C, Lambert B, Atlan M, Missenard G. A giant sacral chordoma resection and reconstruction with a gluteal perforator flap, a case report and literature review. Ann Chir Plast Esthet 64: 271-277. [Crossref]
- Nishizawa K, Mori K, Saruhashi Y, Takahashi S, Matsusue Y (2014) Long-term clinical outcome of sacral chondrosarcoma treated by total en bloc sacrectomy and reconstruction of lumbosacral and pelvic ring using intraoperative extracorporeal irradiated autologous tumor-bearing sacrum: A case report with 10 years followup. Spine J 14: e1-8. [Crossref]
- Domovitov SV, Chandhanayingyong C, Boland PJ, McKeown DG, Healey JH (2016) Conservative surgery in the treatment of giant cell tumor of the sacrum: 35 Years’ experience. J Neurosurg Spine 24: 228-240. [Crossref]
- Martin C, McCarthy EF (2010) Giant cell tumor of the sacrum and spine: series of 23 cases and a review of the literature. Iowa Orthop J 30: 69-75. [Crossref]
- Pan Y, Lu L, Chen J, Zhong Y, Dai Z (2018) Analysis of prognostic factors for survival in patients with primary spinal chordoma using the SEER Registry from 1973 to 2014. J Orthop Surg Res 13: 76. [Crossref]
- Osaka S, Osaka E, Kojima T, Yoshida Y, Tokuhashi Y (2014) Long-term outcome following surgical treatment of sacral chordoma. J Surg Oncol 109: 184-188. [Crossref]
- Clarke MJ, Dasenbrock H, Bydon A, Sciubba DM, McGirt MJ, et al. (2012) Posterior-only approach for en bloc sacrectomy: Clinical outcomes in 36 consecutive patients. Neurosurgery 71: 357-364. [Crossref]
- Hsieh PC, Xu R, Sciubba DM, McGirt MJ, Nelson C, et al. (2009) Long- Term Clinical Outcomes Following En Bloc Resections for Sacral Chordomas and Chondrosarcomas. Spine (Phila Pa 1976) 34: 2233-2239. [Crossref]
- Fourney DR, Rhines LD, Hentschel SJ, Skibber JM, Wolinsky JP, et al. (2005) En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine 3: 111-122. [Crossref]
- Ji T, Guo W, Yang R, Tang X, Wang Y, et al. (2017) What Are the Conditional Survival and Functional Outcomes After Surgical Treatment of 115 Patients With Sacral Chordoma? Clin Orthop Relat Res 475: 620-630. [Crossref]
- Zoccali C, Skoch J, Patel AS, Walter CM, Maykowski P, et al. (2016) Residual neurological function after sacral root resection during en-bloc sacrectomy: a systematic review. Eur Spine J 25: 3925-3931. [Crossref]
- Phukan R, Herzog T, Boland PJ, Healey J, Rose P, et al. (2016) How Does the Level of Sacral Resection for Primary Malignant Bone Tumors Affect Physical and Mental Health, Pain, Mobility, Incontinence, and Sexual Function? Clin Orthop Relat Res 474: 687-696. [Crossref]
- Moran D, Zadnik PL, Taylor T, Groves ML, Yurter A, et al. (2015) Maintenance of bowel, bladder, and motor functions after sacrectomy. Spine J 15: 222-229. [Crossref]
- Zang J, Guo W, Yang R, Tang X, Li D (2015) Is total en bloc sacrectomy using a posterior-only approach feasible and safe for patients with malignant sacral tumors? J Neurosurg Spine 22: 563-570. [Crossref]
- Clarke MJ, Dasenbrock H, Bydon A, Sciubba DM, McGirt MJ, et al. (2012) Posterior-only approach for en bloc sacrectomy: clinical outcomes in 36 consecutive patients. Neurosurgery 71: 357-364. [Crossref]
- Vartanian E, Lynn J, Perrault D, Wolfswinkel E, Patel K, et al. (2018) Abstract QS03: Risk Factors Associated with Postoperative Complications Following Complex Sacrectomy: Outcomes Analysis. Plast Reconstr Surg Glob Open 6: 115-116. [Crossref]
- Kiatisevi P, Piyaskulkaew C, Kunakornsawat S, Sukunthanak B (2017) What Are the Functional Outcomes After Total Sacrectomy Without Spinopelvic Reconstruction? Clin Orthop Relat Res 475: 643-655. [Crossref]


