Background: According to the American Psychiatric Association, anxiety disorders are the most common type of psychiatric disorder. Many patients with anxiety disorders experience physical symptoms related to anxiety and subsequently visit their primary care providers instead of psychiatrists. Despite the high prevalence rates of these anxiety disorders, they often are under-recognized and undertreated clinical problems.
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), anxiety disorders include disorders that share features of excessive fear and anxiety and related behavioral disturbances. These disorders include separation anxiety disorder, selective autism, specific phobia, social anxiety disorder (social phobia), panic disorder, agoraphobia, generalized anxiety disorder, substance/medication-induced anxiety disorder, and anxiety disorder due to another medical condition. Brillia uses the combined sciences of homeopathy and antibodies, which is a new way of treatment. The active ingredient of Brillia, Lapine S-100 immune globulin, is produced using antibodies to the brain-specific S-100 protein (S-100B), which acts as an essential regulator for many different intracellular and extracellular brain processes. To establish the clinical efficacy of Brillia for the treatment of anxiety disorders, the conduct of clinical study was relevant. Thus, to determine the efficacy and safety of Brillia for the treatment of anxiety disorders and Attention- deficit hyperactivity disorders, two clinical studies were designed for each indication. One such clinical research on the patients with anxiety disorders is explained in this article, and results from the other clinical study on the patients with Attention-Deficit/Hyperactivity Disorder is described in another publication/section.
Methods: A double-blind placebo-controlled randomized study of Brillia evaluating clinical efficacy and safety for children with anxiety disorders. Before the patients were recruited in the study, the patients of the legal guardians of the patient were explained about the study and signed informed consent was received. Any patient-related information that was collected during the conduct of the research is handled confidentially.
Prior to inclusion in the study, the patients or their legal guardians were provided with information about the study and signed an informed consent form. Patient information collected during the study is strictly confidential.
brillia, attention-deficit/hyperactivity disorder, ADHD, brain-specific S-100 protein (S-100B), GABA-mimetic and neurotrophic effects
Abbreviations:
ADD: Attention Deficit Disorder; ANCOVA: Analysis of Covariance; AURI: Acute Upper Respiratory Infection; BCM: Behavioral Classroom Management; CGI: Clinical Global Impression scale; CNS: Central Nervous System; DBP: Diastolic Blood Pressure; DSM: Diagnostic and Statistical Manual of Mental Disorders; H.R. : Heart Rate; ICD: International Classification of Drugs; SBP: Systolic Blood Pressure; SCAS: Spence Children'sChildren's Anxiety Scaleanxiety disorders; SSSI: Selective Serotonin Reuptake Inhibitors
Anxiety disorders are the most common type of psychiatric disorders, as per the American Psychiatric Association. Many patients with anxiety disorders experience physical symptoms related to anxiety and subsequently visit their primary care providers. Anxiety disorders have one of the most extended differential diagnosis lists of all psychiatric disorders. Anxiety is a nonspecific syndrome and can be due to a variety of medical or psychiatric syndromes. For example, a 2018 study found that about 30% of those with anxiety also have autoimmune thyroiditis (AIT). Additionally, a variety of anxiety symptoms, such as panic, worry, rumination, and obsessions, can present in a variety of psychiatric illnesses, including mood disorders, psychotic disorders, personality disorders, somatoform disorders, and cognitive impairment disorders (e.g., delirium). Anxiety also can be observed as part of drug withdrawal or drug intoxication effect.
Anxiety disorders are the most common type of psychiatric disorders in the United States Social anxiety disorder (social phobia) is the most common anxiety disorder; it has an early age of onset- by age 11 years in about 50% and by age 20 years in about 80% of individuals that have the diagnosis - and it is a risk factor for subsequent depressive illness and substance abuse.
Effective psychotherapy should be combined with drug therapy of anxiety disorders. Selective Serotonin Reuptake Inhibitors (SSRIs) with broad-spectrum activity are the first-line drugs for the treatment of childhood anxiety disorders but have delayed the onset of action. Benzodiazepines are usually not used as first-line therapy in children and adolescents, as they were shown to cause behavioral disinhibition in younger children. However, the administration of these varieties of drugs available for the treatment of anxiety disorders is limited due to the frequent and severe adverse events. (Arena, Rozenbaum, 2004; Khodarev, 2002).
Brillia uses the combined sciences of homeopathy and antibodies, which is a new way of treatment. This product has shown excellent results for children who have been diagnosed with Attention Deficit Hyperactivity Disorder (ADHD), or who are dealing with anxiety disorders and hyperactivity issues for almost 10 years of usage in Europe (under a different brand name but and similar formulation). Brillia is found both safe and impactful and is widely accepted as a safe alternative to prescription pharmaceuticals that have harmful side effects. It also helps in reducing the symptoms of anxiety and hyperactivity, consequently helping improve attention and focus. It also helps in reducing temper tantrums, obsessive, and defiant behavior.
The active ingredient of Brillia, Lapine S-100 immune globulin, is produced using antibodies to the brain-specific S-100 protein (S-100B), which acts as an essential regulator for many different intracellular and extracellular brain processes, for example, calcium homeostasis, various enzymes activities and communications between neurons, etc. Thus, the clinical study of the efficacy of Brillia for Children for the treatment of anxiety disorders, disturbances of behavior, and attention, accompanied by increased excitability, irritability, and hyperactivity, is very important and promising.
Two clinical trials were performed for indications of anxiety disorder and ADHD each. In this article, the clinical study with the evidence of anxiety disorders is explained. Another clinical study of patients with attention deficit hyperactivity disorder was conducted separately (this study in ADHD was performed as part of another study published as a separate article).
Mechanism of Action: In multiple neurotransmitter networks in the human body, this protein of S-100 is involved, which helps in improving the concentration of the attention, migrate the anxiety, and in reduction of the hyperactivity. Besides, it also restricts the area of damage, and the cognitive function is improved as this product shows a neuroprotective effect. Also, the drug modifies the activity of S-100 protein that integrates synaptic and metabolic processes in the brain. Brain-specific S-100 is expressed in different cell types, including astrocytes and specific neuronal populations. This product stimulates cell proliferation and migration and inhibits apoptosis and differentiation in nanomolar concentrations. The molecular mechanism of regulation of synaptic plasticity by the extracellular brain-specific S-100 proteinis still unknown.
Brillia for Children is a new drug based on ultra-low doses of antibodies to endogenous regulators. S-100 protein is the molecular target of Brillia for Children that couples synaptic and metabolic processes in the brain. Since the drug involves the primary regulatory system of CNS, the drug possesses a broad spectrum of pharmacological activities. Thus, the clinical study of the efficacy of Brillia for Children for the treatment of anxiety disorders, disturbances of behavior, and attention, accompanied by increased excitability, irritability, and hyperactivity, is very important and promising.
To determine the efficacy of Brillia for Children for the treatment of anxiety disorders, disturbances of behavior, and attention, accompanied by increased excitability, irritability, and hyperactivity in children, two clinical studies were carried out. The two studies involved 198 subjects in which 98 of them were administered Brillia for Children. One clinical research on the indication of anxiety disorders is explained in this article, and the other study on ADHD is described in another related article. The use of Brillia was provided to be non-inferior to placebo, indicating a high degree of safety of the drug.
Treatment and Management: Treatment usually consists of a combination of pharmacotherapy and psychotherapy. Antidepressant agents are the drugs of choice in the treatment of anxiety disorders, particularly the newer agents, which have a safer adverse effect profile and higher ease of use than the older tricyclic antidepressants (TCAs), such as selective serotonin reuptake inhibitors (SSRIs). Antidepressants that are not FDA-approved for the treatment of a given anxiety disorder, such as Nefazodone and Mirtazapine, still may be beneficial. Older antidepressants, such as TCAs and monoamine oxidase inhibitors (MAOIs), also are useful in the treatment of some anxiety disorders.
Behavioral therapy and CBT (Cognitive Behavior Therapy) have demonstrated efficacy through controlled studies. Computerized CBT (Fear Fighter) has been recommended for panic and phobia by the National Institute for Health and Clinical Excellence guidelines (NICE). Psychodynamic therapy (or insight-oriented therapy) is rarely indicated as preferential treatment for phobias and is now mostly used for cases of phobic disorders that overlap personality disorders. Interpersonal psychotherapy (IPT) has also shown some efficacy. Eight trials examined the use of IPT for anxiety disorders and found significant effects in comparison with control groups. There was no evidence suggesting that IPT is less effective than CBT for anxiety.
In 2019, the FDA approved a cranial electrotherapy stimulator (CES) for the treatment of anxiety, depression, and insomnia. The prescription device delivers micro pulses of electrical current across the brain, which in clinical trials led to a reduction in anxiety levels, insomnia, and depressed mood. It is the first CES integrated into noise-canceling, Bluetooth-enabled headphones, and the first CES managed through an app.
The outcome of treatment is determined by several factors, including but not limited to the following:
1. The specific type of anxiety disorder
2. Severity of diagnosis
3. Level of functioning prior to the onset of symptoms
4. Degree of motivation for treatment
5. Level of support (e.g., family, friends, work, school)
6. Ability to comply with medication and/or psychotherapeutic regimen
Pharmacotherapy for Anxiety and Panic Disorders:
Selective serotonin reuptake inhibitors (SSRIs) are generally used as first-line agents, followed remotely by tricyclic antidepressants (TCAs). Fluoxetine (Prozac) can be used (mainly if panic disorder occurs with depression); however, patients may poorly tolerate it initially because it may initially increase anxiety, except at low starting doses. Fluoxetine has a long half-life, making it an excellent choice in marginally compliant patients. It alters the metabolism of cytochrome P-450 2D6-cleared agents; this fact should be considered.
- Paroxetine (Paxil) represents a partially sedating SSRI option that is also available in a controlled-release preparation (Paxil C.R.), which may improve tolerability, but paroxetine still inhibits P450 2D6. Paroxetine has a short half-life, which may be a limitation in marginally compliant patients.
- Citalopram (Celexa) carries a risk of dose-dependent Q.T. prolongation. Because of the risk for Q.T. prolongation, Citalopram is contraindicated in individuals with congenital long Q.T. syndrome, and the dose should not exceed 40 mg/day. Do not exceed a dose of 20 mg/day when coadministered with CYP2C19 inhibitors (e.g., cimetidine, fluconazole, omeprazole).
- Escitalopram (Lexapro) is likely to cause fewer hepatic enzyme interactions and may be appropriate initial choices for patients with complicated medical regimens or those who are concerned about drug interactions. Escitalopram also appears to be particularly well tolerated in preliminary studies, although it may be restricted from some formularies due to the significant difference in cost with Citalopram without a corresponding improvement in efficacy or tolerability for many patients.
- Sertraline (Zoloft) represents a similar SSRI option with a slightly different pharmacodynamic profile, including sigma receptor effects, although it has some P450 3A4 interactions.
- Mirtazapine (Remeron) has a much more sedating effect, generally reducing its potential to aggravate initial anxiety. Mirtazapine acts distinctly as an alpha-2 antagonist, consequently increasing synaptic norepinephrine and serotonin, while also blocking some postsynaptic serotonergic receptors that conceptually mediate excessive anxiety when stimulated with serotonin Mirtazapine may cause residual morning sedation that often improves with continued therapy and may cause an increase in appetite or weight gain. A study by Kim et al. suggests among patients with major depressive disorder who have high anxiety symptoms, Mirtazapine (15-30 mg/d) administered in the early weeks of treatment may have an earlier-onset action for anxiety symptoms.
Clinical Study design: Study (MMH-TD-001): Multicenter, double-blind placebo-controlled randomized study of Brillia for Children efficacy and safety in the 12-week treatment of children with anxiety disorders (phase IV).
This study was designed to assess the clinical efficacy and safety as the primary objectives in children with anxiety disorders when treated with Brillia. Analysis of the dynamics of anxiety test (R. Temple, М. Dorky, V. Amen) conducted in phase IV, 12-week, multicenter, double-blind, placebo-controlled, randomized study (MMH-TD-001), proved a significant anxiolytic effect of Brillia for Children compared to placebo. According to patient self-assessment, anxiolytic efficacy of Brillia for Children was mostly manifested in young children, which was confirmed by the decrease of average anxiety index after 12-week treatment (11.9 points versus 8.3 points in the placebo group).
Study Participants: Study (MMH-TD-001)
Materials and Methods:
Patients included those aged in between 05-15 years of both male and females and should at least one of the diagnoses according to ICD-10:
1. Separation anxiety disorder of childhood – F93.0
2. Phobic anxiety disorder of childhood – F93.1
3. Social anxiety disorder of childhood – F93.2
4. Other childhood emotional disorders – F93.8
Below are the other inclusion criteria
- Mild or moderate severity of the disease under the anxiety scale of G.P. Lavrentieva and T.M.
- Titarenko and according to anxiety test of R. Temple, M. Dorky, V. Amen
- Absence of significant impairment of general intelligence
- Lack of pharmacotherapy of anxiety disorders in the last two weeks
- Signed an informed consent form
Below are the exclusion criteria
- Mental retardation
- ASD and
- Comorbid seizure disorder as the exclusion criteria
Scales used for assessment
1. Spence Children's Anxiety Scale (SCAS)
2. Dynamics of anxiety severity by the of test R. Temple, М. Dorky, V. Amen
3. Self-assessment of anxiety form to the kids.
Randomization and Masking:
Study (MMH-TD-001): The study involved 98 patients 5-15 years of old with emotional disorders with onset specific to childhood (F93 under ICD-10). The patients were randomized into two groups
1. Treatment group (n=48) was administered Brillia for Children (1 tablet t.i.d.)
2. Control group (n=50) was administered placebo (1 tablet t.i.d.).
The duration of treatment was 12 weeks
Table 1. Demographic characteristics of patients (abs (%))
Parameter
|
Brillia for Children
|
Placebo
|
Total
|
Clinical Study 01: MMH-TD-001 |
Sex |
М |
29 (60.4%) |
34 (68.0%) |
63 (64.3%) |
F |
19 (39.6%) |
16 (32.0%) |
35 (35.7%) |
Comorbidities |
Hypoxia in perinatal period/
during childbirth |
27 (56.3%) |
27 (54%) |
98 (55.1%) |
Nervous system diseases |
2 (4.2%) |
1 (2%) |
3 (3.1%) |
Mental and behavioral disorders |
4 (8.3%) |
2 (4%) |
6 (6.1%) |
Respiratory system diseases |
4 (8.3%) |
2 (4%) |
6 (6.1%) |
Digestive system diseases |
2 (4.2%) |
2 (4%) |
4 (4.1%) |
Diseases of the
musculoskeletal system |
1 (2.1%) |
3 (6%) |
4 (4.1%) |
Diseases of eye adnexa |
- |
1 (2%) |
1 (1%) |
Congenital anomalies |
1 (2.1%) |
1 (2%) |
2 (2%) |
Age, years (M±m) |
8.1±2.89 (5-15) |
9.0±2.82 (5-15) |
8.6±2.88 (5-15) |
Connection with definite event |
14 (29.2%) |
18 (36.0%) |
32 (32.7%) |
The initial level of anxiety under anxiety scale of Lavrentieva and Titarenko, points (M±m) |
10.9±3.06 |
10.7±3.00 |
- |
Discussion of the Clinical study (MMH-TD-001)
Study Populations (Brillia for Children, tablets): A total of 98 patients were included in the efficacy evaluation in this clinical study. However, among the 98 children, four patients were found to be discontinued from the study (2 patients from the treatment group and two patients from the placebo group) because of the decision of their parents. The final analysis of the efficacy of Brillia for Children in children with anxiety disorders included 94 patients (MMH-TD-001).
Brillia for Children experimental groups were compared with placebo groups
The demographic characteristics of the patients are presented in Table 01. In all cases, treatment and control groups were comparable by sex, age, the severity of the initial state, comorbidities. The sample size was sufficient for statistical analysis and assessment of clinical efficacy (Table 1).
MMH-TD-001 study did not include age randomization criteria. Thus, the assessment of the efficacy of Brillia for Children in children aged 5-7 years with SCAS was carried out in unbalanced groups (13 patients in the treatment group, 23 patients in the control group).
The sample volume was still sufficient for statistical analysis and assessment of clinical efficacy.
Comparison of Efficacy Results of the Study (Brillia for Children, tablets)
Anxiety disorders: The standard scales of questionnaires and specially designed tests were used to assess the severity of anxiety and depression and the efficacy of the treatment of anxiety disorders.
In general, Brillia for children demonstrated high efficacy in the treatment of emotional disorders with the onset specific for childhood (under ICD-10, separation anxiety disorder of childhood – F93.0; phobic anxiety disorder of childhood – F93.1; social anxiety disorder of childhood – F93.2; other childhood emotional disorders – F93.8), exceeding the efficacy of placebo (MMH-TD-001).
ANCOVA showed that anxiety severity under the test of R. Temple, M. Dorky, V. Amen in treatment and control groups significantly differed during the study by drug factor (F1/91=4.31; p=0.04). The results confirmed the superiority of Brillia for children over placebo. According to ANCOVA, anxiety severity in children decreased post eight weeks of treatment with Brillia for children. Noted: The effect steadily increased with treatment with 12 weeks of therapy.
Also, frequency analysis of patients with various anxiety severity showed significant differences between treatment and control groups (χ2(1)=6.0; p=0.014). By week 12 of the treatment with Brillia for Children, 20% (vs. 35% in control group), of the proportion of children with severy anxiety increased to 78% (vs. 65% in control group) the proportion of patients with moderate anxiety
According to self-assessment of patients aged 8-15 years and the results of reports of parents of children aged 5-7 years and 8- 15 years under SCAS confirmed that the average total score of severity of anxiety disorders post-treatment with Brillia for children had a positive influence on the severity of anxiety disorders compared to the initial state. Thus, the overall score in the treatment group (children aged 8-15 years) decreased from 39.2±14.5 to 26.9±15.7 points (standard: 27.38±16.50 points). When analyzing the reports of parents of children aged 5-7 years and 8-15 years, similar results were obtained.
Analysis of results on the efficacy of Brillia for Children showed that the drug had sedative and anxiolytic effects, improved tolerance of psychoemotional stress. The efficacy of Brillia for Children was found to be superior to placebo.
Discussion of Results in Sub-populations (Brillia for Children, tablets)
Efficacy of Brillia For Children in various age groups : Analysis of self-assessment of anxiety severity depending on age showed that the most marked improvement among patients with anxiety disorders (F93) occurred in younger children (5-7 years old) treated with Brillia for Children (MMH-TD-001). After 12 weeks of treatment average anxiety index according to the test of R. Temple, M. Dorky, V. Amen fell by 11.9 points vs. 8.3 points in patients aged 5-7 years treated with placebo. A similar analysis carried out in older children (8-15 years old) also testified the efficacy of Brillia for Children, but positive dynamics of anxiety index was less pronounced (decrease by 11.2 points with Brillia for Children and 11 points with placebo). Thus, the anxiolytic efficacy of Brillia for Children was mainly manifested in young children.
The most significant effect of the treatment regarding anxiety severity level was observed in younger children. By the end of the therapy total, the SCAS score, according to the parent's opinion, was close to standard indices (24.6±12.1 vs. 31.0±15.8 points in the control group). Total SCAS score in older children reached 26.9±15.69 points according to self-assessment and 25.7±10.14 points according to parents' opinion.
However, due to the absence of randomization criteria depending on the patient's age, groups of patients were not equal. Analysis of SCAS results revealed no significant differences between treatment and control groups. Nevertheless, the most distinct effect of Brillia for Children was observed in younger children. The pronounced anxiolytic effect of Brillia for Children was revealed in children of 8-15 years old with separation anxiety, panic attacks, agoraphobia.
Thus, the need for early diagnosis and treatment of anxiety disorders in children was confirmed with the efficacy of Brillia for Children in the younger group (5-7 years old).
Discussion of Clinical Information Relevant to Dosing Recommendations (Brillia for Children, tablets)
The recommended dose is 1 or 2 tablets per intake. Daily, the drug can be taken 2-3 times only. The overall duration of treatment is three months. If necessary, the treatment can be repeated in 1 month.
In the study of the efficacy of Brillia for Children for the treatment of anxiety disorders, in children, the following dosage regimens were used:1tablett.i.d. The total duration of the treatment was 12 weeks. The following regimen for Brillia was used for the study: 2 tablets b.i.d. for 12 weeks.
These schemes of drug administration were proved to be effective. However, an analysis of the correlation of dosage regimen with the efficacy of Brillia for Children was not performed. Although the possibility of flexible-dose titration (2-6 tablets a day), its repeated use during the day, depending on the severity of the disease and current condition of the patient, allows choosing the optimum regimen and achieving positive outcomes in each case.
Thus, the recommended dosage regimen of the drug was found to be efficient enough and confirmed to be used for the treatment of anxiety disorders for children.
Persistence of Efficacy and/or Tolerance Effects (Brillia for Children, tablets)
The duration of the treatment in both studies with Brillia for Children was 12 weeks.
Anxiolytic, antidepressant and anti-asthenic effects of Brillia for Children were gradually increased during the treatment (MMH-TD-001).
Note: - ***p<0.001, significance of differences vs baseline
Thus, the administration of Brillia for Children led to a gradual decrease in anxiety severity in children during the treatment (Figure 01). Besides, the marked reduction of anxiety severity was reported after eight weeks of treatment. The influence of the factor of therapy duration on anxiety severity was obtained using two-way ANOVA. The analysis showed that anxiety severity under the test of R.Temple, M.Dorky, V.Amen significantly decreased by week 8 of the treatment, and this effect was steadily growing to week 12.
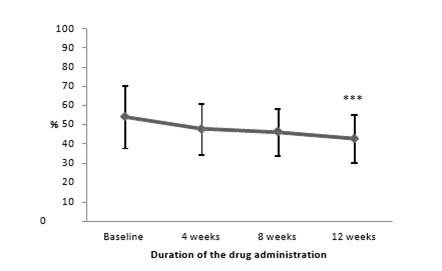
Figure 1. Dynamics of anxiety severity under the test of R. Temple, M. Dorky, V. Amen depending on the duration of the treatment with Brillia for Children
Note: - ***p<0.001, significance of differences vs. baseline
Also, SCAS averaged data proved the positive dynamics of anxiety severity (Figure 02). Post-hoc analysis showed a significant reduction of anxiety by week 04 of the treatment with Brillia for Children. The results obtained after the completion of therapy (12 weeks) were significantly better than the initial values and the results of week 04.
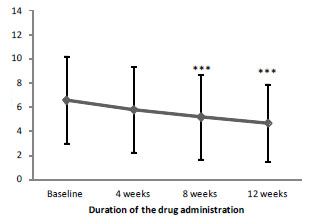
Figure 2. Dynamics of SCA Saveraged data depending on the duration of the treatment with Brillia for Children
In this study, it was observed that the increase in the therapy duration did not lead to a reduction in the efficacy of the treatment with Brillia. Therefore, long-term use of this product in children may not decrease the efficacy of the treatment and/or promote tolerance and/or addiction.
Besides, the marked reduction in anxiety severity was reported after two weeks of the drug administration.
The duration of the treatment with Brillia for Children did not impact the efficacy.
Thus, long-term treatment with Brillia for Children does not lead to a decrease in efficacy, the development of tolerance and addiction, but rather to a positive effect, which confirms the need for a long-term course of Brillia for Children.
Discussion of Clinical Efficacy:
Efficacy Evaluation of Study (MMH-TD-001)
Efficacy criteria:
- Comparison of the therapy with Brillia for Children and placebo therapy in terms of anxiety severity assessed by the test of R. Temple, M. Dorky, V. Amen and SCAS
- Comparison of the treatment with Brillia for Children and placebo therapy in terms of the proportion of patients with improvement of anxiety disorder
- Dynamics of anxiety disorders depending on their types under the subscales of SCAS
- Therapeutic dynamics and dynamics of adverse events by the end of the treatment under the Clinical Global Impression Scale (CGI) compared to placebo.
Results from Clinical Efficacy
Note: - ***p<0.01; *** - p<0.001, significance of differences in comparison to baseline
12-week treatment resulted in a significant reduction of severity of anxiety disorders compared to baseline parameters in both groups (Table 2). The average index of anxiety in the treatment group decreased from 54.3±16.3 to 42.9±12.5 points after completion of therapy (p<0.001).
Table 02. Dynamics of anxiety severity by the of test R. Temple, М. Dorky, V. Amen (points, M±SD)
Drug |
Baseline |
4 weeks |
8 weeks |
12 weeks |
Brillia for Children |
54.3±16.3 |
48.0±13.2 |
46.3±11.9 |
42.9±12.5 *** |
Placebo |
57.3±12.5 |
54.9±11.9 |
50.3±12.2 |
47.6±12.0 ** |
Analysis of parameters of self-assessment of anxiety severity depending on the age of the patients showed a more pronounced positive effect of Brillia for Children in younger children (Table 03).
Table 3. Anxiety index according to self-assessment of children of 5-7 and 8-15 years old (points, M±SD)
Drug
|
Baseline
|
4 weeks
|
8 weeks
|
12 weeks
|
Younger children (5-7 years old) |
Brillia for Children, n=13 |
54.4±12.0 |
49.0±13.0 |
44.6±11.0 |
42.5±10.0 |
Placebo, n=24 |
58.6±11.0 |
57.4±12.0 |
53.8±14.0 |
50.3±13.0 |
Older children (8-15 years old) |
Brillia for Children, n=33 |
54.3±14.0 |
47.6±13.0 |
47.0±12.0 |
43.1±13.0 |
Placebo, n=24 |
56.0±13.0 |
52.3±11.0 |
46.7±9.5 |
45.0±13.0 |
The first incidence of anxiety disorders of different severity was comparable in both groups. By the end of the treatment with Brillia for Children, the proportion of children with severe anxiety was only 20% (vs. 35% in the control group), and the ratio of patients with moderate anxiety increased to 78% (vs. 65% for placebo) (Figure 3).
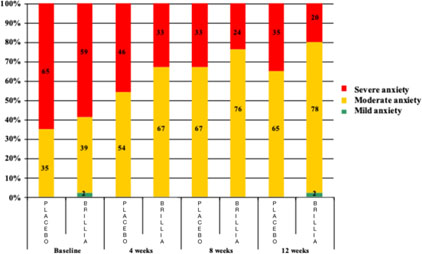
Figure 3. Dynamics of the proportion of patients with various severity of anxiety
Frequency analysis of the proportion of patients with different severity of anxiety disorders using the χ2 test in the modification of Cochran-Mantel-Haenszel revealed significant differences between study and control groups (χ2(1)=6.0; p=0.014) in the dynamics of four consecutive visits. The average total scores of the severity of anxiety disorders (SCAS) according to self-assessment of patients and the results of the reports of their parents at various stages of study are presented in (Table 04).
Table 4. The total score of SCAS (points, M±SD)
Drug
|
Baseline
|
4 weeks
|
8 weeks
|
12 weeks
|
Self-assessment of children 8-15 years old |
Brillia for Children, n=33 |
39.2±14.47 |
32.5±17.63 |
30.3±17.29 |
26.9±15.69 |
Placebo, n=24 |
33.6±12.70 |
27.8±12.49 |
25.7±12.93 |
21.4±12.12 |
Assessment of parents of children 8-15 years old |
Brillia for Children, n=33 |
36.9±14.38 |
32.5±11.22 |
29.0±12.10 |
25.7±10.14 |
Placebo, n=24 |
34.1±16.08 |
30.2±12.41 |
25.3±13.14 |
23.1±14.06 |
Assessment of parents of children 5-7 years old |
Brillia for Children, n=13 |
36.1±13.99 |
32.4±12.95 |
26.6±13.10 |
24.6±12.13 |
Placebo, n=24 |
44.7±13.13 |
37.9±13.07 |
35.7±17.36 |
31.0±15.78 |
Also, four-way analysis of variance (ANOVA) (drug, visit, group, domain) showed a significant improvement of anxiety in all subgroups (both for Brillia for Children and placebo) after four weeks of therapy.
Note: ** - p<0.01; *** - p<0.001, significance of differences vs baseline
The study aimed to evaluate the dynamics of anxiety disorders, depending on their types under the subscales of SCAS. Table 07 contains data on the distribution of patients by types of anxiety disorders, age, and groups.
Efficacy results
Over the observation period, four patients were withdrawn. The analysis of study drug efficacy was based on the data of 94 patients. Two-way analysis of covariance (ANCOVA) showed that anxiety severity under the test of R.Temple, M.Dorky, V.Amen in the study and control groups differed by a drug factor (Brillia for Children and placebo, F1/91=4.31; p=0.04). The results confirmed that Brillia for Children was superior to placebo. Furthermore, ANCOVA revealed that anxiety severity significantly decreased in the treatment group after eight weeks of treatment.
The results of testing of children and their parents during the study indicated that the use of the active drug or placebo had a significant positive impact on the severity of anxiety disorders compared to the baseline state. Three-way ANCOVA (drug, visit, domain) with repeated measures revealed no significant differences between study and control groups in terms of the dynamics of anxiety disorder severity (Table 5-7).
Table 05. ANCOVA of the dynamics of the average SCAS score
Groups |
Factors |
Interactions |
Drug (1) |
Domain (2) |
Visit (3) |
(1)*(2) |
(1)*(3) |
(1)*(2)*(3) |
Parents of children
5-7 years old |
F1/28=0.1; p=0.8 |
F4/112=0.9; p=0.4 |
F2/56=0.3; p=0.7 |
F4/112=1.5; p=0.2 |
F2/56=0.2; p=0.7 |
F8/224=0.9; p=0.5 |
Parents of children
8-15 years old |
F1/49=0.8; p=0.4 |
F5/245=1.7; p=0.15 |
F2/98=0.9; p=0.4 |
F5/245=1.4; p=0.2 |
F2/98=0.2; p=0.8 |
F10/490=0.8; p=0.6 |
Children 8-15
years old |
F1/50=0.1; p=0.8 |
F5/250=1.3; p=0.3 |
F2/100=0.1; p=1.0 |
F5/250=1.3; p=0.3 |
F2/100=0.2; p=0.8 |
F10/500=0.8; p=0.6 |
Table 06. Average data of SCAS in both groups (points, M±SD)
Drug |
Baseline |
4 weeks |
8 weeks |
12 weeks |
Brillia for Children, n=46 |
6.6±3.61 |
5.8±3.53 |
5.2±3.50 *** |
4.7±3.19 *** |
Placebo, n=48 |
6.9±4.17 |
5.9±3.71 ** |
5.4±4.00 *** |
4.7±3.77 *** |
Table 07. Distribution of patients by type of anxiety disorder, age and groups
Group |
Age |
Type of anxiety disorder (ICD-10) |
Total |
F 93.0 |
F 93.1 |
F 93.2 |
F 93.8 |
Brillia for Children |
5-7 years |
1 (8%) |
1 (8%) |
6 (46%) |
5 (38%) |
13 (100%) |
8-15 years |
0 (0%) |
8 (24%) |
12 (36%) |
13 (39%) |
33 (100%) |
Total |
1 (2%) |
9 (20%) |
18 (39%) |
18 (39%) |
46 (100%) |
Placebo |
5-7 years |
2 (8%) |
10 (42%) |
5 (21%) |
7 (29%) |
24 (100%) |
8-15 years |
1 (4%) |
4 (17%) |
8 (33%) |
11 (46%) |
24 (100%) |
Total |
3 (6%) |
14 (29%) |
13 (27%) |
18 (38%) |
48 (100%) |
According to patients and their parents, Brillia for Children had marked anxiolytic effect in children 8-15 years old with panic attacks and agoraphobia. The treatment improved separation anxiety, also mostly in older children. Also, parents of younger children believed that Brillia for Children significantly reduced the fear of injury in their children. Finally, treatment with Brillia for Children had a positive impact on the degree of social phobia in children, regardless of age (Table 8).
Table 08. Values of SCAS subscales according to the assessment of children and their parents (points, M±SD)
Drug |
Baseline |
4 weeks |
8 weeks |
12 weeks |
Panic attacks and agoraphobia, self-assessment of children 8-15 years old |
Brillia for Children, n=33 |
5.4±4.2 |
4.4±3.5 |
3.8±3.5 |
3.5±3.6 |
Placebo, n=24 |
4.4±3.1 |
3.1±2.8 |
2.3±2.9 |
2.0±2.5 |
Panic attacks and agoraphobia, assessment of parents of children 8-15 years old |
Brillia for Children, n=33 |
4.7±3.9 |
3.8±2.6 |
3.3±2.9 |
2.6±2.7 |
Placebo, n=24 |
4.2±4.0 |
4.0±3.3 |
2.7±3.0 |
2.5±3.2 |
Separation anxiety, self-assessment of children 8-15 years of old |
Brillia for Children, n=33 |
6.9±3.8 |
5.4±3.9 |
5.0±3.8 |
4.6±3.6 |
Placebo, n=24 |
5.6±3.5 |
4.5±3.4 |
4.5±3.4 |
3.6±3.5 |
Separation anxiety, assessment of parents of children 8-15 years old |
Brillia for Children, n=33 |
6.3±3.4 |
5.9±3.3 |
5.2±3.0 |
4.6±2.7 |
Placebo, n=24 |
5.7±3.9 |
4.7±2.8 |
4.3±3.1 |
3.6±3.1 |
Fear of injury, self-assessment of children 5-7 years old |
Brillia for Children, n=13 |
8.0±3.9 |
6.1±3.8 |
5.0±3.6 |
5.1±3.6 |
Placebo, n=24 |
11.9±4.5 |
10.1±4.6 |
10.1±6.4 |
9.1±6.1 |
Fear of injury, assessment of parents of children 5-7 years old |
Brillia for Children, n=33 |
8.6±4.0 |
7.1±4.2 |
6.2±3.8 |
5.7±3.4 |
Placebo, n=24 |
6.6±3.4 |
5.7±3.2 |
5.8±3.7 |
5.0±3.5 |
Social phobia, self-assessment of children 5-7 years old |
Brillia for Children, n=33 |
8.9±3.3 |
7.3±3.3 |
6.5±3.6 |
5.7±3.2 |
Placebo, n=24 |
7.0±4.1 |
6.3±3.6 |
5.8±3.422 |
5.4±3.432 |
Social phobia, assessment of parents of children 5-7 years old |
Brillia for Children, n=13 |
9.6±5.5 |
8.1±4.5 |
6.6±3.4 |
5.5±3.9 |
Placebo, n=24 |
9.8±4.6 |
8.2±4.1 |
6.9±4.2 |
6.4±3.9 |
However, in the absence of randomization criteria depending on age, treatment, and placebo groups were not equal regarding age. Analysis of SCAS in whole or at the level of subscales (domains) revealed no significant differences between the two groups. A comparison of average scores of the CGI Scale between the groups using the Student's t-test for independent samples showed no significant differences.
Conclusions from clinical efficacy of clinical study
Thus, Brillia for Children (1 tablet t.i.d. for 12 weeks) is effective in children with anxiety disorders.
Safety Evaluation
Study (MMH-TD-001) SafetyEvaluation:
Safety criteria:
1. Nature and duration of adverse events (A.E.s) and their relationship to study drug
2. Dynamics of vital functions.
During the study, the patients did not report any abnormalities during a physical examination. Respiratory rate, blood pressure, and heart rate of children were in the range of age norms. Significant differences between the two groups were not revealed (Table 9).
Table 09. Dynamics of vital parameters (M±SD)
Drug
|
Baseline
|
4 weeks
|
8 weeks
|
12 weeks
|
Systolic blood pressure, mmHg |
Brillia for Children |
105.8±9.27 |
105.3±10.61 |
105.7±9.25 |
105.5±10.2 |
Placebo |
102.7±12.36 |
102.6±12.01 |
102.3±10.54 |
102.0±10.53 |
Diastolic blood pressure, mmHg |
Brillia for Children |
67.8±6.54 |
66.6±7.14 |
65.4±7.92 |
66.1±7.43 |
Placebo |
66.8±8.59 |
65.6±7.93 |
65.7±7.04 |
65.6±6.22 |
Breathing rate, breaths per minute |
Brillia for Children |
20.1±4.63 |
19.9±4.39 |
19.6±3.78 |
19.5±3.66 |
Placebo |
21.2±7.89 |
21.2±7.02 |
20.6±6.45 |
20.5±6.38 |
Heart rate, bpm |
Brillia for Children |
75.5±10.03 |
76.8±10.3 |
75.8±8.69 |
76.5±8.53 |
Placebo |
76.2±10.77 |
77.7±10.6 |
77.4±8.85 |
77.9±8.26 |
In the course of the study, serious A.E.s were not reported. A.E.s were observed in 15 patients in the treatment group and 12 patients in the control group. A list of A.E.s and their connection with Brillia for Children or placebo are presented in Table 10; the distribution and nature of adverse A.E.s in groups – in (Table 10,11).
Table 10. Adverse events
No. |
Description of adverse event |
Relation to study drug |
Brillia for Children |
01-001-001 |
Acute bronchitis |
Unlikely |
02-008-018 |
Aggravation of chronic maxillitis and chronic tonsillitis |
Unlikely |
03-004-024 |
Cephalgia, spotted sore throat |
Unlikely |
03-005-025 |
Acute nasopharyngitis |
Unlikely |
03-007-027 |
AURI, acute catarrhal otitis, right ear |
Unlikely |
03-010-030 |
AURI |
Unlikely |
03-014-074 |
Increase in frequency of motor tics, vocalisms, twisting of fingers |
Unlikely |
03-017-077 |
AURI |
Unlikely |
04-003-033 |
Allergic rhinitis |
Unlikely |
04-018-058 |
Ambrosial hay fever |
Unlikely |
04-019-059 |
AURI |
Unlikely |
04-021-061 |
AURI |
Unlikely |
04-022-062 |
AURI |
Unlikely |
04-026-066 |
AURI, acute laryngotracheitis, and gastroenteritis |
Unlikely |
04-045-105 |
Punctate rash of unspecified nature and genesis, pruritus |
Possible |
Placebo |
03-002-022 |
AURI |
Unlikely |
03-006-026 |
Increase in frequency of motor spasms, vocalisms, AURI |
Unlikely |
03-008-028 |
Acute intestinal infection |
Unlikely |
03-009-029 |
AURI |
Unlikely |
04-001-031 |
AURI |
Unlikely |
04-011-051 |
AURI |
Unlikely |
04-028-068 |
AURI |
Unlikely |
04-029-069 |
AURI |
Unlikely |
04-030-070 |
AURI |
Unlikely |
04-032-082 |
AURI |
Unlikely |
04-033-083 |
AURI |
Unlikely |
04-035-085 |
AURI |
Unlikely |
Table 11. The number of adverse events (n=98)
AEs |
Brillia for Children |
Placebo |
Cephalgia |
4 cases (1 patient) |
– |
Sore throat, acute nasopharyngitis |
2 cases |
– |
Acute catarrhal otitis |
1 case |
|
AURI |
10 cases (7 patients) |
13 cases (11 patients) |
Increase in frequency of motor spasms |
2 cases (1 patient) |
2 cases (1 patient) |
Increase in frequency of vocalisms |
2 cases (1 patient) |
2 cases (1 patient) |
Increase in frequency of twisting of fingers |
1 case |
– |
Allergic rhinitis |
1 case |
– |
Ambrosial hay fever |
1 case |
– |
Acute bronchitis |
1 case |
– |
Aggravation of chronic tonsillitis and maxillitis |
2 cases |
– |
Acute gastroenteritis |
1 case |
1 case |
Punctate rash of unspecified nature and genesis, pruritus |
1 case |
– |
One patient in the treatment group had A.E.s that were characterized as possibly related – punctate rash and pruritus. Two patients (one in the treatment group and one control group, acceleration of motor tics and vocalisms) had A.E.s with a relationship to study drug characterized as unlikely related since it had an equal frequency in both groups. Other A.E.s were also described as questionable to study medicine.
The majority of patients were administered concomitant therapy for A.E.s, predominantly for acute upper respiratory infections (AURI).
Conclusions from clinical safety of clinical study
Thus, Brillia for Children is safe when used in patients with anxiety disorders in a dose of 1 tablet t.i.d. for 12 weeks.
From the clinical study, Two-way analysis of covariance (ANCOVA) showed that anxiety severity under the test of R. Temple, M. Dorky, V. Amen in the study and control groups differed by a drug factor (Brillia for Children and placebo, F1/91=4.31; p=0.04). The results confirmed that Brillia for Children was superior to placebo. Furthermore, ANCOVA revealed that anxiety severity significantly decreased in the treatment group after eight weeks of treatment.
The results of testing of children and their parents during the study indicated that the use of the active drug or placebo had a significant positive impact on the severity of anxiety disorders compared to the baseline state. Three-way ANCOVA (drug, visit, domain) with repeated measures revealed no significant differences between study and control groups in terms of the dynamics of anxiety disorder severity according to patient self-assessment anxiolytic efficacy of Brillia for children was mostly manifested in young children, which was confirmed by decrease of average anxiety index after 12-week treatment (11.9 points versus 8.3 points in placebo group). After four weeks of treatment, the percentage of children with severe anxiety disorders significantly declined: from 59% to 33%. The average total score of the Spence Children's Anxiety Scale (SCAS) according to self-assessment of patients of 8-15 years of age and the reports of parents of children of 5-7 and 8-15 years during the treatment confirmed that Brillia for Children improved the severity of anxiety disorders. Thus, the clinical study showed anxiolytic effects of Brillia for Children in the treatment of anxiety disorders, and Brillia for Children (1 tablet t.i.d. for 12 weeks) is effective in children with anxiety disorders.
Brillia is an alternate treatment not only as monotherapy, but also as combination therapy with other conventional therapies such as methylphenidate and atomoxetine. The current FDA approved therapies are limited. Additionally, it is useful in patients with unmet medical needs due to treatment emergent events associated with the standard of care. However, more studies are needed with larger sample size and combined non pharmacological therapies; additionally, in patients with unmet medical needs due to treatment emergent events associated with the standard of care.
Changes in clinical and laboratory parameters were not significant and did not go beyond physiological deviations. An increase in the duration of the treatment with Brillia for children did not lead to a decrease in the efficacy. Brillia for Children was proved not to be inferior to placebo, indicating a high degree of safety of the drug. Treatment with Brillia showed improved separation anxiety in older children, and it reduced the fear of injury in younger children (anxiety disorder).
- Siegmann EM, Müller H, Luecke C (2018) Association of depression and anxiety disorders with autoimmune thyroiditis: a systematic review and meta-analysis. JAMA 75: 577-584. [Crossref]
- Arena J, Rozenbaum J (2004) Pharmacotherapy of mental disorders: Tr. from English. M Binom.
- Commission directive 2003/63/EC of June 25, 2003, amending Directive 2001/83/EC of the European Parliament and the Council on the Community code relating to medicinal products for human use.
- Constitution of the World Health Organization. Geneva, Official records of WHO, No.2:100. Directive 2001/83/EC of the European Parliament and the Council of November 6, 2001, on the Community code relating to medicinal products for human use.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition, Text Revision. 4th ed. Washington, DC: APA Press; 2000.
- Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, et al. (2013) Functions of S-100 Proteins. CurrMol Med 13: 24-57. [Crossref]
- Edwards SL, Rapee RM, Kennedy SJ, Spence SH (2010) The assessment of anxiety symptoms in preschool-aged children: the revised Preschool Anxiety Scale. J Clin Child Adolesc Psychol 39: 400-409. [Crossref]
- Shear MK, Bjelland I, Beesdo K, Gloster AT, Wittchen HU (2007) Supplementary dimensional assessment in anxiety disorders. Int J Methods Psychiatr Res 16: 52-64. [Crossref]
- Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents, American Academy of Pediatrics, 2011.
- Zavadenko NN (2006) Attention deficit hyperactivity disorder in children: diagnosis and treatment. Russian medical J. 14: 51-56.
- Khodarev SV (2002) Approaches to the diagnosis and correction of anxiety disorders in children / Khodarev SV, Poddubnaya TM, Simaeva AR. CurrPediat Iss 1: 92-94.
- Guidelines for experimental (preclinical) studies of new pharmacological agents. Ed. Habriev RU. M.: OJSC "Publishing House "Medicine," 2005. 832 p.
- Liberman LC, Lipp OV, Spence SH, March S (2006) Evidence for retarded extinction of aversive learning in anxious children. Behav Res Ther 44: 1491-1502.
- Mosholder AD, Gelperin K, Hammad TA, Phelan K, Johann-Liang R (2009) Hallucinations, and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children. Pediatrics 123: 611–616. [Crossref]
- Sawyer MG, Pfeiffer SS, Spence SH (2009) Life events, coping, and depressive symptoms among young adolescents: a one-year prospective study. J Affect Disord 117: 48-54. [Crossref]
- Keeton CP, Kolos AC, Walkup JT (2009) Pediatric generalized anxiety disorder: epidemiology, diagnosis, and management. Paediatr Drugs 11: 171-183. [Crossref]
- Shmakova OP (2004) School adaptation of children and adolescents with psychiatric disorders: Abs. Ph. D. M 24 p.
- Swanson J, Elliott GR, Greenhill LL (2007) Effects of stimulant medication on growth rates across three years in the MTA follow-up. JAmAcad Child Adolesc Psychiatry 46: 1015-1027. [Crossref]
- Mash E, Wolf D (2003) Children's psychopathology: Violations of the child's mind: Trans. from English. St. Petersburg: Prime-Evroznak 384p.
- Wenar Ch, Kering P (2004) Psychopathology of childhood and adolescence. Developmental psychopathology: Tr. from English. St. Petersburg.: Prime-Evroznak 384p.



