Abstract
The aims of the current study were to evaluate 1) the chemical interactions between a self-adhesive resin cement (SARC) with zirconia by solid-state Nuclear Magnetic Resonance (NMR); 2) the interface SARC/zirconia by scanning electron microscopy energy dispersive X‑ray (SEM-EDX) microanalysis
Materials and Methods: This in vitro study was executed in two parts: (1) Characterization and composition of the SARC - and study of the interface between SARC and a 3Y-TZP zirconia by MAS (Magic Angle Spinning) -NMR spectroscopy in collecting information about molecular organization.31P (CP)MAS (CP: Cross-Polarization MAS), 13C (CP)MAS and 1H MAS were done. (2) Energy dispersive X‑ray microanalysis of SARC tested, 3Y-TZP Zirconia and SARC/zirconia interface. The EDX spectra were collected from the specimens of SARC and zirconia using common procedures and an elemental analysis (weight% and atomic%) is performed. For the SEM-EDX analysis of the SARC/zirconia interface, zirconia is also used in the form of discs.
In our results 31P CPMAS and MAS NMR indicate that 10-MDP is less mobile in the presence of zirconia than in the SARC alone and the presence of a more symetric environment of the phosphorus nuclear charge of 10-MDP due to the interaction with zirconia. These observation proves that 10-MDP strongly interacts with the zirconia surface. Furthermore, 13C CPMAS and MAS NMR show some evidence that acrylates participate also slightly in this interaction. Analysis of the interface SARC/zirconia by SEM-EDX mainly reveals the participation of phosphorus in the interaction.
This work highlighted a strong and stable interaction of 10-MDP of SARC with zirconia and the participation of acrylates in this interaction.
Key words
self-adhesive resin cement, zirconia, solid state NMR, energy dispersive X-ray analysis, chemical organization
Introduction
Zirconia can be luted with conventional cements (zinc phosphate or resin-modified glass ionomer ones) only if the appropriate tooth preparation provides sufficient retention. Resin cements with adhesive capabilities increase retention in many other clinical situations and demonstrate superior mechanical properties and stability with the desired optimal esthetic results [1-3]. The behavior of the bonding cement and the bonding procedure are essential in the clinical success of ceramic restorations [4]. So, the complexity of some adhesive systems (multistep) can lead to technical errors and affect the quality of bonding. Self-adhesive resin cements (SARC) which do not require pretreatment, are a true innovation. They have been clinically used for almost 20 years. They simplify the bonding technique with the consequence of limiting the possibility of technical errors and the working time of the operator and have been preferred clinically [2,5-8]. Since their appearance, many formulations have been developed. 10-MDP (methacryloyloxydecyl dihydrogen phosphate)-based resin cements are considered materials of choice, as phosphate ester monomers may have chemical interaction with zirconium oxide hydroxyl groups. They therefore give better results than adhesives containing other functional monomers [9-12].
Different types of tests are performed to estimate the bond strength [1,15-21]. It must be considered that the type of test can partly influence the result [9,23]. For Comino-Garayoa [24] it is difficult to compare different mechanical studies comparing materials even if they are carried out with the same test method because there are multiple variables often unspecified, especially if they are intraoral simulation.
Studies address the chemical interaction between some of the monomers of SARC and zirconia and sometimes, more rarely, between SARC in its complete formula and zirconia [25-28]. Chen [26] investigated the chemical affinity between Y-TZP and 10-MDP dissolved in different solvents (acetone/ethanol/water or mixture) using X-ray photoelectron spectroscopy, Fourier-transform infrared spectroscopy, and thermodynamic calculations. Several phosphate ester monomer variants were created. Changes included modifications to the length of the spacing chain and installing hydroxyl or carboxyl groups as side chains at different positions along the spacer chain. The study concludes that the change in molecular structure of 10-MDP affects its chemical affinity to Y-TZP. Xie [27] investigated the possibility of chemical bonding between 10- MDP and tetragonal zirconia and the effect of pH reaction conditions on such prospective chemical bonds via a computational modeling approach. Study demonstrates that MDP can form a chemical bond with zirconia and that an alkaline pH favorably modifies the formation of MDP–ZrO2 bonds. Nagaoka, et al. [28] determined by a study using Nuclear Magnetic Resonance (NMR) that not only are ionic bonds between 10-MDP and zirconia, but also hydrogen bonds.
Studies based on different techniques reveal chemical interactions between the 10-MDP monomer and zirconia. However the studies do not take into account the entire structure of the SARC in the possible bonds with zirconia.
Therefore, the aims of the current study were to evaluate 1) the chemical interactions between all the components of SARC (not only 10-MDP) with zirconia by NMR; 2) the interface SARC/zirconia by Energy dispersive X‑ray (EDX) microanalysis.
Materials and methods
The SARC tested is SpeedCEM Plus (Ivoclar Vivadent, Schaan, Liechtenstein) (Table 1). The present in vitro study is executed in two parts: (1) Characterization and composition of the SARC - and study of the interface between SARC and a 3Y-TZP zirconia by solid-state MAS NMR spectroscopy in collecting information about molecular organization, (2) Energy dispersive X‑ray (EDX) microanalysis of SARC tested, 3Y-TZP Zirconia and SARC/zirconia interface (Table 1).
Table 1. Chemical composition of the self-adhesive composite cement
SpeedCEM Plus |
Resin |
Fillers |
Ivoclar Vivadent, Schaan, Liechtenstein,
lot number: ZO2030 |
UDMA,
TEGDMA, DDMA,
MDP,
dibenzoyl peroxide,
stabilizer |
Barium glass
Silica
Ytterbium fluoride |
As we have specified the adhesive monomer is 10-MDP. This monomer has a long hydrocarbon chain with a phosphate ester terminal group on one side and an acrylic functional group on the other.
The zirconia that was used in this study is a 3Y-TZP zirconia (Ivoclar Vivadent, Schaan, Liechtenstein, lot XY 308 573 F). The zirconia used here is mainly composed of zirconium oxide but also includes aluminium oxide and yttrium oxide (3% by molar weight of yttrium oxide in order to stabilize the zirconia at room temperature. This percentage is equivalent to 4.5-6% by weight of yttrium oxide). It is used in the form of powder and discs. Regarding zirconia powder, the size of the grains is of 0.50 μm. We previously analyzed it by NMR spectroscopy [29].
Solid-state NMR experiments were recorded on a Bruker Avance 400 III HD spectrometer operating at a 9.4 T magnetic field. Samples were packed into 4 mm zirconia rotors. The rotors were spun at 10 kHz at 298 K. 31P MAS (Magic Angle Spinning) and CPMAS (Cross-Polarization MAS) were done with recycle delays of 10 s and 3 s respectively and a contact time of 3 ms. 13C MAS and CPMAS have been performed with recycle delays of 3 s and 2 s respectively, and a contact time of 2 ms. 1H MAS performed with the DEPTH pulse sequence and a recycle delay of 2 s.
Samples preparation
The SARC alone and finally the SARC put in contact with the zirconia powder are studied. For the mixture, 80 mg of zirconia powder are mixed with 10 mg of cement, photopolymerized at 1200 mW/cm² (bluephase - Ivoclar Vivadent, Schaan, Liechtenstein) and then transferred and packed into zirconia rotors for MAS NMR experiments.
Energy dispersive X‑ray microanalysis
EDX microanalysis was performed using FEG FEI Quanta 250 Scanning Electron Microscope (Thermo Fisher Scientific) in low vacuum mode (Pressure of 100 Pa), using an accelerating voltage of 20 kV and the backscatter electron detector. On Scanning Electron Microscope (SEM), EDX analysis was performed using EDAX GENESIS Software of the EDS EDAX Octane Elect plus (EDAX Inc., Mahwah, New Jersey, United States). The EDX spectra were collected from the specimens of SARC and zirconia using common procedures and an elemental analysis (weight% and atomic%) is performed.
For the analysis SEM-EDX of the SARC/zirconia interface, zirconia is also used in the form of discs. Zirconia is then machined dry (Machine tool: Select Hybrid 5-axis Ivoclar Vivadent Wieland) in the form of discs of 13 mm in diameter and 2 mm thick. The machining is done from the burs of larger diameter and then reduced when the machining is refined. The burs used are made of tungsten, first of 2.5 mm then of 1 mm. The SARC is applied on zirconia disc following the manufacturer’s instructions and cured at a luminous intensity. A first insolation is of 30 s of about 650 mW/cm2 (bluephase - Ivoclar Vivadent, Schaan, Liechtenstein - LOP mode), followed by a second insolation at 1200 mW/cm² (bluephase, HIP mode) for 20 s. There is no other surface treatment at the end of the machining. The analysis is carried out after 15 days of waiting.
Also for the study of the interface other samples are prepared by mixing cement with zirconia powder and photopolymerized with the same protocol as before. The specimens were mounted on aluminium stubs for SEM and elemental analysis is performed.
Results
We analyze the SARC/zirconia using solid-state NMR. Previous studies exist by NMR but only address the 10-MDP/zirconia interface, the interface between zirconia and all the elements forming part of a self-adhesive cement isn’t approached.
We also study the interface using scanning electron microscopy with energy-dispersive X-ray spectrometry (SEM-EDX) to provide more information on the interface therefore obtained.
NMR of SARC and SARC/Zirconia interface
a) 1H NMR
The 1 MAS NMR spectrum of photopolymerized SARC is shown in show the different hydrogenated components of the self-adhesive cement. This spectrum has been recorded after polymerization of the cement outside the NMR rotor. The 1 spectrum presents two types of signals: i) one very broad with strong spinning sidebands (SSB) characteristic of rigid materials. The SSB are mostly associated to 1-1 dipolar couplings in this case; ii) some sharper signals with smaller SSB that is typical of molecules that show some local mobility. Indeed, local motions for H atoms decrease the strength of the dipolar couplings
The sharp peaks between 1.0 and 1.6 ppm, 3.4 and 3.8 ppm and 5.2 and 5.8 ppm correspond respectively, to alkyl, alkoxy and alkene groups. They indicate the presence of some mobile species even after solidification of the cement. These signals are similar with previous studies on 10-MDP analyzed by NMR [30] and indicate that few 10-MDP molecules can be slightly mobile in the rigid material. The alkene groups correspond to the acrylic functional groups of 10-MDP but also potentially to other methylacrylate compounds [31-33].
In the presence of zirconia (Figure 2), we note a clear decrease of the SSB that indicates a global increase of mobilities inside the cement material at the zirconia/cement interface. We can also observe a clear decrase of the sharper signal of alkyl groups (1.0-1.6 ppm) that are mostly associate to the alkyl chain of 10-MDP. This result evidences a specific interaction between 10-MDP and the zirconia surface.
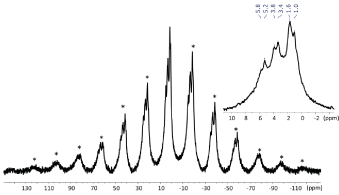
Figure 1. 1H MAS NMR spectra of SARC with a zoom of the 10/-2 ppm area. * = Spinning Sidebands
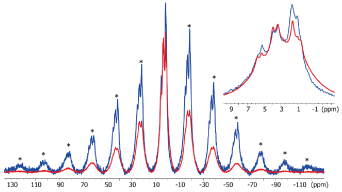
Figure 2.1H MAS NMR spectra of SARC in absence (blue) and in presence (red) of zirconia with a zoom of the 10/-2 ppm area. * =
Spinning Sidebands
b) 13 C NMR
13C CPMAS spectrum of photopolymerized SARC is shown in Figure 3. The CPMAS experiment is adequate for observation of protonated rigid material. The main signal are in the alkyl (10-50 ppm) and alkoxy (60-70 ppm) areas. Notably, the signals at 44 ppm is characteristic of the polymerization reaction of the acrylates. Only weak signals are observed around 125 ppm indicating that most of the acrylate have reacted and been polymerized. Some carbonyl groups typical of ester (~156 ppm) and carboxylate (~178 ppm) groups are also detected and are part of the acrylic resin material. We also performed a MAS experiment with direct polarization of carbon atoms with a short relaxation delay (5 s). This experiment allows to check if some mobile species are present in the UV-irradiated cement as it was observed by 1H MAS NMR. The 13C MAS experiment (Figure 4) shows only weak and broad signals in the alkyl-alkoxy area confirming that only few molecules are slightly mobile.
In the presence of zirconia we note differences between the 13C spectra. In the CPMAS one, the signals are broader pointing an increase of structural heterogeneity for the cement due to the presence of the interface with zirconia. A weaker polymerization reaction of the cement can be proposed due to a weaker signal at 44 ppm. Finally, an increase of the relative intensity of the alkyl signal (at ~20 ppm) can be related to rigidification of 10-MDP and some acrylate at the zirconia surface. The 13C MAS shows several intense and sharp signals notably for acrylate functions and alkoxy groups. It can be associated to the lower polymerisation level of the cement at the zirconia interface. This means that zirconia impacts the organisation of the SARC during the polymerization and indicates that some acrylate species are located at the interface and so slightly interact with the zirconia.
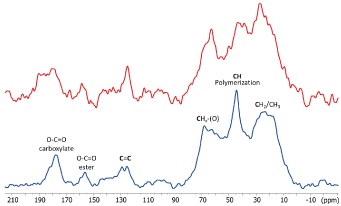
Figure 3. 13 C CP MAS NMR spectra of SARC alone (blue) and in the presence of zirconia (red)
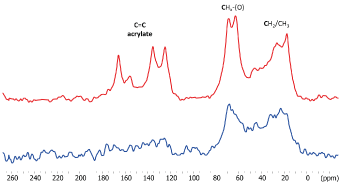
Figure 4. 13 C NMR spectra of SARC alone (blue) and in the presence of zirconia (red)
c) 31P NMR
The 31P MAS NMR spectrum (Figure 5) of SARC displays a broad isotropic resonance at -7 ppm with intense spinning sidebands related to a strong chemical shift anisotropy. This 31P peak is associated to the 10-MDP species and shows an important asymetry of the nuclear charge on the phosphorus atom. In presence of zirconia, there is no significant shift or change of the shape of the isotropic 31P resonance. However, there is a clear decrease of the spinning sideband intensities with zirconia. This decrease can have two origins: a change in the charge symetry or an increase of mobility that reduce the chemical shift anisotropy. The 31P CPMAS NMR signal (Figure 6) is much stronger in presence of zirconia than in its absence. As intensity of CPMAS is strongly affected by the mobility, this indicates that 10-MDP is less mobile in the presence of zirconia than in the SARC alone. The reduction of spinning sidebands in the MAS experiment is so related to a more symetric environment of the phosphorus of 10-MDP due to a the interaction with zirconia. These observation prove that 10-MDP strongly interacts with the zirconia surface.
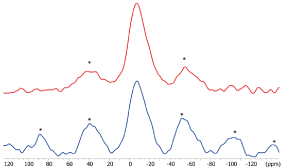
Figure 5. 31 P NMR spectra of SARC alone and in the presence of zirconia. * = Spinning Sidebands
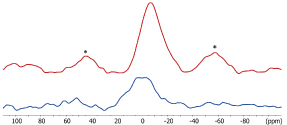
Figure 6. 31 P CPMAS NMR spectra of SARC alone and in the presence of zirconia. * = Spinning Sidebands
SEM-EDX of SARC/ zirconia interface
We analyze the SARC, then the zirconia,to finally study their interface.
a) SEM-EDX of SARC
We analyze two different areas on picture obtained by backscatter electron detector (BSD) that seem to be representative of the structure: dark area 1, bright area 2 (Figure 7: 1,2). With the BSD detector, the light area represents the area where there are more high atomic weight atoms (Figure 7: 3,4). We note that the areas analysed by SEM-EDX punctually do not have the same composition, in particular show a different percentage of ytterbium and O.
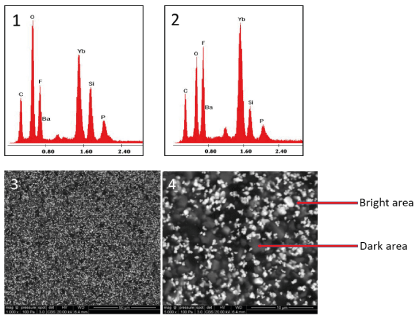
Figure7. (1) Energy dispersive X‑ray spectrum of SARC (dark area), (2) Energy dispersive X‑ray spectrum of SARC (bright particle area 2) Scanning electron microscope image of SARC ( 3 : ×1000, 4 : × 5000)
b) SEM-EDX of zirconia disc
As expected, it is composed mainly of Zr (47.8%) and O (32.7%) but also with a significant amount of C (16.9 %) (Figure 8, Table 2).
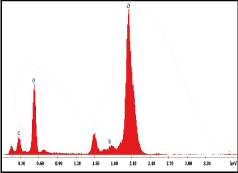
Figure8. Energy dispersive X‑ray spectrum of zirconia disc
Table 2. Weight of main constituents (elements) identified with energy dispersive X-ray microanalysis of SARC (A); of zirconia (B); of interface between Zirconia disc and SARC (C)
SEM-EDX |
SARC |
Zirconia disc |
Interface Zirconia disc/SARC |
Elem |
Wt %area 1 |
Wt % area 2 |
Wt % |
Wt % |
C |
22.41 |
27.34 |
16.99 |
38.55 |
O |
32.90 |
20.33 |
32.70 |
17.14 |
F |
13.18 |
10.81 |
|
7.72 |
Si |
6.16 |
3.95 |
0.19 |
1.02 |
P |
2.62 |
1.94 |
|
0.56 |
Zr |
|
|
47.86 |
20.49 |
Ba |
3.84 |
1.43 |
|
0.40 |
Yb |
18.88 |
31.82 |
|
14.50 |
Y |
|
|
2.25 |
|
Al |
|
|
0.15 |
|
EDX analysis estimates the average composition of the SARC area 1 and of the SARC area 2 Both the materials differed in their composition mainly for Yb (Table 2 ).
c) Interface between zirconia disc and SARC
The observation of the interface between the self-adhesive cement and the zirconia disc having undergone no preparation reveals, at low and higher magnification (×300; ×1500; ×2000), a homogeneous and continuous interface (Figure 9)

Figure9. Scanning electron microscope image of interface between zirconia disc and SARC (×300) (A), (×1500) (B), (×2000) (C)
We note in the EDX analysis carried out on the whole of the interface, the components of the 2 biomaterials. Note the 0.56 wt% P (Figure 10, Table 2).
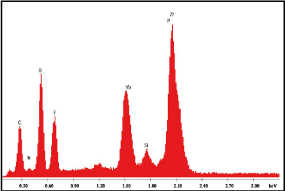
Figure10. Energy dispersive X‑ray spectrum of interface between zirconia disc and SARC
Discussion
In our results 31P CPMAS and MAS NMR spectra show that 10-MDP is less mobile in the presence of zirconia than in the SARC alone. Also, the reduction of spinning sidebands in the MAS experiment is related to a more symetric environment of the phosphorus of 10-MDP due to a the interaction with zirconia. These observation proves that 10-MDP strongly interacts with the zirconia surface. This is in line with previous studies. Meta-analyses and review of bonding to zirconia were previously carried out in particular by Mattiello [34], Inokoshi [35], Özcan [9], Tzanakakis [21], Thammajaruk [10], Scaminaci Russo [22] and Comino-Garayoa [24]. According to the results obtained by the different studies, the authors agree to analyze that phosphate ester monomers, such as MDP, chemically react with ZrO2, promoting a water-resistant bond to densely sintered zirconia ceramics. MDP-based resin cement is advocated as mandatory for better adhesion to ZrO2. The 10-MDP presents a terminal functional group with phosphoric acid, which reacts with zirconia and forms P-O-Zr bonds. The other end of the molecule is occupied by a vinyl terminal group, which allows the copolymerization with the resin [21].
We also highlight that the 13C MAS shows several intense and sharp signals notably for acrylate functions and alkoxy groups for SARC/Zirconia. This means that zirconia impacts the organisation of the SARC during the UV-irradiation and so evidence weak interactions between acrylates and zirconia. These results were also confirmed by 1H NMR which evidence difference of mobilities for SARC alone or in presence of zirconia. The sharp 1H signals are similar with previous studies on 10-MDP analyzed by NMR [30] and indicate that few 10-MDP molecules can be slightly mobile in the rigid material. The alkene groups correspond to the acrylic functional groups of 10-MDP but also potentially to other methylacrylate compounds [31-33].
In an experimental study Ling, in 2022, [23] analyzes that 10-MDP which has a longer ethylene chain is more hydrophobic than other phosphoric acid monomers such as glycerol phosphate dimethacrylate (GPDM) and dipentaerythritol pentaacrylate monophosphate (PENTA). This hydrophobicity of the longer ethylene backbone enhanced the pH neutralization capacity of the cement, which resulted in less susceptibility to hydrolysis over time and greater bonding than other self-adhesive resin cements with different phosphate functional monomers. This is consistent with the results of other authors including Sriamporn [36] who explains that the 10-MDP chemical reaction with the oxide layer of the base metal alloy or zirconium oxide may explain the high shear bond strength on base metal alloys and zirconia. 10-MDP is an acidic phosphate functional monomer along a lengthy chain spacer of -(CH2)n than GPDM [28].
Some authors have suggested using a MDP containing primer before the SARC [15,16]. But studies show that use of MDP-containing primer did not increase the bond strength in the MDP-containing SARC and that the increased MDP concentration in both the primer and SARC did not lead to an additional enhancement in bond strength [37,38,39]. There are a few studies reporting optimal MDP concentrations for maximum bond strength to zirconia ceramics. Nagaoka suggested that a minimum 1-ppb MDP was needed to bond to zirconia [28]. Thus, the 10-MDP concentration should be optimized in future investigations.
The experimental part conducted thanks to EDX microanalysis makes it possible to place the SARC on zirconia discs according to the manufacturer's instructions and to evaluate the interface obtained. EDX is a reproducible and precise technique to identify and quantify major components present in a material. Still, it has limitations for precise detection of low molecular weight elements such as carbon, hydrogen, and oxygen [40]. For the analysis of zirconia discs the high percentage of C may be due to some diamond abrasive remains. The EDX microanalysis reveals on the whole of the interface, the components of the 2 biomaterials which goes in the hypothesis of a good assembly. Note the detection of P which can determine the participation of 10-MDP.
Conclusion
Depending on the results of this study, the conclusions can be given:
- NMR makes it possible to study the local environment of many nuclei and the dynamics in solids. This method of analysis allowed us to highlight a change in the environment when the self-adhesive cement SpeedCEM Plus (SARC) is in contact with the 3Y-TZP Zirconia that has not undergone prior treatment.
- The phosphorus of 10-MDP contained in SARC reacts stably with zirconia.
- Another study has already analyzed the interactions of 10-MDP with zirconia by NMR, however their study exclusively analyzes the interaction of 10-MDP with zirconia and not that of an adhesive-cement including all of its components. This allowed us to highlight the second strong point - which is the participation of the acrylate group in the interaction with zirconia.
- Microscopy, which gives results on a completely different scale, has allowed us to see that SARC-applied in accordance with clinical conditions on zirconia discs not previously prepared- shows a continuous and homogeneous interface. It is also to be noted that the analysis of the interface highlights the participation of phosphorus.
Acknowledgments
The authors thank the manufacturer for the kind support of the materials used in the study.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Pilo R, Folkman M, Arieli A, Levartovsky S (2018) Marginal Fit and Retention Strength of Zirconia Crowns Cemented by Self-adhesive Resin Cements. Oper Dent 43: 151-161. [Crossref]
- Attar N, Tam LE, McComb D (2003) Mechanical and physical properties of contemporary dental luting agents J Prosthet Dent 89: 127-134. [Crossref]
- Ernst CP, Cohnen U, Stender E, Willershausen B (2005) In vitro retentive strength of zirconium oxide ceramic crowns using different luting agents. J Prosthet Dent 93: 551-558. [Crossref]
- da Silva EM, Miragaya L, Sabrosa CE, Maia LC (2014) Stability of the bond between two resin cements and an yttria-stabilized zirconia ceramic after six months of aging in water. J Prosthet Dent 112: 568-575. [Crossref]
- Behr M, Rosentritt M, Wimmer J, Lang R, Kolbeck C, et al. (2009) Self-adhesive resin cement versus zinc phosphate luting material : A prospective clinical trial begun 2003. Dent Mater 25: 601-604. [Crossref]
- Ferracane JL, Stansbury JW, Burke FJ (2011) Self-adhesive resin cements - chemistry, properties and clinical considerations. J Oral Rehabil 38: 295-314. [Crossref]
- Manso AP, Carvalho RM (2017) Dental cements for luting and bonding restorations: self-adhesive resin cements. Dent Clin North Am 61: 821-834. [Crossref]
- Liu JF, Yang CC, Luo JL, Liu YC, Yan M, et a. (2022) Bond strength of self-adhesive resin cements to a high transparency zirconia crown and dentin. J Dent Sci 17: 973-983. [Crossref]
- Özcan M, Bernasconi M (2015) Adhesion to zirconia used for dental restorations: a systematic review and meta-analysis. J Adhes Dent 17: 7-26. [Crossref]
- Taniş MC, Akçaboy C (2015) Effect of different surface treatment methods and MDP monomer on resin cementation of zirconia ceramics an in vitro study. J Lasers Med Sci 6: 174-181. [Crossref]
- Thammajaruk P, Inokoshi M, Chong S, Guazzato M (2018) Bonding of composite cements to zirconia: a systematic review and meta-analysis of in vitro studies. J Mech Behav Biomed Mater 80: 258-268. [Crossref]
- Yoshida K, Tsuo Y, Atsuta M (2006) Bonding of dual-cured resin cement to zirconia ceramic using phosphate acid ester monomer and zirconate coupler. J Biomed Mater Res B Appl Biomater 77: 28-33. [Crossref]
- Oyagüe RC, Monticelli F, Toledano M, Osario E, Ferrar M, et al. (2009) Effect of water aging on microtensile bond strength of dual-cured resin cements to pre-treated sintered zirconium-oxide ceramics. Dent Mater 25: 392-399. [Crossref]
- Gomes AL, Castillo-Oyagüe R, Lynch CD, Montero J, Albaladejo A (2013) Influence of sandblasting granulometry and resin cement composition on microtensile bond strength to zirconia ceramic for dental prosthetic frameworks. J Dent 41: 31-41. [Crossref]
- Kitayama S, Kitayama S, Nikaido T, Takahashi R, Zhu L, Ikeda M, et al. (2010) Effect of primer treatment on bonding of resin cements to zirconia ceramic. Dent Mater 26: 426-432. [Crossref]
- Koizumi H, Nakayama D, Komine F, Blatz MB, Matsumura H (2012) Bonding of resin-based luting cements to zirconia with and without the use of ceramic priming agents. J Adhes Dent 14: 385-392. [Crossref]
- Zorzin J, Belli R, Wagner A, Petschelt A, Lohbauer U (2014) Self-adhesive resin cements: adhesive performance to indirect restorative ceramics. J Adhes Dent 16: 541-546. [Crossref]
- Chen L, Suh B I, Brown D, Chen X (2012) Bonding of primed zirconia ceramics: evidence of chemical bonding and improved bond strengths. Am J Dent 25: 103-108. [Crossref]
- Noda Y, Nakajima M, Takahashi M, Mamanee T, Hosaka K, et al. (2017) The effect of five kinds of surface treatment agents on the bond strength to various ceramics with thermocycle aging. Dent Mater J 36: 755-761. [Crossref]
- Le M, Larsson C, Papia E (2019) Bond strength between MDP-based cement and translucent zirconia. Dent Mater J 38: 480-489. [Crossref]
- Tzanakakis EG, Tzoutzas IG, Koidis PT (2016) Is there a potential for durable adhesion to zirconia restorations? A systematic review. J Prosthet Dent 115: 9-19. [Crossref]
- Scaminaci Russo D, Cinelli F, Sarti C, Giachetti L (2019) Adhesion to Zirconia: A Systematic Review of Current Conditioning Methods and Bonding Materials. Dent J(Basel) 7: 74. [Crossref]
- Ling L, Ma Y, Chen Y, Malyala R (2022) Physical, Mechanical, and Adhesive Properties of Novel Self-Adhesive Resin Cement. Int J Dent 2022: 4475394. [Crossref]
- Comino-Garayoa R, Peláez J, Tobar C, Rodríguez V, Suárez MJ (2021) Adhesion to Zirconia: A Systematic Review of Surface Pretreatments and Resin Cements. Materials (Basel) 14: 2751. [Crossref]
- Shimoe S, Hirata I, Otaku M, Matsumura H, Kato K, et al. (2018) Formation of chemical bonds on zirconia surfaces with acidic functional monomers. J Oral Sci 60: 187-193. [Crossref]
- Chen Y, Lu Z, Qian M, et al. (2017) Chemical affinity of 10-methacryloyloxydecyl dihydrogen phosphate to dental zirconia: effects of molecular structure and solvents. Dent Mater 33: e415-e427. [Crossref]
- Xie H, Tay FR, Zhang F, Lu Y, Shen S, et al. (2015) Coupling of 10-methacryloyloxydecyldihydrogenphosphate to tetragonal zirconia: effect of pH reaction conditions on coordinate bonding. Dent Mater 31: e218-e225. [Crossref]
- Nagaoka N, Yoshihara K, Feitosa VP, Tamada Y, Irie M, et al. (2017) Chemical interaction mechanism of 10-MDP with zirconia. Sci Rep 7: 45563. [Crossref]
- Tavernier B, Coppel Y, Prigent Y, Grégoire G (2021) Zirconia interaction with zinc phosphate cement studied by solid-state NMR spectroscopy. Oral Health and Care 6: 1-5.
- Yoshihara K, Nagaoka N, Yoshida Y, Van Meerbeek B, Hayakawa S (2019) Atomic level observation and structural analysis of phosphoric-acid ester interaction at dentin. Acta Biomaterialia 97: 544-556. [Crossref]
- Grégoire G, Sharrock P, Prigent Y (2016) Performance of a universal adhesive on etched and non-etched surfaces: Do the results match the expectations? Mater Sci Eng C Mater Biol Appl 66: 199-205. [Crossref]
- Coppel Y, Prigent Y, Grégoire G (2022) Dentin interaction with universal adhesive containing isopropanol solvent studied by solid-state NMR spectroscopy. Dent Mater 38 : 7-18. [Crossref]
- Coppel Y, Prigent Y, Grégoire G (2021) Characterization of hydrogenated dentin components by advanced 1H solid-state NMR experiments. Acta Biomater 120: 156-166. [Crossref]
- Mattiello R, Coelho T, Insaurralde E, Coelho A, Terra G, et al. (2013) A review of surface treatment methods to improve the adhesive cementation of zirconia-based ceramics. ISRN Biomaterials 185376: 1-10.
- Inokoshi M, De Munck J, Minakuchi S, Van Meerbeek B (2014) Meta-analysis of bonding effectiveness to zirconia ceramics. J Dent Res 93: 329-334. [Crossref]
- Sriamporn T, Thamrongananskul N, Klaisiri A (2022) The Effectiveness of Various Functional Monomers in Self-adhesive Resin Cements on Prosthetic Materials. J Int Soc Community Dent 12: 332-335. [Crossref]
- Go EJ, Shin Y, Park JW (2019) Evaluation of the microshear bond strength of MDP-containing and non-MDP-containing self-adhesive resin cement on zirconia restoration. Oper Dent 44: 379-385. [Crossref]
- Van Landuyt KL, Peumans M, Munck JD, Lambrechts P, Meerbeek BV (2006) Extension of a one-step self-etch adhesive into a multi-step adhesive. Dent Mater 22: 533-544. [Crossref]
- Blatz M, Sadan A, Martin J, Lang B (2004) In vitro evaluation of shear bond strengths of resin to densely-sintered high-purity zirconium-oxide ceramic after long-term storage and thermal cycling. J Prosthet Dent 91: 356-362. [Crossref]
- Saxena S, Tiwari S (2016) Energy dispersive X-ray microanalysis, fluoride release, and antimicrobial properties of glass ionomer cements indicated for atraumatic restorative treatment. J Int Soc Prevent Communit Dent 6: 366-372. [Crossref]










