Abstract
Background: Subclinical atrial fibrillation (AF) can be defined as asymptomatic AF of short duration and detected by long-term, continuous monitoring. This condition has been best studied in patients who receive a cardiovascular implantable electronic device for reasons other than monitoring of arrhythmia.
Methods: We conducted a prospective, longitudinal study in 50 controlled hypertensive subjects with dual chamber pacemaker, subclinical AF which underwent pulmonary vein isolation (PVI) by radiofrequency ablation. From the 50 patients enrolled, half of them had the atrial lead positioned in the right atrial appendage (RAA), and the other half had the atrial lead positioned in the right atrial lateral wall (RALW).
Results: We observed that the right atrial sense (mV) in patients that had the atrial lead positioned in the RAA decreased from 3.4±1.1 to 1.4±1.1 mV, one month post PVI (P<0.0001). However, in the group with the atrial lead positioned in the RALW, no changes occurred. The right atrial sense comparison between 2 groups at the first month after ablation was significantly different (P<0.0001).
Conclusion: We observed that post PVI the patients that had the right atrial lead positioned in the RAA presented changes in the leads parameters in comparison with them positioned in the RALW.
Key words
subclinical atrial fibrillation, stroke Evaluation, dual-chamber pacemaker, arrhythmia, Stroke Risk study
Introduction
Subclinical atrial fibrillation (AF) can be defined as asymptomatic AF of short duration and detected by long-term, continuous monitoring. This condition has been best studied in patients who receive a cardiovascular implantable electronic device (CIED) for reasons other than monitoring of arrhythmia [1-3]. The Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT) [4] enrolled patients with a dual-chamber pacemaker for sinus node or atrioventricular node disease or with an implantable cardioverter- defibrillator for any reason. The Relationship Between Daily Atrial Tachyarrhythmia Burden From Implantable Device Diagnostics and Stroke Risk study (TRENDS) [2] enrolled patients with a clinical indication for a pacemaker or implantable cardioverter defibrillator and at least 1 stroke risk factor. Both populations would be expected to have an AF prevalence above that of the general population. In ASSERT, at least 1 atrial tachyarrhythmia was detected by an implanted device among 10.1% of patients within 3 months and in an additional 24.5% of patients within approximately 2.5 years [4]. In TRENDS, atrial tachyarrhythmias occurred in 12 of 163 patients during more than 10% of the surveillance period; an additional 33 patients had atrial tachyarrhythmias during the 1.1-year of follow-up [2]. Long-term continuous monitoring increases the chance of detecting not only AF that is present at the time of monitoring initiation but also AF that develops subsequently.
Study
Oral anticoagulation is recommended in patients with clinical AF who are at high risk for stroke, irrespective of AF subtype [5]. Because oral anticoagulation has been proven to reduce stroke risk in patients with clinical AF, oral anticoagulation may reduce stroke risk in patients with subclinical AF; however, the absolute risk of stroke associated with subclinical (vs. clinical) AF is lower, thereby possibly mitigating the treatment effect and net clinical benefit of anticoagulation. While awaiting empirical evidence, patients with a CHA2DS2-VASc score of less than 2 are unlikely to benefit from oral anticoagulation regardless of the duration of subclinical AF. Those with a CHA2DS2-VASc score of 2 or greater who experience longer episodes of AF (e.g., >24 hours) may be similar enough to patients with AF enrolled in historical clinical trials to warrant anticoagulation. For episodes shorter than 6 minutes in patients with a CHA2DS2-VASc score of at least 2, the risk of stroke is very low, and not starting anticoagulation therapy is reasonable; however, for episodes ranging from 6 minutes to 24 hours in the setting of a CHA2DS2-VASc score of at least 2, little agreement exists on the risks vs. benefits of starting anticoagulation treatment.
Based on this information, we conducted a prospective, longitudinal study in 50 controlled hypertensive subjects with dual chamber pacemaker, subclinical AF presenting episodes ranging from 6 minutes to 24 hours, a CHA2DS2-VASc score of less than 2, in which all the patients underwent pulmonary vein isolation (PVI) by radiofrequence ablation. From the 50 patients enrolled, half of them had the atrial lead positioned in the right atrial appendage (RAA), and the other half had the atrial lead positioned in the right atrial lateral wall (RALW). The study was piloted in accordance with the Helsinki Declaration and approved by the Ethics Committee. All patients signed the written informed consent before inclusion. In the present study, we aim to evaluate the effects of radofrequence energy in the atrial leads parameters accordingly to their position in the right atrium one month after PVI, during de pacemaker evaluation.
This study was conducted in the state of Rio de Janeiro, Niterói in the Cardiostim. Patients were recruited from January 2015 to December 2016 and were derived from Arrhythmias and Artificial Cardiac Pacing Service of the same institution. Patients who had the combination of the following criteria were consecutively enrolled: (i) mean 24-hour systolic ambulatory blood pressure measurements (ABPM) <130/<180 mmHg; (ii) age between 18 and 80 years; (iii) structurally normal heart to myocardial scintigraphy and transthoracic echocardiography, without ischemia, fibrosis area or any other illness, with a left ventricular ejection fraction of >50% as measured by echocardiography (Simpson's method); (iv) subclinical AF presenting episodes ranging from 6 minutes to 24 hours detected by pacemaker; (v) CHA2DS2-VASc score of less than 2; (vi) not having undergone previous AF treatment; (vii) glomerular filtration rate estimated by the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation, eGFR [6] >60mL/min/1.73 m2 without microalbuminuria; and (viii) be able to read, understand and sign the informed consent form.
Patients with any of the following criteria were excluded: (i) pregnancy; (ii) valvular disease with significant hemodynamic repercussions; (iii) myocardial infarction, unstable angina, stroke or transient ischemic attack within the previous 6 months; (iv) mobility deficit; (v) psychiatric disease; (vi) patients unable to be followed clinically; (vii) patient known to have drug addiction or alcohol, can affect the ability to understand or follow medical instructions; (viii) patient has a serious disease, which in the opinion of the investigator, may adversely affect the safety and / or efficacy of the participant or the study.
Results
The results were expressed as the mean and standard deviation (mean ± SD) of the mean in the case of normal distribution and as the median with inter-quartile range otherwise. Statistical tests were all two sided. Comparisons between two-paired values were performed by the paired t-test in case of a Gaussian distribution or, alternatively, by the Wilcoxon test. Comparisons between more than two-paired values were performed by ANOVA for repeated measures or with Kruskal– Wallis ANOVA as appropriate complemented by a post hoc test. Frequencies were compared with Fisher’s exact test. P-values < 0.05 were considered significant. All statistical analysis was performed using the program Graphpad Prism v 7.0 (Graphpad software, La Jolla, CA, USA).
General features of the 50 subjects divided into two groups are listed in Table 1. We observed that the right atrial sense (mV) in patients that had the atrial lead positioned in the RAA decreased from 3.4±1.1 to 1.4±1.1 mV, one month post PVI (P<0.0001). However, in the group with the atrial lead positioned in the RALW, no changes occurred from baseline 3.4±1.1 to 3.3±1.0 mV (P=0.9975) in relation the first month after PVI. The right atrial sense comparison between 2 groups at the first month after ablation was significantly different (P<0.0001), as shown in Figure 1A. The atrial threshold (V) @ 0.5 ms in the subjects presenting the atrial lead positioned inside the RAA augmented from 0.6±0.3 to 1.0±0.2 V, one month post PVI (P<0.0001), and in the individuals with the atrial lead positioned in the RALW no changes occurred from baseline 0.7±0.3 to 0.7±0.2 V (P=0.9556) in relation the first month after PVI. The atrial threshold comparison between 2 groups at the first month post ablation was significantly different (P=0.0003), as shown in Figure 1B. We also evaluated, the atrial resistance (Ω) in the subjects presenting the atrial lead positioned inside the RAA reduced from 475.4±154.6 to 360.2±121.5 Ω, one month post PVI (P=0.0351), and in the patients with the atrial lead positioned in the RALW no changes occurred from baseline 466.7±152.2 to 476.9±160.7 V (P=0.9949) vs. the first month after PVI. The atrial resistance comparison between 2 groups at the first month post PVI was significantly different (P=0.0321), as shown in Figure 1C.
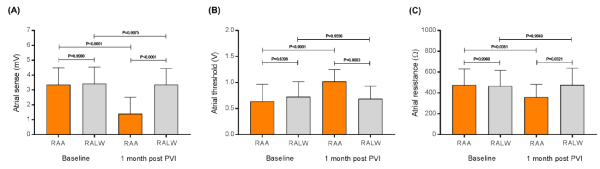
Figure 1. Right atrial leads sense (A), the threshold (B), and resistance (C) at baseline and one month after pulmonary vein isolation (PVI); RAA, right atrial appendage (n=25); RALW, right atrial lateral wall (n=25). Values are presented as mean ± SD.
Table 1. General features of patients at baseline.
|
Atrial lead position |
Parameters |
RAA |
RALW |
P value |
N |
25 |
25 |
--- |
Age, years |
65±6 |
68±6 |
0.0785 |
Body mass index, kg/m2 |
26.6±2.3 |
25.8±2.2 |
0.2354 |
Male gender (%) |
16 (64%) |
13 (52%) |
0.5672 |
White ethnicity (%) |
20 (80%) |
17 (68%) |
0.5202 |
Dual chamber pacemaker |
25 (100%) |
25 (100%) |
1.0000 |
Subclinical paroxysmal AF |
25 (100%) |
25 (100%) |
1.0000 |
Controlled hypertension |
25 (100%) |
25 (100%) |
1.0000 |
Type 2 Diabetes Mellitus |
8 (32%) |
12 (48%) |
0.3868 |
Creatinine, mg/dL |
0.80±0.21 |
0.78±0.11 |
0.9928 |
eGFR, mL/min/1.73 m2 |
96.7±20.0 |
99.3±16.7 |
0.6201 |
Albumin:creatinine ratio, mg/g |
20.4±7.2 |
18.0±9.1 |
0.3063 |
Antihypertensive |
|
|
|
ACE-inhibitors/ARB |
25 (100%) |
25 (100%) |
1.0000 |
Diuretics |
25 (100%) |
25 (100%) |
1.0000 |
β-blockers |
25 (100%) |
25 (100%) |
1.0000 |
Mean 24-hour ABPM, mmHg |
125±3/76±3 |
124±3/75±3 |
0.5013/0.2357 |
Echocardiographic parameters |
|
|
|
Indexed left atrial volume, mL/m2 |
31.2±3.4 |
32.0±3.9 |
0.4433 |
IST, mm |
10.9±1.0 |
11.5±1.2 |
0.0607 |
LVPWT, mm |
9.9±1.0 |
9.4±0.8 |
0.0568 |
LVEF, Simpson (%) |
67.3±8.5 |
66.0±7.8 |
0.5758 |
LVEDD, mm |
44.1±3.0 |
45.0±2.2 |
0.2324 |
LVESD, mm |
32.0±5.0 |
33.5±6.8 |
0.3787 |
LV mass index, g/m2 |
95.3±15.0 |
89.4±19.2 |
0.2319 |
Values are expressed as mean ± SD or n (%); ABPM, ambulatory blood pressure measurements; ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; IST, interventricular septum thickness; LV, left ventricular; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVPWT, left ventricular posterior wall thickness; RAA, right atrial appendage; RALW, right atrial lateral wall.
Conclusion
Pacemakers are very effective in diagnosing of subclinical AF presenting episodes ranging from 6 minutes to 24 hours. We observed that post PVI the patients that had the right atrial lead positioned in the RAA presented changes in the leads parameters in comparison with them positioned in the RALW, probably due to the right PVI in the left atrium, causing possible interatrial transmural necrosis sites.
Conflict of Interest
None declared.
Acknowledgements
The authors thank all the participants in this study.
References
- Ziegler PD, Koehler JL, Mehra R (2006) Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm 3: 1445-1452. [CrossRef]
- Ziegler PD, Glotzer TV, Daoud EG, Wyse DG, Singer DE, et al. (2010) Incidence of newly detected atrial arrhythmias via implantable devices in patients with a history of thromboembolic events. Stroke 41: 256-260. [CrossRef]
- Charitos EI, Stierl2021 Copyright OAT. All rights reserv DR, et al. (2012) A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation 126: 806-814. [CrossRef]
- Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, et al. (2012) Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 366: 120-129. [CrossRef]
- January CT,Wann LS, Alpert JS, Calkins H, Cigarroa JE, et al. (2014) AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 130: e199-e267. [CrossRef]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, et al. (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604-612. [CrossRef]

