Background: Alzheimer’s dementia (AD) is aggravated in the presence of vascular risk factors. On the other hand, it is well known, that vascular risk factors, particularly diabetes mellitus are independent risk factors for hippocampal atrophy, respectively neurodegeneration.
Objective: To investigate possible interactions of vascular risk factors and markers of vascular encephalopathy and Alzheimer’s pathology.
Methods: In this retrospective analysis subjects with mild cognitive impairment (MCI), AD, mixed dementia and vascular dementia (VD) were included as well as a group of controls. As a measure of vascular damage white matter hyperintensities (WMHI) and for Alzheimer pathology hippocampal volume (HV) and cerebrospinal fluid (CSF) markers were analyzed.
Results: CSF markers were altered in AD and mixed dementia (p<.001), while in VD and mixed dementia more WMHI were present (p<.001). HV was smaller in all groups compared to controls (p≤.001). Patients with diabetes mellitus had smaller HV (p=.01), lower Abeta ratio (p=.05) and a higher tau (p=.03), while there was no relation to vascular abnormalities. Similar findings were present in hypercholesterolaemia with a smaller HV (p=.001). In contrast patients with arterial hypertension showed more WMHI (p=.004).
Conclusion: Our data deliver some hints that different vascular risk factors have different influence on vascular and Alzheimer pathology.
Many studies showed that cerebrovascular risk factors (CVRF) such as hyperlipidemia, diabetes and hypertension interact with the risk of dementia [1-3].
Whereas diabetes increases the risk for vascular dementia (VD) and Alzheimer’s disease (AD) [4,5], the connection of hypertension and hypercholesterolemia to the development of dementia is less clear. Hypertension in mid-life seems to raise the risk for dementia, but in older patients already suffering from AD a lowered blood pressure can be observed. A current hypothesis states, that this might be caused by the reduction of physical activity. Nevertheless, it can be stated that hypertension is linked to VD as well as to AD [3,6-8]. The link between hypercholesterolemia and dementia tends to be similar. While hypercholesterolemia in mid-life increases the risk of getting AD [9], some studies show that this association is not valid in late-life prior to dementia onset [10]. Moreover, even a protective effect of a high cholesterol level is discussed [11,12] Very recently high LDL-cholesterol and low HDL-cholesterol were linked to Aβ-accumulation in the brain in a same manner as for cardiovascular diseases [13] Regarding VD, hypercholesterolemia is also a discussable risk factor [8,14].
Up to now most studies have focused on the influence of the above outlined risk factors to develop a dementia. Data on their influence on vascular abnormalities and AD pathology once the disease is manifest are scarce.
Biomarkers reflecting AD pathology are available [15,16], beside neuropsychological tests.
Besides Amyloid-PET [16] there are measurable markers of neurodegeneration or neuronal injury in cerebrospinal fluid (CSF) like Amyloid-ß-42 (Aß42) and tau-protein. In neurodegenerative disorders like Alzheimer’s disease (AD) lowered CSF Aß42 indicates increased cortical Aß load detectable using PET [17]. In contrast to Aß42 total Tau-protein (T-tau) and its hyperphosphorylated form tau181P (P-Tau181P) are elevated in CSF with P-Tau181P to be more specific to the diagnosis of AD [18,19].
Measuring hippocampal volume (HV) by magnetic resonance imaging suites as another correlate of neurodegeneration. Former studies constrained that AD goes along with HV atrophy [24,25]. Also a reduced hippocampus volume can be observed in patients diagnosed with diabetes [26].
On the other hand magnetic resonance neuroimaging indicates vascular damage, e.g. cerebral small vessel disease (CSVD). Several patients suffering from VD present with white matter hyperintensities (WMH) in certain regions of the brain e.g. comprising centrum semiovale or periventricular areas [20]. The exact pathogenesis remains still unclear, but they might be caused by incomplete infarctions due to different reasons e.g. from acute lacunar infarcts [21-23].
The objective of this study was to determine the impact of individual CVFRs on several biomarkers comprising CSF Aß42, Abeta-ratio, CSF T-tau, CSF P-Tau181P, WMH and HV in various patient groups suffering from cognitive decline. Analysis was conducted on a well-characterized cohort of aged controls (CON), mild cognitive impaired (MCI) and various dementia cases comprising AD and VD enrolled at the memory clinic of the Department of Neurology, Otto-von-Guericke-University Magdeburg
Participants
We conducted a retrospective cross-sectional study comprising 120 subjects diagnosed at the Department of Neurology’s memory clinic of the Otto-von-Guericke-University Magdeburg. During 2007 to 2014 all subjects underwent a diagnostic work-up including neuropsychological testing, magnetic resonance imaging (MRI) and lumbar puncture. The participants were categorized in five diagnosis groups: MCI (n=22), AD (n=28), mixed dementia (n=26), VD (n=15) and non-demented CON (n=28). Patients were diagnosed with MCI when the Petersen criteria [27] were fulfilled.
For the diagnosis of AD the recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup were applied [19], whereas VD was diagnosed according to the NINDS-AIREN criteria [28]. If participants had evidence for both VD and AD, e.g. WMH and lowered CSFAß42 or elevated CSF tau, according to our local references (Aβ42 below 485 pg/ml and hTau above 350 pg/ml or pTau181P above 70 pg/ml) the type of dementia was classified as mixed dementia.
We moreover included CSF and MRI data of n = 28 aged control subjects who were free of memory complaints, had a MMSE score between 24 and 30 and a CDR of 0. Participants were recruited from the Department of Neurology, University of Magdeburg. They were without a prior history or a current diagnosis of neurodegenerative or psychiatric diseases but underwent lumbar puncture for other reasons (e.g. disc prolapse, exclusion of neuroborreliosis) in the context of diagnostic workup.
In order to be diagnosed and categorized the patients got CSF measures of Aß42, T-tau and P-Tau181P.
CSF was obtained during clinical work-up of the patient by lumbar puncture at the L3/L4 or L4/L5 interspaces. A minimum sample volume of 1 ml was collected and stored at -20°C or lower until analysis.
CSF levels of Aß42, T-tau, and P-tau181P were determined with commercially available single-parameter ELISA kits, additionally Aβ40 was measured to estimate the Abeta ratio (Aβ42/Aβ40x10) (respectively Innotest ß-amyloid(1-42) and ß-amyloid(1-40), Innotest hTauAg, Innotest phospho-Tau(181P), (Innogenetics, Ghent, Belgium)). With each assay, the clinical samples, together with a blank (sample diluent), the (prepared) calibrator solutions and the appropriate controls, were tested strictly following the instructions provided in the kit inserts. All samples were run in duplicate. If the intra-assay coefficient of variance was >30% (calculated as range x 100/average), or if concentrations obtained were out-of-range.
(values not between mean values of highest and lowest calibration concentration), samples were retested (by extension of the calibrator concentration range for some samples). Dilution of samples with measured concentrations above the highest calibration concentration for possible reanalysis was not performed, as this is not recommended in the manufacturer’s instructions. The concentration ranges of the test kits are described in the package inserts (P-tau181P: 15.6-500 pg/ml, T-tau: 75-1200 pg/ml, Aß42: 125-2000 pg/ml).
Neuroimaging techniques
All participants received neuroimaging, preferentially an MRI or, if contraindicated a CT. Due to the retrospective design only semi quantitative measures could be applied. WMHI were evaluated in the periventricular space (WMHI-PV) and in the basal ganglia (WMHI-BG) according to the rating scale for age-related white matter changes (ARWMC) applicable for CT and MRI [29]. The scale consists of 4 values from 0 (no lesions) to 3 (lesions of an entire region or confluent lesions in basal ganglia).
Hippocampal atrophy was assessed in MRI or CT using the Schelten’s scale [30]. The hippocampal formation was rated visually from 0 (normal width of the choroid fissure and the temporal horn and normal height of the formation) to 4.
All neuroimaging ratings were performed by an experienced neurologist from the memory clinic (DB).
Assessment of cerebrovascular risk factors
In this retrospective study we incorporate the three major cardiovascular risk factors hypertension, diabetes and hyperlipidemia. Hypertension was diagnosed by the intake of antihypertensive medications or a documented former diagnosis. Diabetes was defined by the use of insulin, intake of antidiabetic medications or glycated hemoglobin (HbA1c) > 6.5%, whereas hyperlipidemia was diagnosed by the intake of statins. Former documented diagnosis of these risk factors was comprised, too.
Statistical analyses for two-group comparisons were performed with the Mann-Whitney U test. Analyses including more than 2 groups were performed with a one-way ANOVA. To remove some of the variance multivariate analysis of covariance (MANCOVA) was applied including significant variables. The limit of significance was set as lower than 0.05.
Participants’ demographics, clinical and biomarker data are given in Table 1 Included were 26 neurological controls (age: 65.2 ± 10.1 years), 22 subjects with MCI (age: 70.6 ± 7.0 years), 29 patients with AD (age: 75.2 ± 5.8 years), 26 with mixed dementia (age: 75.9 ± 5.9 years) and 17 with VD (age: 72.6 ± 8.9 years). There was a significant group effect on age (F = 8.4, p < .001) that was attributed to a younger age in CON (bonferroni post-hoc: CON vs. AD p < .001; vs. mixed dementia p < .001; vs. VD p = .02).
Albeit diabetes, hypertension and hyperlipidemia appear to be most frequent in VD, differences not get significant apart from a higher frequency of patients with diabetes in VD compared to MCI (Χ2 = 9.6; p = .04). Corresponding blood pressure was higher in mixed dementia and VD compared to the other groups without reaching significance (data not shown).
There were significant age-adjusted group differences on CSF Aβ42 (F = 5.5, p < .001), CSF P-tau181P (F = 12.7, p < .001) and T-Tau (F = 12.0, p < .001). CON had lower CSF Aβ42 levels.compared to AD (p = .001), mixed dementia (p < .001) and VD (trend with a p = .09). Compared to the remaining groups mixed dementia and AD subjects revealed significantly higher CSF P-tau181P and T-Tau levels. For the Abeta ratio the only difference was observed for mixed dementia that displayed a reduced CSF level compared to controls (p = .02) Regarding MRI markers there were significant group differences for WMH-PV (F = 37.5, p < .001), WMH-BG (F = 4.4, p = .002) and hippocampal size (F = 15.8, p < .001). Compared to the remaining subgroups there was significantly more WMH-PV in VD and mixed dementia (p < .001) while WMH-BG were more pronounced in VD compared to MCI (p = .01) and AD (p = .01), only Interestingly hippocampal volume was smaller in all disease groups compared to controls (p = .004 vs. MCI, p < .001 vs. all other groups). Additionally, compared to MCI in AD (p = .05) and mixed dementia (p = .03) HC atrophy was more pronounced.
Since there were no group differences concerning the frequency of diabetes, hypertension and hyperlipidemia beside more frequent diabetes in VD compared to MCI (p = .04), all subjects were sampled for an analysis to assess whether the CVRF’s predict vascular or neurodegenerative pathology. Compared to participants without arterial hypertension subjects with arterial hypertension revealed more WMH-PV (Z = -3.5, p = .004; Figure 1A) while there were no differences on CSF markers or degree of hippocampal atrophy. Participants suffering from diabetes compared to subjects without diabetes displayed smaller hippocampal size/more pronounced HV atrophy (Z = -1.9, p = .01; Figure 1B), higher CSF T-Tau (Z = -2.1, p = .03; Figure 1C) and a lower beta-amyloid ratio (Z = -1.8, p = .05 Figure 1D). An association between WMH and diabetes (p > 0.2 Figure 1E) could not be detected.
In subjects diagnosed with hyperlipidemia the hippocampus was smaller compared to those without (Z = -2.9, p = .001; Figure 1F, 1G, 1H, 1I). No further differences were found for CSF or MRI markers (Figure 1). In a MANOVA no interaction of the CVRF occurred.
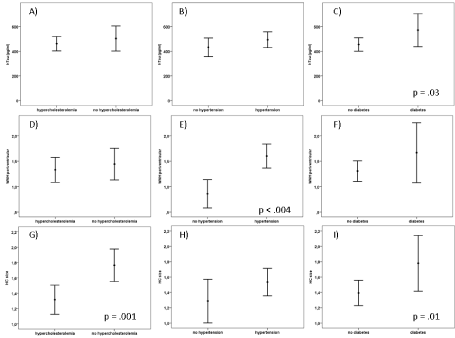
Figure 1 A-I: Cerebrovascular risk factors and relation to CSF total Tau (T-Tau) (A-C), periventricular white matter hyperintensities (WMH) (D-F) and hippocampal size (G-I).
Table 1. Demographics, clinical and biomarker data for the different groups under consideration.
|
Controls (n=28)
|
MCI (n=22)
|
AD (n=28)
|
mixed dementia (n=26)
|
VD (n=15)
|
p-value
|
Age
|
64.4±10.7a,c,d
|
70.6±7.0
|
75.0±5.9c
|
75.9±5.9d
|
71.7±9.1a
|
<.001
|
Gender male (%)
|
12 (42.9)a
|
13 (59.1)
|
13 (46.4)
|
10 (38.5)b
|
13 (86.7)a,b
|
.02
|
MMSE
|
na
|
26.6±2.1
|
21.6±4.0
|
20.1±3.3
|
24.3±2.9
|
<.001
|
YoE (years)
|
13.8±2.8
|
12.2±3.0
|
11.7±2.7
|
11.0±2.3
|
13.0±3.0
|
ns
|
pTau (pg/ml)
|
56.6±24.8c,d
|
68.9±30.3e,g
|
99.6±36.9c,ef
|
97.4±33.7d,g,h
|
46.6±16.4f,h
|
<.001
|
hTau (pg/ml)
|
286±160 c,d
|
412±210 e,g
|
670±2799c,ef
|
607±2717d,g,h
|
295±143 f,h
|
<.001
|
Aβ42 (pg/ml)
|
673±200d
|
666±344
|
496±210
|
464±249d
|
554±230
|
.006
|
Aβ ratio
|
2.01±1.43c,d
|
1.29±0.92
|
1.11±0.89c
|
0.80±0.46d
|
1.41±1.02
|
.001
|
WMH-BG
|
0.30±0.57
|
0.05±0.21 i
|
0.00±0.00f
|
0.35±0.63
|
0.55±0.69 f,i
|
.003
|
WMH-PV
|
0.80±0.95a,d
|
0.95±0.90g,i
|
0.71±0.54f,k
|
2.31±0.47d,g,k
|
2.46±0.66a,i,k
|
<.001
|
HC size
|
0.75±0.72a,c,d
|
1.18±0.73e,g
|
1.75±0.52c,e
|
1.77±0.71d,g
|
1.77±0.93a
|
<.001
|
MCI: mild cognitive impairment; AD: Alzheimer’s disease; VD: vascular dementia; MMSE: Mini Mental State Exam; YoE: years of education; WMH: white matter hyperintensities; BG: basal ganglia; PV: periventricular; HC: hippocampus; Ab, ptau, T-Tau
a: controls vs. VD: p <.05; b: mixed dementia vs VD: p < .05; c: controls vs AD: p < .001; d: controls vs. mixed dementia: p < .001; e: AD vs. MCI: p <.01; f: AD vs. VD: p < .001; g: mixed dementia vs. MCI: p < .05; h: mixed dementia vs. VD: p < .001; i: VD vs. MCI: p <.05; k: mixed dementia vs. AD: p < .001.
In this study we explored the prevalence of vascular and neurodegenerative abnormalities in the spectrum of neurodegenerative and vascular dementias under special consideration of CVRF.
Not surprisingly CSF biomarkers, e.g. an elevated ptau and htau and reduced Aβ42, were abnormal in AD and mixed dementia only while vascular pathology was abnormal in VD and mixed dementia. This proves the validity of the used diagnostic criteria and the applicability of the different groups for the performed analyses.
More irritating is the finding of normal CSF values in MCI that is regarded a risk factor for AD and where a conversion to AD can be observed in up to 80% of amnestic MCI cases [31]. A preferential selection of MCI with no evidence for an AD-pathology might be a possible explanation.
Another interesting result was the finding of similar hippocampal atrophy in AD and VD. There is not much evidence about hippocampal size in VD, but a recent study reported a similar result. Hippocampus turned out to be smaller in VD compared to healthy controls, albeit bigger than in AD [25]. Our rather rough method of a visual assessment of hippocampal size might attribute for the similar ratings in AD and VD. More precise methods for its analysis might have elaborated a difference between AD and VD, as well.
Finally, when assessing the association of CVRF on neurodegeneration or vascular pathology we obtained the surprising result that solely hypertension was associated with WMHI while diabetes beside hyperlipidemia were related to neurodegeneration. The presence of hyperlipidemia and diabetes were associated with hippocampal atrophy whereas for diabetes there was an additional association to CSF htau and Aβratio, as well. In accordance with our data WMHI restricted to the periventricular region has been shown in hypertensive healthy, MCI and AD subjects [32]. It is well known that CVRF overlap for AD and VD. Both diabetes [33,34] and hypertension [35] increase the risk to develop these types of dementia. While in VD the mechanism seems to be vascular pathology, in AD it remains an open question whether it is secondary due to the vascular pathology or caused by a direct damage through another mechanism.
Arterial hypertension is discussed as a risk factor for hippocampal damage, although existing literature is query. In the Honolulu-Asia Aging study untreated hypertensive subjects had smaller hippocampi [36]. On the other hand, treated hypertensive patients with lower diastolic blood pressure had a smaller hippocampal volume as well [37]. Also, in elderly subjects with hypertension there was only a non-significant trend for a smaller hippocampus [38].
In healthy controls an association of diabetes and hippocampal atrophy is well described. In diabetic subjects regardless of their age a higher level of HbA1c was related to cognitive impairment and hippocampal atrophy [39,40]. In the Honolulu-Asia Aging study in elderly people with diabetes the hippocampus was smaller than in those without [41]. Possible mechanisms include vascular damage and neural pathology with amyloid-β plaques and neurofibrillary tangles among others. From our data the latter mechanism seems to be more important for hippocampal atrophy in dementia because we did not found, an association to WMHI but to CSF neurodegenerative markers.
To date there is only limited knowledge about hypercholesterolemia and hippocampal size. However, one study with a cholesterol-lowering treatment in patients with atrial fibrillation demonstrated a lower rate of hippocampal decline [42].
In summary from our data one might assume that different CVRF act differentially in the brain with hypertension affecting preferentially white matter, while diabetes and hypercholesterolemia are more likely to induce neurodegenerative changes.
Important limitations of our study are the retrospective design with no follow-ups, the relatively small number of subjects caused by a lack of pure VD and the applied methods to measure hippocampal size and vascular pathology in the brain.
Nevertheless, our results offer some new aspects in the research of cerebrovascular risk factors and dementia that encourage further studies with a prospective design, a higher number of subjects and more detailed analysis of neuroimaging markers.
The authors have nothing to disclose. There are no competing financial interests concerning the study.
- Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, et al. (2014) Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 129: 1560-1567.
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C (2014) Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 13:788-794.
- Barnes DE, Yaffe K (2011) The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 10: 819-828.
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5: 64-74. [Crossref]
- Cheng G, Huang C, Deng H, Wang H (2012) Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 42: 484-491. [Crossref]
- Duron E, Hanon O (2008) Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag 4: 363-381. [Crossref]
- de Bruijn RF, Ikram MA (2014) Cardiovascular risk factors and future risk of Alzheimer's disease. BMC Med 12: 130 [Crossref]
- Román GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC (2002) Subcortical ischaemic vascular dementia. Lancet Neurol 1: 426-436.
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K (2005) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64: 277-281. [Crossref]
- Tan ZS, Seshadri S, Beiser A, Wilson PW, Kiel DP, Tocco M, et al. (2003) Plasma total cholesterol level as a risk factor for Alzheimer disease: the Framingham Study. Arch Intern Med 163: 1053-1057.
- Reitz C, Tang MX, Luchsinger J, Mayeux R (2004) Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol 61: 705-714. [Crossref]
- Mielke MM, Zandi PP, Sjogren M, Gustafson D, Ostling S, Steen B, et al.: High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology 64: 1689-1695.
- Reed B, Villeneuve S, Mack W, DeCarli C, Chui HC, et al. (2014) Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol 71: 195-200. [Crossref]
- O'Brien JT, Thomas A (2015) Vascular dementia. Lancet 386: 1698-1706. [Crossref]
- Jack CR, Hampel HJ, Universities S, Cu M, Petersen RC (2016) A new classification system for AD, independent of cognition A / T / N?: An unbiased descriptive classification scheme for Alzheimer disease. biomarkers 1: 1-10.
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. (2014) (Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13: 614-629.
- Landau SM, Horng A, Fero A, Jagust WJ (2016) Amyloid negativity in patients with clinically diagnosed Alzheimer disease and MCI. Neurology 86: 1377-1385.
- Jack CR, Albert MS, Knopman DS, Mckhann GM, Sperling R a, Carrillo MC, et al. (2011) Introduction to the recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 7: 257-262.
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer’s Dement 7: 263-269.
- Prins ND, Scheltens P (2015) White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol 11: 157-165.
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12: 822-838.
- Pantoni L (2010) Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9: 689-701.
- Wardlaw JM, Smith C, Dichgans M (2013) Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol 12: 483-497.
- Gosche KM, Mortimer JA, Smith CD, Markesbery WR, Snowdon DA (2016) Hippocampal volume as an index of Alzheimer neuropathology: Findings from the Nun Study. Neurology 58: 1476-1482.
- Kim GH, Lee JH, Seo SW, Kim JH, Seong J-K, Ye BS, et al. (2014) Hippocampal volume and shape in pure subcortical vascular dementia. Neurobiol Aging 58: 1476-1482.
- Fotuhi M, Do D, Jack C (2012) Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol 8: 189-202. [Crossref]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, et al. (2001) Current concepts in mild cognitive impairment. Arch Neurol 58: 1985-1992. [Crossref]
- Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. (2015) Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43: 250-260.
- Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, et al. (2001) A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 32: 1318-1322. [Crossref]
- Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, et al. (1992) Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 55: 967-972.
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, et al. (2001) Current concepts in mild cognitive impairment. Arch Neurol 58: 1985-1992. [Crossref]
- Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, et al. (2006) Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology 67: 2192-2198. [Crossref]
- Knopman DS, Roberts R (2010) Vascular risk factors: imaging and neuropathologic correlates. J Alzheimers Dis 20: 699-709. [Crossref]
- Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, et al. (2007) Relation of diabetes to mild cognitive impairment. Arch Neurol 64: 570-575. [Crossref]
- Iadecola C, Davisson RL (2008) Hypertension and cerebrovascular dysfunction. Cell Metab 7: 476-484. [Crossref]
- Korf ES, White LR, Scheltens P, Launer LJ (2004) Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension 44: 29-34. [Crossref]
- den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, et al. (2016) Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology 64: 263-267.
- Wiseman RM, Saxby BK, Burton EJ, Barber R, Ford GA, O’Brien JT, et al. (2004) Hippocampal atrophy, whole brain volume, and white matter lesions in older hypertensive subjects. Neurology 63: 1892-1897.
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, et al. (2003) Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 46: 1604-1610. [Crossref]
- Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, et al. (2007) Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 50: 711-719.
- Korf ES, White LR, Scheltens P, Launer LJ (2006) Brain aging in very old men with type 2 diabetes: the Honolulu-Asia Aging Study. Diabetes Care 29: 2268-2274. [Crossref]
- Tendolkar I, Enajat M, Zwiers MP, van Wingen G, de Leeuw F-E, van Kuilenburg J, et al. (2016) One-year cholesterol lowering treatment reduces medial temporal lobe atrophy and memory decline in stroke-free elderly with atrial fibrillation: evidence from a parallel group randomized trial. Int J Geriatr Psychiatry 27: 49-58.

