Abstract
The protein thiol contents of two human prostate cell lines, LNCaP (malignant) and PNT2 (a virally transformed prostate cell line) were quantified and analysed. The former was found to contain twice the thiol content of the latter.
After removal of the cellular glutathione by trichloroacetic acid precipitation the protein precipitate was dissolved in buffered 8M urea containing the Ellman reagent (ESSE); this reagent reacts with thiols (RSH) to give a yellow anion (ES) and a mixed disulfide (RSSE). Fractionation of the latter by gel filtration chromatography on Biogel P2 revealed that the excluded protein components eluted in the void volume (Mr > 1500) could only account for a fraction (18-19%) of the total thiol originally present. A low molecular weight fraction liberated from the protein matrix accounted for 56-61% of the total cellular thiol detected with the Ellman reagent. This fraction contained the excess ESSE, possible RSSE and the yellow anion liberated (ES). After removal of the ES, HPLC analysis revealed that only one major component was present. This was shown by MS analysis to be ESSE (m/z 395); traces of some other derivatives were found on the chromatogram of mass 592m/z and 791m/z, probably artefacts formed by the addition of ES molecules to ESSE. No amino acids or cysteine could be detected in this low molecular weight ESSE/RSSE fraction.
It was concluded that a considerable amount of “labile” low molecular weight thiol had been released from the protein matrix by extraction with buffered 8M urea which did not form an RSSE adduct with the Ellman reagent. This may be a simple divalent sulfur moiety, possibly sulfide (S2-), polysulfides or derivatives of sulfane sulfur (S0) associated with vital metabolically active/regulatory cellular proteins, such as those involved in respiratory functions.
Key words
Protein thiols; human prostate cells
Abbreviations
ASF acid soluble fraction obtained after deproteinization of cells; ESSE Ellman’s reagent 5’5 dithiobis (-2-nitrobenzoic acid); DE 52 Whatman cellulose anion exchanger; ES yellow anion 5 thio- 2-nitrobenzoic acid; GSH glutathione; HPLC high pressure liquid chromatography; RSSE aromatic mixed disulphide (s) formed on reaction of cellular thiols with Ellman’s reagent; TCA trichloracetic acid; MS mass spectroscopy; MES 2-Mercaptoethane sulfonic acid (sodium salt)
Introduction
Apart from their established role in the maintenance of the redox milieu thiols play many pivotal roles in cellular metabolism. Over the past 120 years there have been many published research papers and reviews on the numerous facets of the role of thiol compounds in cell division, apoptosis etc. These cellular components also have important roles in cellular resistance/defence against to toxic materials and, in medicine, resistance to ionizing radiation and chemotherapeutic agents used, for example, against cancer. Some research has shown that oncogenic transformation is frequently associated with a shift in the cytosolic thiol redox balance to a more powerful oxidized state, which may enhance the proliferative phenotype [1]. In addition, the work of Oberley’s group [ 2] has clearly established the importance of thiol redox factors in prostate cancer metabolism. Knowledge of the nature of these factors could be invaluable in the search for more effective drug forms to treat cancer and to counter drug resistance with those drugs already used in cancer treatment.
Because of their extreme reactivity and sensitivity to oxidation the analysis of these vital cellular molecules provides a formidable challenge to investigators. This challenge has led to the evolution of several sensitive analytical techniques to study their role in cellular metabolism.
One of the most popular techniques for thiol estimation utilises the reagent 5’5 dithiobis (-2-nitrobenzoic acid) (ESSE) [3]. In addition to providing a colorimetric estimation of thiols by generating a quantifiable yellow anion (ES-) this reagent is also forms mixed aromatic disulfides (RSSE) which can be utilized for thiol identification as shown below (Figure 1).
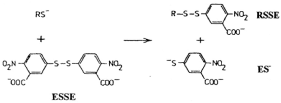
Figure 1. Reagent is also forms mixed aromatic disulfides (RSSE) which can be utilized for thiol identification.
There have been several publications on the preparation and analysis of the Ellman mixed disulfide derivatives obtained from a variety of known naturally occurring low molecular weight thiols [4-7]. However, although this reagent has been used to block protein thiols in order to demonstrate the metabolic importance of reduced thiols, protein SSE derivatives per se have not been used for identification purposes.
In earlier research work, while investigating the labelling of non-histone protein fraction of isolated nuclei from different tissues and tumours with 35S labelled Ellman reagent, this author observed that, on extraction of these nuclei with the strong chaotropic reagent 8M urea 50mM phosphate pH 7.6, the bulk of the sulfhydryl containing protein could be solubilized leaving an insoluble residue consisting of the DNA: histone complex (nucleosomes) [8]. The measured thiol content of these nuclei doubled on extraction with this reagent revealing a considerable amount of total thiol is buried within the secondary and tertiary structure of the nuclear protein mass. Further investigations on the nuclear proteins dissolved in buffered 8M urea containing an excess of the Ellman regent showed that the bulk of the mixed disulfide derivatives formed from this reagent were of low molecular weight and could be dissociated from the isolated non-histone proteins [9]. These “labile” thiol components which seemed to be trapped within a hydrophobic matrix as the 14C-N-ethylmaleimide derivatives could not be dissociated from the protein mass whereas those labelled with the negatively charged 35S labelled Ellman reagent were easily removed. At the time it was not possible to identify these moieties.
More recently this protein extraction technique has been applied to whole tumour cells showing that the thiol content of these cells doubled on extraction with buffered 8M urea [10]. Using the Ellman reagent in this solvent the thiol contents of two human prostate cell lines, PNT2 (a virally transformed line) and LNCaP (a malignant metastatic line isolated from lymph nodes of a cancer patient), have been analysed by newer analytical techniques which were not available at the time of the original discovery.
Materials
All reagents and chemicals were of analytical or higher grade. Ellman reagent, (5,5’-dithio-bis-(2-nitrobenzoic acid), 2-mercaptoethanesulfonic acid, sodium salt (MES) and other chemicals used were obtained from VWR Chemicals (BDH Prolab) and Sigma Aldrich.
ODS AQ silica gel (product 12S50) was purchased from YMC Co. Ltd. Kyoto, Japan. Bio-Gel P2 from Bio-Rad Laboratories Inc.
All solvents used were of analytical grade supplied by Sigma Aldrich or Merck. Solvents for HPLC were Merck LiChroSolv grade. Europe GMBH.
Large glass columns were supplied by Soham Scientific, Fordham, Ely, Cambs., UK
LNCaP (androgen-sensitive human prostate adenocarcinoma cells, clone FGC-ECACC no. 89110211) and PNT2 cells (a normal human prostate epithelial cell line immortalized with SV40 virus) were purchased from the Public Health England Laboratories (ECACC-HPA) at Porton Down, Salisbury, England. Cells were grown to confluence in cell factories in a medium consisting of RPMI 1640 + 2mM glutamine + 1mM sodium pyruvate containing 10% Zone 2 FBS. The confluent cells were given a two-hour incubation in fresh medium before harvesting by trypsinization (Tryple Express). Cell counts were performed on a Nucleocounter NC3000 and the cells collected by centrifugation. The resultant cell pellets were snap frozen and stored at -800C until required.
Methods
A schematic of the method for the preparation and isolation of protein bound RSSE is shown in (Figure 2) below.
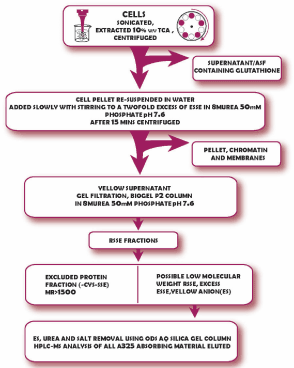
Figure 2. A schematic of the method for the preparation and isolation of protein bound RSSE.
Generally, for batches of up to 109 cells the following procedure was employed:
The cell pellet was re-suspended in water at 0-40C, then enough 100% TCA to give a 10% solution added. The resulting mixture was sonicated at full power in an ice bath until no whole cells were left; this usually took 1-2 mins. After centrifuging the mixture at 3,000 x g for 3mins the supernatant (ASF) was aspirated off and the cell residue extracted with a further volume of 10%TCA, usually half as much as the original volume. The combined TCA extracts removed > 95% of the non-protein low molecular weight thiol of these cells to give an acid soluble fraction (ASF) containing the cellular glutathione and some other thiols (Gronow 2010) The thiol content was measured on 100 and 200µl aliquots using the Ellman reagent in 2ml of 8M urea 0.5M phosphate pH 7.6.
The cell residue was then washed with water and after centrifugation the pellet was re-suspended again in this volume of water. The thiol content was measured on 10 and 20µl aliquots of the cell residue suspension using the Ellman reagent as described above. Following this, the cell residue suspension was added drop wise, with stirring, into 8M urea 50mM phosphate pH 7.6 containing a twofold excess of the Ellman Reagent (ESSE). The resulting mixture was centrifuged as before to remove the membranes and the DNA/histone (nucleosome) pellet [8]. The concentration of thiol in the supernatant (at suitable dilutions) was calculated from the yellow anion (thionitrobenzoate) present; λmax 412nm, molar extinction coefficient 13,600 M-1cm-1.
After a brief wash in 8M urea 50mM phosphate the colorless insoluble nucleoprotein pellet was dissolved in 8M urea 50mM phosphate containing 1MNaCl. Addition of excess 2-Mercaptoethane sulfonic acid (MES) liberated yellow anion revealing the amount of thiol in this fraction.
The supernatant (clear solution) was immediately loaded onto a 15 x 6cm chromatography column of BioGel P2 made up in 8M urea 50mM phosphate pH7.6. The column was eluted with this solvent and the effluent monitored by the absorbency at 325nm (λmax Ellman mixed disulphide).
A colourless protein fraction was eluted in the void volume. The thiol content of this protein fraction was determined as before by the release of the yellow anion after the addition of excess 2-Mercaptoethane sulfonic acid (sodium salt)(MES).
After the protein had eluted the A325 dropped to a low value and then rose again as lower molecular weight mixed disulfides emerged, together with the yellow anion generated in the reaction.
If the ES is not removed immediately, it will slowly react with any mixed disulfides formed from the Ellman reagent giving rise to unwanted artefacts. Therefore, this fraction was immediately put through a 15 x 5cms column of ODS AQ silica gel in 5% v/v aqueous ethanol and eluted with this solvent. The yellow anion, urea and any salts present were not adsorbed and passed straight through the column. After the conductivity of the eluate dropped below 50 microsiemens the adsorbed A325 (ESSE or RSSE) was eluted in 30% ethanol.
Reduction of protein fraction eluted from Biogel P2
It was thought possible that the Biogel P2 isolated cellular protein SSE could give a low value for thiol content if large amounts of vicinal dithiols, possibly bound α lipoic acid derivatives, were present in the protein mass. This is because these would tend to exchange any Ellman mixed disulphide formed on one thiol with the other giving an internal disulphide which would not adsorb at 325nm [11].
To check this an aliquot of the isolated P2 protein was taken, a tenfold excess of MES (or mercaptethanol) added and the thiol concentration recorded from the yellow anion released. Then TCA was added to a concentration of 10%w/v and the precipitated protein centrifuged down. Excess MES was removed from the protein pellet after washing twice with 10% TCA. The final precipitate was dissolved in 8M urea 0.5M phosphate pH 7.6 containing excess ESSE and the thiol content determined again from the A412 released. This method measures total thiol and disulfide content of the protein mixture. This value would be expected to be slightly higher than the original protein thiol value due to the reduction of the disulfide bonds present in the secondary structure of these proteins.
Results
The thiol contents of the various cell fractions determined using the Ellman reagent are given in table 1
Table 1.Thiol contents of cells.
Distribution of cellular thiols in LNCaP(red) and PNT2 (green) cells |
| Femtoles thiol*/cell | | % of total thiols | |
Total thiol content | 56.3 ± 3.6 | 28.5 ± 1.7 | 100.0 | 100.0 |
ASF 10% TCA soluble | 13.1 ± 0.8 | 4.9 ± 0.3 | 23.3 | 17.2 |
Total protein pellet | 43.2 ± 2.9 | 23.6 ± 1.4 | 76.7 | 82.8 |
Protein pellet after extraction with 8 M urea containing ESSE |
Chromatin bound- SSE (insoluble fraction) | 1.3 ± 0.2 | 0.7 ± 0.1 | 2.3 | 2.4 |
Biogel P2 Protein-SSE (soluble fraction) | 10.1 ± 0.6 | 5.4 ± 0.4 | 17.9 | 19.0 |
Reduced P2 protein thiol content (includes protein disulphide) | 12.2 ± 1.0 (+20.8%) | 6.5 ± 0.4 (+20.3%) | | |
Thiol released from protein by difference- "labile thiol" | 31.8 ± 2.2 | 17.5 ± 1.6 | 56.5 | 61.4 |
| | | | | |
*Average of 4 results
Amino acid analysis of the protein “labile” thiol fraction
Aliquots of the Ellman disulfide fractions isolated from the OD-AQ chromatography as described, were analysed for amino acids by the Department of Biochemistry Protein and Nucleic Acid Chemistry Facility at the University of Cambridge. No amino acids were detected in concentrates of these fractions. Cysteine analysis after oxidative hydrolysis (performic acid) was also negative (Table 2).
Table 2. Instrument: Waters Alliance 2695 LC (LIMS 1108, SOP006.52)
Description | Parameter |
Column: | 100x3mm Accucore C18 2.6µm (Ref 14/SEPSCI/39) |
Column Temperature: | 40ºC |
PDA Detector: | 200 to 550nm |
Eluent A: Eluent B: | 1% (v/v) formic acid in deionised water 1% (v/v) formic acid in acetonitrile |
Flow: | 1.0 mL/min |
Gradient (LC-MS): | T(mins)= 0 2.0 3.5 7.5 8.0 12.0 %B = 0 30 95 95 0 0 |
Stop time: | 12 minutes |
Injection volume: | 5µl and 10µl |
HPLC-MS analysis
This was carried out by Intertek, ITS Testing Services (UK) Ltd. Manchester UK
The A325 absorbing fraction eluted after the protein on P2 was separated from the yellow anion on an ODS-AQ column and then analysed by HPLC with RP 18C column as shown in figure 3 below.
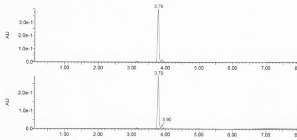
Figure 3. HPLC analysis of post protein A325 absorbing materials (aromatic disulfides) isolated on ODS-AQ (upper trace) and ESSE labelling agent used (lower trace).
An identical pattern was obtained from the post protein fraction to that of the ESSE used in the preparation. Minor contaminant peaks at 3.23 and 3.96 mins were found in both samples. The major peak at 3.79 mins was further analysed by MS as shown in figure 4.
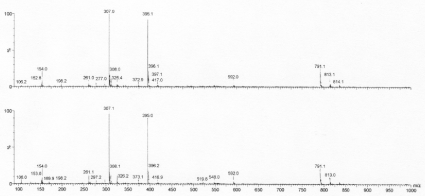
Figure 4. Negative ion electrospray spectra from the main peak at RT 3.79 minutes (scan range m/z 90-1000). Upper trace - post protein disulfide; lower - ESSE labelling agent.
Essentially identical MS patterns were obtained, and the same result was obtained by positive ion analysis. In the negative ion analysis, the main component present in the 3.79 min peak is ESSE with a mass of 395 m/z.
Further LC- MSMS of the post protein A325 fraction revealed only fragments derived from the breakdown of ESSE.
The analysis also revealed traces of derivatives of mass 592m/z and 791m/z in the original ESSE and in the material isolated from the reaction of the cellular protein with the Ellman reagent. These are probably artefacts formed by the addition of ES molecules to ESSE to give ESSE(ES) x; where x=1 in the 592m/z peak and 2 in the 791m/z peak (Table 3).
Table 3. Instrument: Micromass Quattro Ultima (LIMS 1107, SOP 010.09)
Description | Parameter |
Interface | Electrospray and APCI |
Polarity | Positive and negative ion |
m Calibrated range m/z | 50 to 2000 |
m Scan range m/z | Electrospray: 90-1000 190-850 800-1500 APCI: 190-850 800-1500 |
Scan time | 2 seconds |
Discussion
The presence of cellular protein “labile” thiol material has been reported in earlier analytical work carried out on isolated nuclei from normal and tumour cells [9], where it was also observed, using the Ellman reagent, that the thiol value of these nuclei of doubled in the presence of buffered 8M urea. However, it was found that the bulk of the RSSE (ca 70%) formed from non-histone protein extracted by the above method was “labile” and could be dissociated from the protein. This was confirmed by labelling of the nuclear protein with S35 labelled Ellman reagent to give RS-S35E. After isoelectric focussing of the protein mixture in polyacrylamide gel the 35S label was found to have been released into the acid anode solution; however, this dissociation did not occur if the thiols were labelled with another thiol reagent 14C N-ethylmaleimide [12] indicating that these “labile” thiols are tightly bound on hydrophobic regions of the protein. In these nuclear studies the RSSE dissociated fraction, derived from the “labile” protein thiol, constituted some 80-90% of the measured protein thiol.
In the current studies a similar result was obtained with whole cells; a significant proportion of the measured protein thiol did not co-elute with the protein on gel filtration. Unexpectedly this amounted to 56.5% of the total cellular thiol of the LNCaP cells and 61.4% in the PNT2 cells. It appears that a major portion of the reducing sulfur embedded in the proteins of these cells is loosely bound in some way to the matrix and not in the form of cysteine residues; that is, not chemically (covalently) bound to the peptide chains of the denatured protein. The extent of this hidden “labile” reducing sulfur present in the secondary or tertiary structure of cellular protein has not been reported.
According to Ellman [3] several sulfur containing natural products other than thiols can react with his reagent that would not produce a mixed disulphide. There are several possibilities to account for this “labile” sulfur which are as follows:
a) The presence free sulphide in the cell
However, despite the huge deviations of measured sulphide concentrations found in biological specimens Nagy et al. have stated [13] that biological sulphide concentrations, even at the largest reported values, are relatively low compared to protein thiol and reduced glutathione concentrations. Additionally, this author later states that “favourable reduction of ESSE (DTNB) by sulfide yields stoichiometric amounts of inorganic polysulfides and 5-thio-2-nitrobenzoic acid (ES or TNB), even when sulfide is in deficit”.
b) Hydrogen Sulfide
Although normally considered to be highly toxic hydrogen sulphide (H2S) has recently been established as an important gas transmitter/signalling molecule in many cell types [14].
According to Nashef et al. [15], H2S reacts with ESSE at neutral pH; one mole of hydrogen sulfide reacting with only one mole of DTNB, producing two moles of the thiol anion, 5-thio-2-nitrobenzoate, and one mole of free sulfur.
c) Thiosulfoxide or Sulfane sulfur (S0) derivatives which may play important roles in cellular metabolism [16].
Earlier workers [17] suggested this form of sulfur in the cell could be responsible for the formation of persulfides such as thiolated cysteines or thiocysteines). Sulfane sulfur does not occur in the free form in biological tissues but is always carried on another sulfur atom present in carrier proteins and enzymes, e.g. serum albumin, rhodanese and mercaptopyruvate sulfur transferase, to which it attaches as a persulfide or trisulfide [18].
It is not known whether S0 moieties would form stable derivatives with the Ellman reagent, but reaction may produce a yellow anion adduct derivative which would not have been identified in these studies.
d) Other possibilities are thiosulfate (S2O32- ), which has also been shown to react rapidly with ESSE [19] and sulphite (SO32-) which reacts with ESSE to give a “bunte” salt (ESSO32-); in the experiments reported here this “salt” would probably elute from an OD-AQ column along with the yellow ES fraction which was not analysed in these studies. Similarly, any reaction products of any simple polysulfides present would have not been detected.
e) It could arise from iron sulfur clusters. Iron-sulfur (Fe-S) clusters are ubiquitous cofactors (prosthetic groups) found in various forms all living cells and tissues [20]. These clusters can consist of iron-cysteine-sulfide combinations, mainly with molar ratios of cysteine to sulfur of 4:2; this might release two S2- (four reducing equivalents) on the disruption of the protein tertiary structure in buffered 8M urea. However, from the ratio of “labile” reducing sulfur to the protein containing cysteine sulfur given in table 1 it seems unlikely that this form of sulfide can account for all the labile thiol detected.
This issue may be resolved using the new sensitive and specific probes being developed for H2S, and sulfane sulfur. There is, now, considerable debate as to whether sulfane sulfur species, rather than hydrogen sulfide are the true messenger molecules which are responsible for several important regulatory effects in biological systems [21].
Although the LNCaP cells contained twice as much total thiol as the PNT2 cells a similar percentage of protein labile thiol was present. The amounts detected in this study represent a significant proportion of the total reducing thiol present; 31.8 ± 2.2 femtomoles per cell in the LNCaP cells and 17.5 ± 1.6 in the PNT2 cells. In terms of the reducing capacity of these cells the amount of this labile sulphur far outweighs the contribution of the glutathione present (about 8.3 femtomoles per cell in the LNCaP cells and 4.9 femtomoles per PNT2 cell, [7], This represents a significant amount of the cellular thiol when compared to the glutathione present which is widely considered to be the major redox controlling low molecular weight thiol in cells.
It remains to be determined whether the composition of this labile sulfur fraction is different in the LNCaP and PNT2 cell lines. Further investigations are required, possibly using different thiol labelling compounds, to identify these proteins associated thiols which probably represent the most important part of the reducing/redox controlling elements of the cell. In addition, they could be playing a vital role in cellular metabolism such as in respiration, messaging or signal transduction pathways [22] or in the control of reactive oxygen species (ROS) or H2O2 [23].
It is well known that the toxicity of drugs and radiation is largely dependent on the level of cellular thiols. The identification of such chemically reactive thiols within the matrix of cellular proteins could be a significant factor in the design and development of future anticancer therapeutic drugs. [24]
Acknowledgement
I thank the Cambridge Cancer Research Fund (UK Charity no.328087) for its generous support for this work and Christopher Allan for help with the illustrations.
Declaration of conflicting interest
The author has no conflicts of interest (political, personal, religious, ideological, academic, intellectual, commercial or any other) to declare in relation to this manuscript.
References
- Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, et al. (2006) Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell 10: 241-252. [Crossref]
- Chaiswing L, Zhong W, Oberley TD (2014) Increasing discordant antioxidant protein levels and enzymatic activities contribute to increasing redox imbalance observed during human prostate cancer progression. Free Radic Biol Med 67: 342-352. [Crossref]
- Ellman GL (1959) Tissue Sulfhydryl Groups. Arch Biochem Biophys 82: 70-77. [Crossref]
- Russell J, Rabenstein DL (1996) Speciation and quantitation of underivatized and Ellman’s derivatized biological thiols and disulphides by capillary electrophoresis. Anal Biochem 242: 136-144. [Crossref]
- Russell J, McKeown JA, Hensman C, Smith WE, Reglinski J (1997) HPLC determination of biologically active thiols using pre-column derivatization with 5,5’-dithiobis (2-nitrobenzoic acid). J Pharm Biomed Anal 15: 1757-1763. [Crossref]
- Chen W, Zhao Y, Seefeldt T, Guan G (2008) Determination of thiols and disulfides via HPLC quantification of 5-thio-2-nitrobenzoic acid. J Pharm BioMed Anal 48: 1375-1380. [Crossref]
- Childs S, Haroune N, Williams L, Gronow M (2017) Investigation of the Low Molecular Weight Thiol Composition in a Metastatic Prostate Cancer Cell Line (LNCaP) by LC-UV-MS and NMR after labelling with the Ellman Reagent. Am J Analyt Chem 8: 1-18.
- Gronow M (1969) Solublization and partial fractionation of the sulphur-containing nuclear proteins of hepatoma 223 ascites cells. Eur J Cancer 5: 497-508. [Crossref]
- Gronow M, Lewis FA (1975) Thiols attached to rat liver non-histone nuclear proteins. Exptl Cell Res 93: 225-229. [Crossref]
- Gronow M (2010) Studies on the Non-Protein Thiols of a Human Prostatic Cancer Cell Line: Glutathione Content. Cancers 2: 1092-1106. [Crossref]
- Gilbert HF (1990) Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol 63: 69-172. [Crossref]
- Gronow M, Thackrah T (1973) The non-histone nuclear proteins of some rat tissues. Arch Biochem Biophys 158: 377-386. [Crossref]
- Nagy P, Palinkas Z, Nagy A, Budai B, Toth I, et al. (2014) Chemical aspects of hydrogen sulphide measurements in physiological samples. Biochim Biophys Acta 1840: 876-891. [Crossref]
- Cadenas E and Packer L(eds) Methods in Enzymology, Hydrogen Sulfide in Redox Biology, Part A Elsevier: 554.
- Nashef AS, Osuga DT and Feeney RE (1997) Determination of hydrogen sulfide with 5,5′-dithiobis-(2-nitrobenzoic acid), N-ethylmaleimide, and parachloromercuribenzoate. Anal Biochem 79: 394-405. [Crossref]
- Toohey JI, Cooper AJ (2014) Thiosulfoxide (Sulfane) Sulfur: New Chemistry and New Regulatory Roles in Biology. Molecules 19: 12789-12813. [Crossref]
- Iciek M, Wlodek L. (2001) Biosynthesis and biological properties of compounds containing highly reactive, reduced sulfane sulfur. Pol J Pharmacol 53: 215-225. [Crossref]
- Toohey JI (2011) Sulfur signalling; is the agent sulfide or sulfane? Anal Biochem 413: 1-7. [Crossref]
- Riddles PW, Blakeley RL, Zerner B. (1983) Reassessment of Ellman’s reagent. Methods Enzymol 91: 49-60. [Crossref]
- Rouault TA (2015) Mammalian iron-sulfur proteins: novel insights into biogenesis and function. Nat Rev Mol Cell Biol 16: 45-55. [Crossref]
- Wang K, Peng H, Wang B (2014) Recent Advances in Thiol and Sulfide Reactive Probes. J Cell Biochem 115: 1007-1022. [Crossref]
- Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, et al. (2009) H2S signals through protein S-sulfhydration. Sci Signal 2: ra72. [Crossref]
- Sabharwal SS, Schumacker PT (2014) Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer 14: 709-721. [Crossref]
- Castelado SA, Freitis JR, Conchina NV, Madureira PA (2016) The Tumorigenic Roles of the Cellular Redox Systems. Oxid Med Cell Longev. [Crossref]




