Abstract
Aim: For decades, endocrine therapy (ET) has been the standard of care in the management of hormone-positive, HER2-negative advanced breast cancer. The addition of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors to ET has significantly improved progression-free survival (PFS) for patients with that disease subtype. A meta-analysis to quantify the magnitude of benefit in all patients’ population and in relevant subgroups is warranted.
Methods: Comprehensive literature search identified 8 eligible randomized controlled trials (RCTs) comprising 4,580 patients and were included in the meta-analysis. Seven studies were placebo-controlled. All analyses were based on fixed-effects models.
Results: In five RCTs in the first-line setting, CDK4/6 inhibitors were associated with 44% improvement in PFS, (hazard ratio [HR] = 0.56; 95% confidence interval [CI] 0.50-0.62; P <0.0001). Furthermore, in the second-line setting (3 RCTs), CDK4/6 inhibitors demonstrated a 47% improvement PFS (HR of 0.53; 95% CI 0.46-0.60; P <0.0001). Irrespective of patients’ subgroups, the benefit achieved with CDK4/6 inhibitors was consistent in either the first- or second-line setting. Compared with controls, CDK4/6 inhibitors achieved higher objective response rate (ORR) and the pooled odds ratio (OR) was 1.97 (95% CI, 1.68-2.30; P <0.0001), with numerically higher OR in the second-line setting.
Conclusion: Combining CDK4/6 inhibitors with standard ET significantly improved PFS and ORR in hormone-positive, HER2-negative advanced breast cancer. The benefit was achieved in all patients’ subgroups.
Keywords
breast cancer, advanced, CDK4/6 inhibitor
Introduction
Hormonal receptor (HR)-positive breast cancer is the most common disease subtype as it represents 60-80% of all malignant breast neoplasms [1], moreover, emerging data suggest that the incidence of HR-positive disease may be also rising in premenopausal women [2].
In recent years, the outcome of patients with advanced HR-positive disease has improved by combining endocrine therapy (ET) with targeted agents that enhance the activity of ET and overcome resistance. The first class of such agents has been the mTOR inhibitors, however, the extent of benefit has been influenced by the chosen agent from this class [3,4].
Currently, we now have three cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, i. e. palbociclib, abemaciclib, and ribociclib, approved for the management of HR-positive HER2-negative advanced breast cancer in combination with ET in the first- and second-line settings [5-7]. Moreover, based on data driven from a single-arm study, abemaciclib was approved as single agent in heavily pretreated patients who have never had CDK4/6 inhibitors [8].
To assess the benefit achieved with the use of CDK4/6 inhibitor, a meta-analysis was reported [9]. However, this meta-analysis only included 6 randomized clinical trials (RCTs) [5-7,10-14], while two RCTs were recently published and their data were not included. The two studies incorporated large data sets (MONALEESA-7 [15], 672 patients; and MONALEESA-3 [16], 726 patients).
We believe that performing an updated meta-analysis with inclusion of more data would better quantify the benefit associated with the use of CDK4/6 inhibitors, and it would more accurately determine the advantage achieved in relevant patients’ subgroups.
Materials and Methods
Search Strategy
We identified relevant studies using an electronic literature search of the following databases: MEDLINE, EMBASE, and the Cochrane Library. We also searched for relevant abstracts in conference proceedings of the American Society of Clinical Oncology, San Antonio Breast Conference, and the European Society for Medical Oncology.
We used Medical Subject Heading terms or keywords: “breast cancer”, “cyclin-dependent kinase 4 and 6 (CDK4/6) OR CDK4/6 inhibitor OR palbociclib OR ribociclib OR abemaciclib”, “clinical trial [mh] OR randomized controlled trial (RCT) [mh]”. Throughout the search processes we targeted randomized trials for the three approved CDK4/6 inhibitors, i.e. palbociclib, ribociclib, and abemaciclib.
Selection Criteria
We included all studies that met the following criteria: (i) published in English language (ii) included patients with hormone receptor (HR)-positive and HER-2 negative advanced or metastatic breast cancer; (iii) investigated the efficacy of CDK4/6 inhibitor; (iv) reported hazard ratio (HR) for PFS or overall survival (OS), or reported adequate data allowing the HR to be computed; (v) based on RCT; and (vi) published as original articles or abstracts. For published duplicate data, we included most recent data, the study with mature follow-up, or the most applicable study. However, we included studies that have used the same data set but reported additional outcome.
Quality of the Included Studies
The quality of the studies was assessed by the Jadad scale [17]. In this model, randomization, double-blinding, and the reporting of withdrawals and dropouts were recorded; each scored one point (optimal score of three).
Data Extraction
Two authors (EMI and WME) independently inspected identified item and applied the inclusion/exclusion criteria. All authors reviewed and discussed potential eligible articles. For each identified study we extracted the following fields: the study name, first author’s last name, publication year, brief study description, study design, number of patients, patients median age, menopausal status, de novo vs. recurrent disease, disease-free interval (DFI), prior therapy, metastatic sites, follow-up duration, objective response data (ORR), median PFS, and HR and its 95% confidence interval (CI).
Outcome Measures
The primary outcome was the pooled analysis of HR and its 95% CI for PFS for patients receiving CDK4/6 inhibitor-containing regimens versus those in control arms in the first- and second-line settings. PFS was defined as defined as the time from randomization to either the first documented disease progression or death from any cause. The secondary outcome was to examine the pooled effect for various subgroups classified based on pertinent climicopathologic criteria. We also computed the pooled odds ratio (OR) for ORR of patients in experimental versus those in control groups. In the current meta-analysis we didn’t analyze toxicity or quality of life data.
Statistical Analysis
The pooled estimate of the HR and its 95% CI was computed. A HR less than 1 favored CDK4/6 inhibitor. Pooled OR and its 95% CI for ORR was calculated using the Mantel–Haenszel method [18]. If the HR was not provided in the original publication, the natural log HR and its standard error were calculated from the Kaplan–Meier survival curves or by the indirect method described by Parmar, et al. [19]. Where appropriate, we also used the built-in calculator of the Review Manager for Windows software version 5.3.5 to compute pertinent data (The Cochrane Collaboration, Oxford, UK). We used fixed-effects models because of the assumption that the effect sizes in our meta-analysis differ only because of sampling error and all studies share a common mean [20,21]. We performed subgroup analyses to assess the potential contribution of various variables to the main outcome.
Heterogeneity between trials and groups of trials was tested using X2 statistics [22] and measured with the I2 statistic which represents the proportion of total variation in study estimates that is due to heterogeneity [23]. Statistically significant heterogeneity was defined as a X2 P value less than 0.1 or an I2 statistic greater than 50%. We planned to perform meta-regression analysis to determine to what extent the effects of clinical variables could explain any demonstrated heterogeneity.
All statistical tests were two-sided and a statistical test result with a P value of less than 0.05 was defined as significant. We used Comprehensive Meta-analysis software (Biostat, version 3.3.070, Englewood New Jersey, USA) for all analyses.
Examining Publication Bias
A funnel plot estimating the precision of trials (plots of logarithm of the HR against its inverse standard error) was examined for asymmetry to determine publication bias [24]. Because of the small number of included studies, we used fail-safe N [25], and trim and fill methods [26] to quantify publication bias. The first method determines how many missing studies needed to be incorporated in the analysis before the P value becomes non-significant. While the latter gives the approximate number of studies to be imputed to make the funnel plot symmetric.
Results
Figure 1 shows the results of literature search. Targeted research identified five [5-7,11,15,27], and three studies [12-14,16] where CDK4/6 inhibitors were combined with ET versus ET only in the first- and second-line, respectively. Tables 1 and 2 show patients and disease characteristics of the eight included studies. All patients had hormone-positive and HER2-negative advanced or metastatic breast cancer disease. A total of 2664 patients were included in the first-line studies (1526 in experimental and 1139 patients in control arms), whereas 1916 patients were enrolled to the second-line studies (1277 in experimental and 639 patients in control arms).
Table 1. Patients and disease characteristics of included studies in first-line setting (5 studies). ABE: abemaciclib; CTX: chemotherapy; DFI: disease-free interval; LET: letrozole; m: months; NR: not reported; NSAI: non-steroidal aromatize inhibitor; PAL: palbociclib; PLB: placebo; RIB: ribociclib; T: tamoxifen.
|
Experimental/Control |
Study, design |
Year |
Stratification |
Experimental |
Control |
Number |
Median age (m) |
Premenopausal % |
Prior adj(neo)adjuvant CTX % |
Prior adj(neo)adjuvant ET % |
Visceral
disease % |
Bone only disease % |
Median follow-up (m) |
PALOMA-1 [10, 11]
Randomized, open-label,
phase 2. Global, multicenter |
2016 |
|
LET + PAL |
LET + PLB |
84/81 |
NR |
0/0 |
48/54 (any systemic therapy) |
44/53 |
20/25 |
29.6 |
PALOMA-2 [5]
Randomized, double-blind, placebo-controlled,
phase 3 trial. Global, multicenter |
2016 |
Visceral disease (Yes/No), DFI (de novo, ≤12 m, 12 m), prior therapy |
LET + PAL |
LET + PLB |
444/222 |
62/61 |
0/0 |
48/49.1 |
56.1/56.8 |
48/50 |
23/22 |
23
|
MONARCH-3 [6]
Randomized, double-blind, placebo-controlled,
phase 3 trial. Global, multicenter |
2017 |
Metastatic site, prior therapy |
NSAI + ABE |
NSAI + PLB |
328/165 |
63/63 |
0/0 |
38.1/40 |
45.7/48.5 |
52/54 |
21/24 |
17.8 |
MONALEESA-2 [7, 27] Randomized, double-blind, placebo-controlled,
phase 3 trial. Global, multicenter |
2016
2018 |
Visceral disease (Yes/No) |
LET+
RIB |
LET + PLB |
334/334 |
62/63 |
0/0 |
43.7/43.4 |
52.4/51.2 |
59/59 |
21/23 |
26.4 |
MONALEESA-7 [15]
Randomized, double-blind, placebo-controlled,
phase 3 trial. Global, multicenter |
2018 |
Visceral disease (Yes/No), prior CTX (advanced disease), T vs. NSAI |
T or NSAI
+
PLA
(all + goserelin) |
T or NSAI
+
PLB
(all + goserelin) |
335/337 |
43/45 |
100/100 |
41/41
14/14 (CTX for advanced disease) |
38/42 |
58/56 |
24/23 |
19.2 |
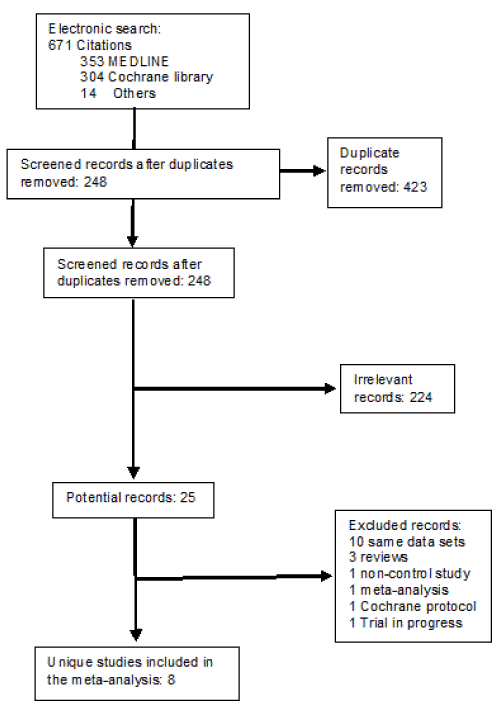
Figure 1. Flowchart of literature search and the selection of the 11 included studies.
Table 2. Patients and disease characteristics of included studies in second-line setting (3 studies). ABE: abemaciclib; CTX: chemotherapy; ET: endocrine therapy; FUL: fulvestrant; m: months; PAL: palbociclib; PLB: placebo; RIB: ribociclib.
|
Experimental/Control |
|
Study, design |
Year |
Stratification |
Experimental |
Control |
Number |
Median age (m) |
Premenopausal % |
Prior CTX % |
Prior ET % |
Visceral
disease % |
Bone only disease % |
Median follow-up (m) |
PALOMA-3 [12, 14]
Randomized, double-blind, placebo-controlled,
phase 3 trial. Global, multicenter |
2015
2016 |
Visceral disease (Yes/No), ET sensitivity, menopausal status |
FUL + PAL |
FUL + PLB |
347/174 |
57/56 |
21/21 |
40/43 (adjuvant / neoadjuvant)
33/37 (advanced disease) |
100/100 (adjuvant/
neoadjuvant) |
59/60 |
21/23 |
8.9
|
MONARCH-2 [13]
Randomized, double-blind, placebo-controlled,
phase 3 trial. Global, multicenter |
2017 |
Metastatic site, ET resistance (primary vs. secondary) |
FUL + ABE |
FUL + PLB |
446/223 |
59/62 |
16/19 |
60/60 (adjuvant / neoadjuvant)
|
71/66.8
(adjuvant/
neoadjuvant) |
55/57 |
28/26 |
19.5 |
MONALEESA-3 [16]
Randomized, double-blind, placebo-controlled,
phase 3 trial. Global, multicenter |
2018 |
Visceral disease (Yes/No), ET for advanced disease (Yes/No) |
FUL+RIB |
FUL + PLB |
484/242 |
63/63 |
0/0 |
56.6/54 (adjuvant / neoadjuvant)
|
59.7/58.7
(adjuvant/
Neoadjuvant)
22.7/16.5 (advanced disease) |
61/60 |
21/21 |
21 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
First-line Studies
Palbociclib, abemaciclib, and ribociclib were the CDK4/6 inhibitor used in PALOMA-1 (Palbociclib: Ongoing Trials in the Management of Breast Cancer) and PALOMA-2, MONARCH-3, and MONALEESA-2 (Mammary Oncology Assessment of LEE011’s [Ribociclib’s] Efficacy and Safetyand) and MONALEESA-7, respectively. Except for the MONALEESA-7 study, all first-line studies entirely included postmenopausal patients with a median age of about 63 years. Patients in MONALEESA-7 were all premenopausal and have a median age of 43 and 45 years among ribociclib and placebo groups, respectively [15] (Table 1).
Almost half of the patients received prior adjuvant or neoadjuvant chemotherapy and a similar proportion received prior adjuvant or neoadjuvant ET. About 40 to 50% of the included patients had visceral metastases, while approximately one-fifth had bone-only disease. All patients received non-steroidal aromatase inhibitors as the ET partner except for about 175 patients received tamoxifen in the MONALEESA-7 trial. In all studies the primary end point was PFS.
Second-line Studies
Approximately one-fifth of patients in the PALOMA-3 and MONARCH-2 studies were premenopausal or perimenopausal [13,14], while all patients in the MONALEESA-3 were postmenopausal [16]. Prior adjuvant/neoadjuvant chemotherapy or ET was offered to 40-60% and 60-100% of patients, respectively. About 60% of the included patients had visceral metastases, while 21-26% had bone-only disease. All patients had fulvestrant as the ET partner. It is worth noting that in the MONALEESA-3 study, 238 and 129 treatment-naïve patients in the ribociclib and placebo groups, respectively were included, and therefore, their data were incorporated into the pooled analysis of the first-line studies (Table 2).
Quality of the Included Studies
Assessment of the methodologic quality of the included studies concerning randomization, double-blinding, and the description of withdrawals and dropouts showed that all studies attained optimal Jadad score of 3 except the PALOMA-1 study as it was an open-label trial (2-point score).
Analysis of PFS for First-line Studies
Table 3 shows the median duration of PFS of CDK4/6 inhibitor-containing arms versus that in control arms. Meta-analysis to estimate the pooled effect of PFS for first-line studies is shown in Figure 2. The fixed effects model showed that CDK4/6 inhibitor was associated with 44% improvement in PFS (HR = 0.56; 95% CI 0.50-0.62; P <0.0001). The analysis included the effect size achieved in the treatment-naïve patients from the MONALEESA-3 study. Excluding the later date resulted into a HR = 0.56 (95% CI 0.50-0.63; P <0.0001). The model showed no heterogeneity (I2 = 0%; P = 0.99).
Table 3. Median progression-free survival. PFS: progression-free survival.
|
|
Median PFS (months) |
|
Study |
Experimental |
Control |
First-line |
PALOMA-1 |
|
|
All patients |
20.2 |
10.2 |
<65 years |
18.8 |
7.7 |
≥ 65 years |
26.2 |
12.9 |
PALOMA-2 |
24.8 |
14.5 |
MONARCH-3 |
NR |
14.7 |
MONALEESA-2 |
25.3 |
16.0 |
MONALEESA-7 |
23.8 |
13.0 |
Second-line |
PALOMA-3 |
9.5 |
4.6 |
MONARCH-2 |
16.4 |
9.3 |
MONALEESA-3 |
20.5 |
12.8 |
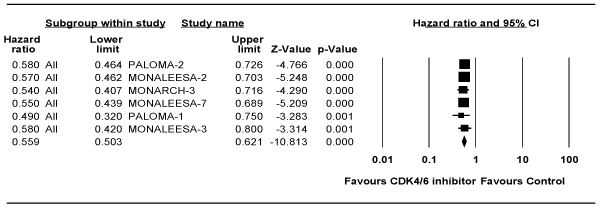
Figure 2. Forest plot of the hazard ratio for progression-free survival for studies in first-line setting. Squares represent the hazard ratio of each single study (size of the square reflects the study-specific statistical weight); horizontal lines represent 95 % confidence intervals; diamonds represent the pooled estimates, based on a fixed-effects model. All statistical tests were two-sided. CDK4/6, cyclin-dependent kinase 4/6.
Subgroup Analyses for First-line Studies
Several subgroup analyses were made, and it demonstrated a consistent statistically significant benefit attained with CDK4/6 inhibitor in all subgroups (Figure 3). Of note is the favorable outcome regardless de novo or recurrent disease, presence or absence of visceral disease, and prior ET or chemotherapy or no prior therapy. Although the benefit of CDK4/6 inhibitor was shown regardless of the length of DFI, different studies have used different cutoff duration. It was also shown that Asian population achieved numerically greater benefit compared with non-Asian patients (HR = 0.36 vs. 0.62). Such data were derived from four studies for Asians [5-7,11,15,27] and three studies for non-Asian patients [5,6,11,15].
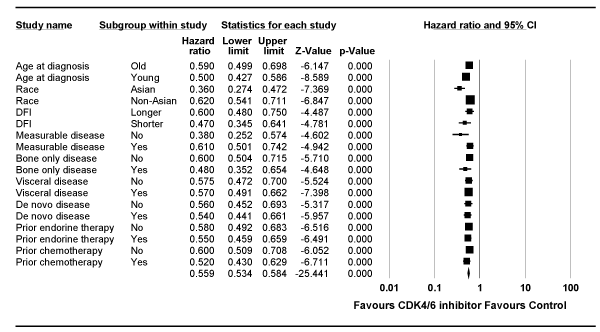
Figure 3. Forest plot of the hazard ratio for progression-free survival for subgroup analysis for studies in first-line setting. Squares represent the hazard ratio of each single study (size of the square reflects the study-specific statistical weight); horizontal lines represent 95 % confidence intervals; diamonds represent the pooled estimates, based on a fixed-effects model. All statistical tests were two-sided. CDK4/6, cyclin-dependent kinase 4/6.
Publication Bias
There was no evidence of publication bias. The shape of the funnel plot was symmetrical. Quantitatively, the fail-safe N method showed that 172 studies are required to accept the null hypothesis, and the trim and fill procedure indicated that there is zero study required to be imputed to make the funnel plot symmetric.
Analysis of PFS for Second-line Studies
Table 3 shows the median duration of PFS of CDK4/6 inhibitor-containing arms versus that in control arms. Meta-analysis to estimate the pooled effect of the PFS for second-line studies is shown in Figure 4. The fixed effects model showed that CDK4/6 inhibitor was associated with 47% improvement in the risk of progression (HR = 0.53; 95% CI 0.46-0.60; P <0.0001). The model showed no heterogeneity (I2 = 0%; P = 0.44).
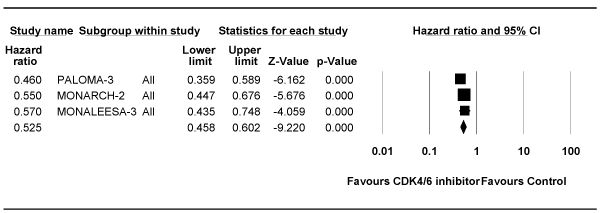
Figure 4. Forest plot of the hazard ratio for progression-free survival for studies in second-line setting. Squares represent the hazard ratio of each single study (size of the square reflects the study-specific statistical weight); horizontal lines represent 95 % confidence intervals; diamonds represent the pooled estimates, based on a fixed-effects model. All statistical tests were two-sided. CDK4/6, cyclin-dependent kinase 4/6.
Subgroup Analyses for Second-line Studies
Several subgroup analyses were made, and it demonstrated a consistent benefit attained with CDK4/6 inhibitor in all subgroups (Figure 5). Only the PALOMA-3 study reported on difference in HR based on the DFI of < 24 months vs. >24 months (HR = 0.84; 95% CI 0.41-1.75 vs. HR = 0.45; 95% CI 0.30-0.67, respectively). All three studies provided data for Asian vs. non-Asian population and the pooled analysis showed similar benefit (HR = 0.60 vs. 0.53, respectively).
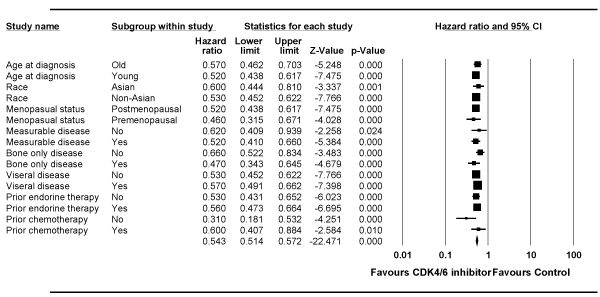
Figure 5. Forest plot of the hazard ratio for progression-free survival for subgroup analysis for studies in second-line setting. Squares represent the hazard ratio of each single study (size of the square reflects the study-specific statistical weight); horizontal lines represent 95 % confidence intervals; diamonds represent the pooled estimates, based on a fixed-effects model. All statistical tests were two-sided. CDK4/6, cyclin-dependent kinase 4/6.
Publication Bias
The shape of the funnel plot was symmetrical. Quantitatively, the fail-safe N method showed that 63 studies are required to accept the null hypothesis, while the trim and fill indicated that there is zero study needed to make the funnel plot symmetric. Therefore, there was no evidence of publication bias.
Overall Survival (OS)
OS was not analyzed as none of the included study reported mature OS data.
Analysis of ORR in First- and Second-line Trials
As shown in Table 4 the ORR was consistently higher in CDK4/6 inhibitor-containing arms as compared with that in the placebo arms with significant OR in all analyses. The pooled OR was 1.97 (95% CI, 1.68-2.30; P <0.0001). Moreover, the ORs were higher in the second-line setting as compared with the ORs in the first-line studies. In none of the comparison was a heterogeneity detected with I2 = 0% and non-significant P values were computed in all comparisons.
Table 4. Analysis of objective response rates. CI: confidence interval.
|
|
Objective response rate % |
Objective response rate % |
|
|
All disease sites |
Measurable disease |
First-line |
Studies |
Experimental |
Control |
Experimental |
Control |
|
PALOMA-1 |
|
|
|
|
|
< 65 years |
82.5 |
63 |
|
|
|
> 65 years |
72.2 |
42.1 |
|
|
|
PALOMA-2 |
42.1 |
34.7 |
55.3 |
44.4 |
|
MONARCH-3 |
48.2 |
34.5 |
59.2 |
43.8 |
|
MONALEESA-2 |
42.5 |
28.7 |
54.5 |
38.8 |
|
MONALEESA-7 |
41 |
30 |
51 |
36 |
Odds ratio (95%CI) |
|
1.82 (1.40-2.37) |
1.78 (1.34-2.36) |
Second-line |
|
|
|
|
|
|
PALOMA-3 |
19 |
9 |
25 |
11 |
|
MONARCH-2 |
35.2 |
16.1 |
48.1 |
21.3 |
|
MONALEESA-3 |
32.4 |
21.5 |
40.9 |
28.7 |
Odds ratio (95%CI) |
|
2.54 (1.81-3.56) |
2.03 (1.27-3.24) |
First- and second-line
Odds ratio (95% CI) |
|
1.97 (1.68-2.30)
|
Discussion
The current meta-analysis clearly demonstrated that the addition of CDK 4/6 inhibitors to standard ET represents a new standard of care in HR-positive HER2-negative advanced breast cancer in the first- or second-line setting. CDK 4/6 inhibitors were associated with significant improvement in ORR and PFS and the benefit was across all analyzed subgroups, emphasizing use of CDK4/6 inhibitors for a broad range of patients. Such advantage was shown regardless of age, menopausal status, de novo versus recurrent disease, DFI, presence or absence of visceral disease, and prior ET or chemotherapy or no prior therapy. Similar to the benefit gained in postmenopausal patients, premenopausal and perimenopausal have also benefited from CDK4/6 inhibitor in both first- and second-line settings [13-15].
It was also intriguing to observe a greater benefit attained in Asian population as compared with non-Asian patients in the first-line setting. On the other hand, both races achieved an almost similar benefit in the second-line setting. It is well known that ethnicity could partially explain differences in drug effect. In the CLEOPATRA study, the incidence of febrile neutropenia of grade 3 or higher associated with pertuzumab, trastuzumab, and docetaxel in Asian patients was 26% compared with a 10% incidence among non-Asians. This observation suggests that pertuzumab may have more effect on Asians as the incidence of febrile neutropenia was 16% and 10% among Asian and non-Asian patients, respectively with the use of trastuzumab and docetaxel only [28].
Despite the remarkable benefit achieved with the introduction of CDK 4/6 inhibitors in the management of in HR-positive HER2-negative advanced breast cancer, there are several issues to be addressed. First, this class of drugs is associated with a wide range of significant, albeit, manageable toxicity such as neutropenia, fatigue, diarrhea specifically linked to abemaciclib, or QTc prolongation related to ribociclib [29]. Second, while there has been consistent PFS benefit in all subgroups, the effect on OS is still unknow and longer follow-up or additional studies may provide the answer.
Third, the cost of CDK4/6 inhibitors. Recently, Mamiya et. al. reported a simulation model that showed that the addition of palbociclib to letrozole in the first-line setting would cost an estimated $768,498 per additional quality-adjusted life year (QALY) gained. While the addition of palbociclib to fulvestrant in patients with prior endocrine therapy would cost an estimated $918,166 per QALY [30].
One of the best ways to offset such high cost is to identify patients that would most benefit from the addition of CDK 4/6 inhibitors to standard ET. No predictive biomarkers have been identified so far. Patients selection based on amplification of cyclin D1 or loss of p16 was not found predictor of benefit [10]. In a small randomized study (74 patients), Arnedos et. al. used palbociclib for 14 days prior to breast cancer surgery and they reported that early decrease of retinoblastoma gene phosphorylation corelates to palbociclib effect on cell proliferation as reflected by changes in Ki67 level [31].
In conclusion, using CDK4/6 inhibitors in advanced HR-positive, HER-2 negative disease is a new standard of care. Future research should be able to answer essential questions about predictor biomarkers, overcoming primary and secondary resistance to CDK4/6 inhibitors, potential use of CDK4/6 inhibitors in other settings, i.e. adjuvant and neoadjuvant and in HER-2 positive disease, and the combination of CDK4/6 inhibitors with chemotherapy or other agents.
Conflict of interest
None
References
- DeSantis C, Ma J, Bryan L, Jemal A (2014) Breast cancer statistics, 2013. CA Cancer J Clin 64: 52-62. [Crossref]
- Anderson WF, Katki HA, Rosenberg PS (2011) Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst 103: 1397-1402. [Crossref]
- Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, et al. (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366: 520-529.
- Wolff AC, Lazar AA, Bondarenko I, Garin AM, Brincat S, et al. (2013) Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol 31: 195-202. [Crossref]
- Finn RS, Martin M, Rugo HS, Jones S, Im SA, et al. (2016) Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 375: 1925-1936. [Crossref]
- Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, et al. (2017) MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol 35: 3638-3646. [Crossref]
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, et al. (2016) Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med 375: 1738-1748. [Crossref]
- Dickler MN, Tolaney SM, Rugo HS, Cortes J, Dieras V, et al. (2017) MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR(+)/HER2(-) Metastatic Breast Cancer. Clin Cancer Res 23: 5218-5224.
- Ding W, Li Z, Wang C, Ruan G, Chen L, et al. (2018) The CDK4/6 inhibitor in HR-positive advanced breast cancer: A systematic review and meta-analysis. Medicine (Baltimore) 97: e10746.
- Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, et al. (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16: 25-35.
- Finn RS, Crown JP, Ettl J, Schmidt M, Bondarenko IM, et al. (2016) Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res 18: 67.
- Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, et al. (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17: 425-439.
- Sledge GW, Toi M, Neven P, Sohn J, Inoue K, et al. (2017) MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol 35: 2875-2884.
- Turner NC, Huang Bartlett C, Cristofanilli M (2015) Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med 373: 1672-1673.
- Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, et al. (2018) Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 19: 904-915. [Crossref]
- Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, et al. (2018) Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol 24: 2465-2472.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1-12.
- MANTEL N, HAENSZEL W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719-748. [Crossref]
- Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815-2834.
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1: 97-111. [Crossref]
- Pignon JP, Hill C (2001) Meta-analyses of randomised clinical trials in oncology. Lancet Oncol 2: 475-482. [Crossref]
- Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127: 820-826. [Crossref]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557-560. [Crossref]
- Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629-634. [Crossref]
- Orwin RG (1979) A Fail-Safe N for Effect Size in Meta-Analysis. Journal of Educational Statistics 8: 157-159.
- Duval S, Tweedie R (2000) Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56: 455-463. [Crossref]
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, et al. (2018) Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 29: 1541-1547.
- Baselga J, Cortés J, Kim SB, Im SA, Hegg R, et al. (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366: 109-119. [Crossref]
- Wander SA, Mayer EL, Burstein HJ (2017) Blocking the Cycle: Cyclin-Dependent Kinase 4/6 Inhibitors in Metastatic, Hormone Receptor-Positive Breast Cancer. J Clin Oncol 35: 2866-2870.
- Mamiya H, Tahara RK, Tolaney SM, Choudhry NK, Najafzadeh M (2017) Cost-effectiveness of palbociclib in hormone receptor-positive advanced breast cancer. Ann Oncol 28: 1825-1831.
- Arnedos M, Bayar MA, Cheaib B, Scott V, Bouakka I, et al. (2018) Modulation of Rb phosphorylation and antiproliferative response to palbociclib. The Preoperative-Palbociclib (POP) randomized Clinical Trial. Ann Oncol 29:1755-1762.





