A number of perovskite-like oxides La1-xCaxCoO3-δ (x = 0-1) prepared by the mechanochemical method were studied in respect of their catalytic activities to methane oxidation. Mechanochemical route as compared with ceramic route results in increase a substitution degree in La1-xCaxСoO3-δ oxides from x=0.3 up to x=0.5 even at lower temperature and duration of calcination. The introduction of calcium resulted in a non-monotonic decrease in the catalytic activity upon an increase in x with the intermediate maximum at x=0.3-0.5. The catalytic activities decreased in the series: LaCoO3> La0.6Ca0.4CoO3 > La0.8Ca0.2CoO3 > Ca2Co2O5. The observed changes in the catalytic activity of the calcium-containing samples correlate with variations in the proportion of weakly bonded oxygen species (or Cо+4 cations) only in the composition range x=0.1-0.4. The higher activity of lanthanum cobaltite may be accounted for by the presence of cobalt oxide particles on the perovskite surface, while the decrease in the activity at x>0.4 by the emergence of calcium oxide on the perovskite surface and by the appearance of the less active brownmillerite phase.
La1-xCaxСоO3-δ, perovskites, mechanochemical route, catalytic oxidation of methane
The high thermal and chemical stability of transition and rare earth metal oxides with the perovskite structure La1-xMex1Me2O3--d (Ме1=Сa, Sr, Ba; Me2=Fe, Co, Mn, Ni) make them widely applicable for high temperature processes such as catalytic combustion of hydrocarbons, steam reforming of methane, ammonia oxidation, reduction of sulfur dioxide and a number of the other processes [1-3]. Properties of the oxides depend on the nature of the transition (Me2) and substituting (Me1) cations, as well as on the preparation conditions.
Substituted perovskites La1-xCaxСoO3-δ are highly active to propane oxidation [4-5], attract interest as bifunctional oxygen electrodes [6], and demonstrate exiting magnetic and electric behavior [7]. Literature data in the field give evidence of the substantial dependence of the phase composition and catalytic properties of La1-xCaxСoO3-δ on the preparation conditions. It was shown that the homogeneity range in substituted lanthanum cobaltites La1‑xCaxСoO3‑d is expanded upon temperature elevation during the ceramic synthesis. For example, calcinations of the initial reactants at 885°C allowed the one-phase samples to be produced at x=0-0.25 [8], while calcinations at 1100 °C resulted in an increase in the solubility up to x=0.3 [9-10] that agrees with the statement [11] on the impossibility of the preparation of solid solutions with x>0.3 by the ceramic method (calcination temperature 1100°C). The temperature elevation up to 1200°C led to the expansion of the solubility range to x=0.5 [12]. Synthesis from solutions allows perovskites with a higher substitution degree to be prepared at lower calcinations temperatures [4,11,13-17]. For example, the solid solutions with x up to 0.8 can be prepared at 700-900°C of the calcinations temperature according [16,17].
The differences in the phase composition of perovskites La1-xCaxСoO3-δ prepared by different methods are reported in literature; these differences can be the result of the different degrees of homogeneity of initial components at their mixing, calcinations duration and atmosphere. Again, by analogy with the studied earlier La1-xСаxFeO3-δ system [18-19], the reason may be a change in the calcium solubility due to ordering of the oxygen vacancies upon temperature elevation.
The observed diversity of catalytic properties of the oxides under consideration prepared using different methods may be accounted for by their different phase compositions, microstructures, textures, and surface composition. The non-linear changes in the catalytic activity to propane oxidation depending on x were shown [4-5] the for the one-phase perovskites prepared by the citrate method, the least active being the sample with x =0.2 (0.5>0.4>0>0.2). Generally, the activity increased with an increase in the proportion of weakly bonded oxygen (detected using TPR and XPS techniques) whiles the sample with x=0.2 fell out of the dependence. Enrichment of the surface of all the prepared samples with lanthanum compounds and a high stability of the oxides in the reaction medium was reported. Another series of the activity to methane oxidation was observed with the samples prepared by the Pechini method: 0.3>0.2=0.6>0.4>0>1 [20-21]. The surface enrichment with calcium compounds due to the destruction of perovskite surface layers during the reaction to release calcium oxide was established. The authors reported correlation between the initial catalytic activity (at x=0-0.3) and the amount of the weakly bonded oxygen that indicated the stepwise (suprafacial) mechanism of the reaction, the sample with x=0.2 being most stable.
The mechanochemical method implies calcination of a mechanically pre-activated mixture of initial reactants; the better disintegration, homogenization and formation of defects to accelerate sintering makes it possible to synthesize complex oxides at low temperature and shorter treatment times against those of the ceramic method [22-23]. The energy effective and environmentally friendly mechanochemical method is expected to provide the preparation of wide latitude of homogeneous solid solutions in the La1-xCaxСoO3-δ system. The present work was aimed at mechanochemical preparation of La1-xCaxСoO3-δ (x=0-1) samples and at characterization of their physicochemical and catalytic properties.
Initial compounds for synthesis were La2O3, СаO, Co3O4 of the chemical purity grade.
The mechanochemical (MC) method for synthesis of La1-xCaxCoO3-d (х=0; 0.1, 0.2; 0.3, 0.4; 0.5, 0.6; 0.7, 0.8; 0.9, 1) included the stage of mechanical treatment of initial oxides in the necessary proportion followed by the stage of thermal treatment. The mechanical treatment was carried out in air using a centrifugal planetary ball mill APF-5; the weight ratio of the loaded powder and 5 mm steel balls was 5:10. The mill drums provided the acceleration equal to ~40 g. The mechanical treatment (MT) took 3 min. The mechanically activated mixture underwent thermal treatment at 1100°C in air for 5 hours.
The phase composition was studied using a X’TRA (Thermo ARL, Швейцария) instrument. The spectra were acquired at the 2θ range of 10о-80о at 0.05 step and 43 s accumulation time. BET surface area, Ssp, was determined using argon desorption at 300°C.
Thermoprogrammed reduction with hydrogen (H2-TPR) was carried out using a flow setup equipped with a thermal conductivity detector for the 0.25-0.5 mm fraction. The samples were pretreated in oxygen at 500°C for 0.5 h and cooled in oxygen to room temperature. The sample weight was 50 mg; flow rate of the reducing mixture (10% H2 in Ar) was 40 cm3min1. The samples were heated to 900°C at the rate of 10°/min. The Origin 6.0 program package was used for calculation of the peak areas underneath the TPR curves. H2 consumption of MC prepared La1-xCaxCoO3-δ was calculated for peaks in the temperature fields: 40-150°C, 40- 500oC, 500-900oC and 40-900оС.
The catalytic activity to methane oxidation was studied at 350-600°C in a flow reactor using 1 g (0.6 cm3) 0.25-0.5 mm fraction of the catalyst. The reaction mixture (0.5 CH4 + 9 % O2 in He) was fed at the rate of 2.4 l/h. The reaction rate for the initial methane concentration Co=0.5 was calculated by formula (1):
W, [molecules of CH4m2·s-1] = k·Co·2.69·1019, (1)
Where k is effective rate constant of the first order reaction calculated for a plug-flow reactor using equation k = -2.3·lg(1-x)/(t·Ssp·q) (x is the conversion of CH4, q is weighed sample, t is contact time). The chromatographic error of measuring concentrations of the gas mixture components was no more than 20%.
Phase composition and specific surface area of MC prepared La1-xCaxCoO3-δ (0 ≤ x ≤ 1).
From XRD data (Figure 1), the prepared samples with x ≤ 0.5 are practically one-phase perovskites with a small admixture of cobalt oxides. The lattice parameter and unit cell volume decreased with increasing the x value to 0.4, which indicated the intercalation of Ca cations into lanthanum sublattice (Table 1). In the samples with x ≥ 0.6, the phase of calcium cobaltite with the brownmillerite structure is detected along with the perovskite phase, the former phase being increased in proportion with increasing x to become the main phase with cobalt and calcium oxides as impurities at x=1. The XRD data indicate the morphotropic phase transition from hexagonal (x<0.4) to cubic (x>0.4) modification of the perovskite structure. Thus, the mechanochemical method, against the ceramic method, allows really the substituted solutions with larger x to be prepared during shorter time of the calcination at 1100°C. The data on the specific surface area of La1-xCaxCoO3-δ samples (no more than 1 m2/g) show their good sintering, see Table 1.
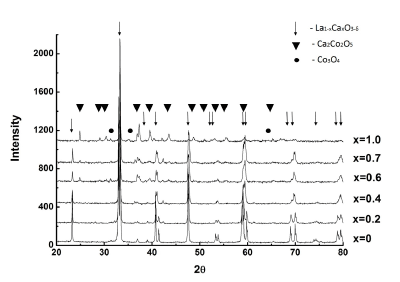
Figure 1. X-ray data for MC prepared La1-xCaxCoO3-δ.
Table 1. Specific surface area (Ssp, m2g-1) of MC prepared La1-xCaxCoO3-δ samples and unit cell parameters for the perovskite phases.
Sample |
aÅ |
cÅ |
VÅ3 |
Ssp, m2g-1 |
LaCoO3 |
5.444 |
13.097 |
56.027 |
0.24 |
La0.9Ca0.1CoO3 |
5.443 |
13.104 |
56.018 |
0.16 |
La0.8Ca0.2CoO3 |
5.437 |
13.109 |
55.938 |
0.14 |
La0.7Ca0.3CoO3 |
5.429 |
13.120 |
55.825 |
0.26 |
La0.6Ca0.4CoO3 |
3.818 |
|
55.652 |
0.2 |
La0.5Ca0.5CoO3 |
3.816 |
|
55.564 |
0.4 |
La0.4Ca0.6CoO3 |
3.818 |
|
55.669 |
0.34 |
La0.3Ca0.7CoO3 |
3.819 |
|
55.702 |
1.0 |
La0.1Ca0.9CoO3 |
3.819 |
|
55.700 |
0.36 |
CaCoO3 |
|
|
|
0.35 |
Microstructure of MC prepared La1-xCaxCoO3-δ
From HRTEM data, samples of La1-xCaxCoO3-δ at x= 0-0.3 are mainly built up by particles of rhombohedral perovskite phase of 100 nm to micron in size (Figure.2). There are ensembles (10–50 nm in size) of fine cobalt oxide particles (x=0), as well as lanthanum and calcium oxides (x=0.2-0.4 and x>5, respectively) on the perovskite surface. With an increase in the amount of calcium, the amount of admixtures in the samples (Figure. 2) increases. The size of such particles is ca. 10 nm (Figure. 2). Sample with x=0.4 is cubic perovskite. The cubic phase is La0.6Ca0.4CoO3-δ with some deviations (5-7 at %). In the samples with x ≥ 0.5 an additional brownmillerite phase (figure. 3b) along with the cubic perovskite modification (figures 2, 3a) occurs. From HRTEM and EDX data, the composition of the rhombohedral phase is changes according to the Vegard rule up to x<0.4. The HRTEM data allow the compositions at x=0.3–0.4 to be assigned to the region of morphotropic phase transition.
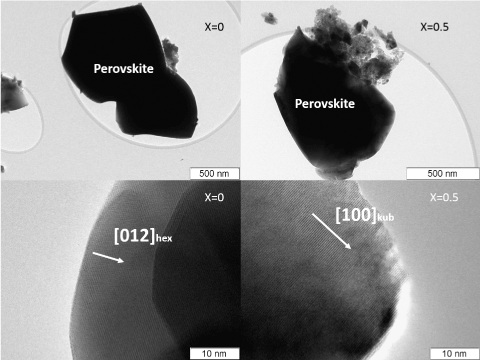
Figure 2. Microstructure of two kinds of particles of MC prepared: LaCoO3-δ particle with R3m perovskite structural modification (a); particle La0.5Ca0.5CoO3-δ with Pm3m perovskite structural modification (b).
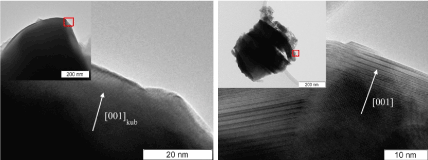
Figure 3. Microstructure of two kinds of particles of MC prepared La0.4Ca0.6CoO3-δ: particle with Pm3m perovskite structural modification (a); particle with brawnmillerite structural modification (b).
H2-TPR of MC prepared La1-xCaxCoO3-δ
TPR curves (dependencies of hydrogen absorption on the temperature of sample reduction) are shown in Figure. 4. The complex shapes of the TPR curves indicate the stepwise process of the sample reduction with hydrogen with the temperature rise; the first low-temperature peak is observed at ca. 400°C, and the second high-temperature peak at 600°C. The substitution leads to splitting and increasing in intensity of the first peak, to lowering of the reduction onset temperature at increasing x, while the high-temperature peak is shifted towards low temperature region. The reduction of cations Co3+→Co2 and Co4+→Co0 at below 500°C, and Co2+→Co0 at above 500°C is reported elsewhere [24]. By analogy with literature data [25], we calculated quantities of hydrogen absorption at the ranges assigned, formally, to different steps of the sample reduction:
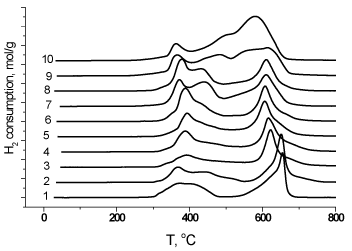
Figure 4. H2-TPR curves of MC prepared La1-xСаxCoO3-δ: 1- x=0; 2- x=0.1; 3-x=0.2; 4 - x=0.3; 5 – x=0.4; 6 – x=0.5; 7 – x=0.6; 8 – x= 0.7; 9 – x=0.9; 10 – x=1.
- The lowest-temperature hydrogen absorption corresponds to removal of no more than one surface oxygen monolayer (40-150°C)
- The low-temperature absorption with maxima at the range no more than 500 °C corresponds quantitatively to the reduction of cations Co4+→ Co0 and Co3+→ Co2+ (40-500°C)
- The high-temperature absorption with maxima at above 500°C corresponds quantitatively to the reduction Co2+→ Co0 in the sample bulk and is accompanied by the perovskite destruction (500-900°C).
Figure. 5 illustrates hydrogen absorption as dependent on the catalyst compositions calculated for the identified TPR ranges. The data argue for an increase in the total hydrogen absorption and, correspondingly, for an increase in the oxygen contents; in accordance with the rule of preserving electroneutrality, this must result in an increase in the charge of a part of cobalt cations, i.e. in an increase in the number of Co4+ cations at increasing the calcium content (x). Similar changes were observed before in the La1-xSrxCoO3-δ system [25]. However, the absorption in the low-temperature range related to the reduction Co4+→ Co0 and Co3+ → Co2+ goes through a maximum to indicate the corresponding change in the quantity of Co4+ cations. The discrepancy in the Co4+ contents calculated from the total or low-temperature absorption may result from incorrect calculation of the absorption at low temperature because of overlapping of the low- and high-temperature peaks.
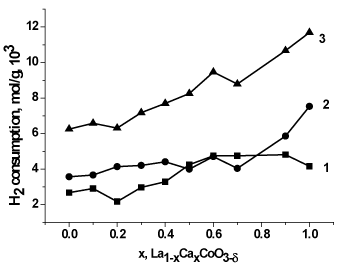
Figure 5. H2 consumption of MC prepared La1-xCaxCoO3--δ versus x in the temperature fields: 40-500°C (1), 500-900°C (2) and 40-900°C (3).
Notice that the lowest-temperature hydrogen absorption, which is assigned to variations in the most weakly bonded oxygen upon calcium introduction, also changes in non-monotone manner but, in general, increases with increasing x in the calcium-containing samples after its initial decrease at x=0.1.
Catalytic activity of MC prepared La1-xCaxCoO3-δ in methane oxidation
Figures 6 and 7 show dependencies of the methane conversion and catalytic activity (CA) on the composition (x) of samples La1-xCaxСoO3-δ at T=350-600оC. The substitution of calcium for lanthanum results in a non-monotone (with an intermediate maximum at x=0.2-0.5) decrease both in the methane conversion (Figure 6) and in CA (Figure 7). The maximal conversion at 600°C (x=0) is ca. 60% but no more than 40% over the calcium-substituted perovskites(x>0). The temperature elevation leads to an increase in the conversion to reach 100% at 750°C even over the least active sample (x=0.9).
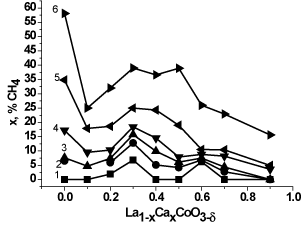
Figure 6. Conversion of methane of MC prepared La1-xCaxСoO3-δ versus x at: 350°C (1), 400°C (2), 450°C (3), 500°C (4), 550°C (5), 600°C (6).
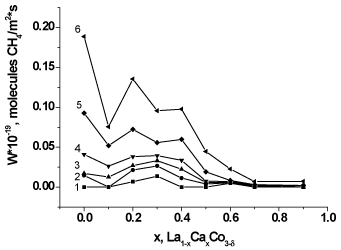
Figure 7. Catalytic activity (reaction rate) in methane oxidation of MC prepared La1-xCaxСoO3-δ versus x at: 350°C (1), 400°C (2), 450°C (3), 500°C (4), 550°C (5), 600°C (6).
The observed variations in the catalyst activity to methane oxidation do not correlate with variations in the quantity of the most weakly bonded surface oxygen species, probably, due to the absence (desorption) of these species under conditions of the catalytic studies. Again, in general, the catalyst activity correlates neither with calcium content nor with an increase in the proportions of weakly bonded oxygen and Co4+ cations (determined from the total absorption and from the first peak). Hence, not only quantity of weakly bonded oxygen determined the catalyst activity. For example, some researchers [4-5] reported the correlation between the catalytic activity to propane oxidation and the quantity of oxygen removed during TPR but mentioned also that this is not the case of the sample at x=0.2, probably, for some additional reasons.
Apart from the sample with x=0, there is observed the correlation between the activity of calcium-containing catalysts and the content of Co4+ in the range of compositions x=0.1-0.4. At the same time, the correlation was observed with the catalysts prepared by the Pechini method at all x=0-0.3 range including the sample with x=0 free of surface cobalt [20]. The further decrease in the activity at an increase in the Co4+ content may be accounted for by the appearance of calcium oxide on the particle surface [20]. The activity of the prepared by us sample of lanthanum cobaltite is 20·1016 CH4 molecules/m2·s against 7·1016 CH4 molecules/m2·s showed by the Pechini sample [20]; this is almost 3 times difference. The catalytic activity of the other samples is only a little higher than that of the Pechini samples.
It is not impossible that the higher catalytic activity of lanthanum cobaltite prepared by the mechanochemical method is the result of presence of dispersed cobalt oxide on the surface while the other samples of the series are free of this oxide. Enrichment of the surface with cobalt oxide also was observed before upon mechanochemical treatment of lanthanum cobaltite [26]. It seems like this is the specific feature of the mechanochemical method since the other methods, e.g. the Pechini method, produce lanthanum cobaltite enriched with surface lanthanum compounds. It is reasonable to suppose that the initial decrease in the activity observed upon calcium introduction results from the decrease (absence) in the content of cobalt oxide on the surface of calcium-containing samples. The further increasing activity up to the intermediate maximum at x=0.3-0.5 may be accounted for by an increase in the content of Co4+ cations, while the activity lowering at x>0.5 by the emergence of the less active brownmillerite phase in the samples and calcium oxide on the surface. Hence it was shown that catalytic activity in oxidative process depends not only content of low bounded oxygen form but on surface enrichment with different oxides that may increase (LaCoO3) or decrease (La1-xCaxCoO3-δ x>0.4) activity.
The mechanochemical method followed by thermal treatment at 1100°C was demonstrated to allow preparation of homogeneous solid solutions in the La1-xCaxCoO3-δ system at x ≤ 0.5. Among the prepared samples, non-substituted lanthanum cobaltite is the most active to methane oxidation due to, probably, the presence of disperse cobalt oxide on the sample surface. This phenomenon is the specific feature of the mechanochemical method. With substituted perovskites, introduction of calcium results in lower activity probably due to absent of cobalt oxide on the sample surface and appearance of calcium oxide impurity. An increase in the catalytic activity at the composition range of x=0.1-0.3 up to the intermediate maximum at x=0.3-0.5 correlates with increase of low bounded oxygen form in perovskites or Co+4 content. The further decrease in the activity may be accounted for by the formation of the vacancy-ordered less active brownmillerite phase. The following series of the catalyst activity is discovered: LaCoO3 > La0.6Ca0.4CoO3 > La0.8Ca0.2CoO3 > Ca2Co2O5
This work was conducted within the framework of budget project АААА-А17-117041710090-3 for Boreskov Institute of Catalysis
- Tejuca LG, Fierro JLG, Tascon JMD (1989) Structure and Reactivity of Perovskite-Type Oxides. Adv Catal 36: 237.
- Baran EJ (1990) Structural Chemistry and Physicochemical Properties of Perovskite-like Materials. Catal Today 8: 133.
- Yamazoe N, Teraoka Y (1990) Oxidation Catalysis of Perovskites – Relationships to bulk structure and Composition (Valence, Defects, ets.). Catal Today 8: 175.
- Merino N, Barbero BP, Grande P, Cadus LE (2005) La1-xCaxCoO3 Perovskite-Type Oxides: Preparation, Characterization, Stability, and Catalytic Potentiality for the Total Oxidation of Propane. J Catal 231: 232.
- Merino N, Barbero BP, Eloy P, Cadus LE (2006) La1- xCaxCoO3 perovskite-type oxides: Identification of the surface oxygen species by XPS. Appl Surf Sci 253: 1489.
- Haas O, Holzer F, Muller S, McBreen JM, Yang XQ, et al. (2002) X-Ray Absorption and Diffraction Studies of La0.6Ca0.4CoO3 Perovskite, a Catalyst for Bifunctional Oxygen Electrodes. Electrochemica Acta 47: 3211.
- Yazdanbakhsh M, Tavakkoli H, Hosseini SM (2011) Electrical and Optical Properties of Nanosized Perovskite-type La0.5Ca 0.5MO3 (M=Co, Ni) prepared using Sol-Gel Method. Afr J Chem 64: 71.
- Wong NW, Lawz WJ, Yan YG (2013) Phase diagram and crystal chemistry of the La–Ca–Co–O system. Solid State Sci 17: 107.
- Cherepanov VA, Gavrilova LY, Barkhatova LY, Voronin VI, Trifonova MV, et al. (1998) Phase Equilibria in the La-Me-Co-O (Me=Ca, Sr, Ba) Systems. Ionics 4: 309.
- Gavrilova LY, Cherepanov VA, Surova TV, Baimistruk VA, Voronin VI (2002) Phase equilibria and oxygen nonstoichiometry in complex oxide phases of the La-Ca-Co-O system. Russian J Phys Chem 76: 150.
- Mastin J, Einarsrud MA, Grande T (2006) Crystal Structure and Thermal Properties of La1-xCaxCoO3-δ (0 ≤ x ≤ 0.4). Chem Mater 18: 1680.
- Kononyuk IF, Tolochko ASP, Lutsko VA, Anishchik VM (1983) Preparation and properties of La1−xCaxCoO3 (0.2 ≤ x ≤ 0.6). J Solid State Chem 48: 209.
- Melo DS, Marinho EP, Soledade LEB, Melo DMA, Lima SJG, et al. (2008) Lanthanum-based perovskites obtained by the polymeric precursor method. J Mater Sci 43: 551.
- Pathak S, Kuebler J, Payzant A, Orlovskaya N (2010) Mechanical behavior and electrical conductivity of La1−xCaxCoO3 (x = 0, 0.2, 0.4, 0.55) perovskites. J Power Sources 195: 3612.
- Merino NA, Barbero B, Eloy P, Cadus LE (2006) La1−xCaxCoO3 perovskite-type oxides: Identification of the surface oxygen species by XPS. J Appl Suf Science 253: 1489.
- Kumar DA, Selvasekarapandian S, Nithya H, Leiro J, Masuda Y (2013) Effect of calcium doping on LaCoO3 prepared by Pechini method. Power Technology 235: 140.
- Haas O, Ludwig C, Bergmann U, Singh RN, Braun A, Graule T (2011) X-ray absorption investigation of the valence state and electronic structure of La1−xCaxCoO3−δ in comparison with La1−xSrxCoO3−δ and La1−xSrxFeO3−δ. J Solid State Chem 184: 3163.
- Nadeev AN, Tsybulya SV, Belyaev VD, Yakovleva IS, Isupova LA (2008) Weakly bound oxygen and its role in stability of solid solutions La1−x Sr x FeO3−δ. Russ J Structural Chem 49: 1077.
- Gerasimov EY, Isupova LA, Tsybulya SV (2015) Microstructural features of the La1-хCaxFeO3-δ solid solutions prepared via Pechini route. Mater Res Bull 70: 291.
- Isupova LA, Kulikovskaya NA, Saputina NF, Gerasimov EY (2018) Catalytic activity of La1-xCaxCoO3-δ (х = 0-1) perovskites prepared by Pechini route in deep oxidation of methane. Kinetics Catalysis 59: 407.
- Gerasimov E, Kulikovskaya N, Chuvilin A, Isupova L, Tsybulya S (2016) Microstructural Changes in La1−xCaxCoO3−δ Solid Solutions Under the Influence of Catalytic Reaction of Methane Combustion. Top Catal 59: 1354.
- Boldyrev VV (1988) Mechanical activation and its application to technology. J Chem Phys 11: 821.
- Avvakumov EG, Senna M, Kosova NV (2001) Soft Mechanochemical Synthesis: A Basis for new Chemical Technologies; Kluwer Acad. Publ: Boston-Dordrecht-London.
- Futai M, Yonghua C (1986) Characterization of perovskite-type oxide catalysts RECoO3 by TPR. React Kinet Catal Lett 31: 47.
- Yakovleva IS, Isupova LA, Rogov VA (2009) Oxygen species and their reactivity in the mechanochemically prepared substituted perovskites La1 - xSrxCoO3-y (x = 0-1). Kinetics Catalysis 50: 275.
- Pauli IA, Avvakumov EG, Isupova LA, Sadykov VA, Poluboyarov VA (1993) Effect of mechanical activation on synthesis and catalytic properties of lanthanum cobaltite. Conference on Mechanochem. (InCoMe -93 Kosice); Cambridge Intersci. Publ.







