Abstract
Fibrolamellar hepatocellular carcinoma (FL-HCC) is a rare primary hepatic cancer which has a preference for children and adolescents without fundamental liver disease or cirrhosis. A fusion gene, DNAJB1‐PRKACA, the only investigated recurring genomic abnormality found in nearly 100% of FL-HCC patients. Studies have revealed that DNAJB1-PRKACA fusion protein is essential to induce oncogenic transformation of hepatocytes, indicating that this fusion protein is potentially a key driver of FL-HCC. Further investigations will be needed to determine the precise tumorigenic mechanism from the cooperation of DNAJB1-PRKACA and β-catenin/WNT signaling pathway. Although most FL-HCC patients have advanced disease when initially diagnosed, treatments of curative intent are possible to most of the cases. Surgical resection or liver transplantation is the only potentially curative option in the present. FL-HCCs have generally been thought not much chemo-responsive, however, multimodality treatments can be efficient in advanced disease. FL-HCC microenvironment expresses multiple-immune suppressive molecules including B7-H3, CD8, CD11c, CD68, Foxp3, IDO, LAG3, PD-1, and PDL-1, while PDL-1 expression is significantly higher in FL-HCC tumor tissues than that of normal hepatocytes. CD8+ T cell density was observed at much higher at tumor interface zone. These finding support the use of immune checkpoint inhibitors in treating this disease. In this review, we reported two cases of FL-HCC patients who had combination treatment of immune modulators, targeted therapy and chemotherapy post resection, achieved long term disease control.
Keywords
fibrolamellar hepatocellular carcinoma, immune modulators, targeted therapy, chemotherapy
Background
FL-HCC is an uncommon liver tumor that only makes up 1% of all liver neoplasms [1]. FL-HCC is a prominent histological type of hepatocellular carcinoma (HCC) and is seen predominantly in children and adolescents without cirrhotic livers and underlying liver diseases [2-4]. Typical histological characteristics of FL-HCC include large polygonal cells with dark nucleoli, an eosinophilic cytoplasm, and thick fibrous collagen bands adjacent to the tumor cells [5]. Histological examinations, immunohistochemical studies using tumor and liver markers, imaging techniques, and medical oncology can all be utilized to diagnose FL-HCC.
FL-HCC is generally non-specific and vague in Clinical presentation. Symptoms such as fever, nausea, abdominal pain and distention, and sudden weight reduction are commonly seen in FL-HCC[4,6]. Although FL-HCC has high rates of recurrence and metastasis, the prognosis of FL-HCC is better than that of conventional HCC [5,6]. Five-year survival rates for FL-HCC patients with and without metastasis at presentation are 39% and 86%, respectively [7].
The most effective treatments for localized FL-HCC are surgical resection and liver transplant. Chemotherapy is reserved for metastatic disease [4]. However, traditional chemotherapy is generally not very effective in this disease. Thus, the therapeutic options for those with advanced disease are very limited. In this article, we report that combination of immune modulator, targeted therapy and chemotherapy are able to achieve long period disease control.
Case presentation
Case #1
A 24-year-old female was diagnosed with fibrolamellar carcinoma of the liver s/p right hepatectomy in 8/2017. 11/2017, she was found to have recurrence in the lungs and remanent liver. She was placed on 5-FU (200mg/m2/day one week on and one week off), Nivolumab (3mg/kg every 2 weeks) and interferon alpha 2b (4million units/m2 on days 1, 3, 5 and 7). 5/2018, staging study showed stable disease. She had cryoablation of the bigger hepatic lesion. 1/2019, 5-FU was changed to 1000mg capecitabine twice daily7 days on and 7 days off. 6/2019, her capecitabine was reduced to 500mg twice daily and she switched from regular interferon to peginterferon (240 mcg every 2 weeks) for neutropenia, tolerance and convenience. 10/2020, disease progressed in the lungs. Her regimen was changed to Lenvatinib, nivolumab and quercetin. Her disease has been stable since then.
Case #2
A 37-year-old female had a history of FL-HCC when she had left hepatectomy in 5/2018. The tumor was 9cm in size and surgical margins were negative. 8/2019, she had recurrence and underwent segmentectomy of segment 7. 3/2020, MRI of the abdomen revealed a 1.4cm lesion in segment 5 which was likely recurrence; 0.9 cm lesion in segment 6, which was FNH. She had cryoablation to the segment 6/caudate lesion and Y90 embolization to the segment 5 lesion. Post operatively, she started peginterferon alpha 2a 240mcg s.q q 2 weeks; nivolumab 240mg q2weeks; capecitabine 1000mg PO bid 7 days on and 7 days off. After 4 months, capecitabine was reduced to 500mg bid for neutropenia and peginterferon alpha 2a was reduced to 180mcg due to thrombocytopenia. She has remained disease-free since.
Molecular pathogenesis
The rarity of FL-HCC and the lack of both cell lines and animal models have posed various challenges for understanding its molecular pathogenesis. Unlike patients with HCC who often have loss-of-function mutations such as TP53 and PTEN, studies in Genetics suggest that patients with FL-HCC do not show those genetic lesions [8]. In 2014, Honeyman et al [9]. detailed a novel fusion gene called DNAJB1‐PRKACA found in nearly 100 percent of patients with FL-HCC. A full genome sequencing showed that the fusion stemmed from a deletion of 400-kilobase on chromosome 19. The fusion chimera integrates a component of heat shock protein DNAJB1 with protein kinase A (PKA) 's catalytic domain while displaying full PKA activity retention (Figure 1). Prominently, this fusion gene has been recognized in approximately 80-100% of patients documented in numerous studies and is the sole known genetic aberration recurring in FL-HCC; meanwhile, there has been no identification of it in any other cancer [10-13]. It is 100% specific for FL-HCC in the setting of primary hepatocellular neoplasms [5].
Evidence has shown that the presence of the DNAJB1-PRKACA fusion protein is enough to promote the oncogenic transformation of hepatocytes. Xu et al. showed that the chimeric gene overexpression especially intensifies colony formation and proliferation in transformed liver cell lines, suggesting the gene’s oncogenic role [11]. In a study where CRISPR/Cas9 technique was used to engineer DNAJB1–PRKACA fusion protein in young adult mouse livers, the development of tumors shared many characteristics with FL-HCC in humans in 12 among the 15 mice [14]. Similar results were observed in another research group’s mice models with FL-HCC lesions induced by an akin technique [15]. Moreover, this DNAJB1-PRKACA chimera was sufficient at driving tumorigenesis in vivo in adult zebrafish models [16]. In addition, a patient-derived xenograft tumor model confirmed that the DNAJB1–PRKACA chimera was a key inducement of FL-HCC and remarkably rich in liver cancer stem cells [17].
The overexpression of DNAJB1-PRKACA in FL-HCC makes its number of transcripts 10-fold greater than the WT PRKACA transcript. When the fusion protein overexpresses, it raises cAMP-dependent PKA activity to a higher level than that in normal livers [18]. However, although the restoration of WT PRKACA induced few differences in liver cells' histology, it did not prompt the development of FL-HCC in a CRISPR/Cas9–mediated mouse model, indicating that FL-HCC does not solely stem from elevated PRKACA activities [15]. A variety of candidate drivers were tested in this model, but remarkably, only one activated type of β-catenin synergized with the DNAJB1–PRKACA chimera and significantly enhanced tumorigenesis [19]. Interestingly, phospho-β-catenin, an indicator of the hyperactivate status in the Wnt pathway, has appeared in patients with FL-HCC [20]. It was hypothesized that β-catenin might defend cells from DNAJB1–PRKACA-inflicted oxidative stress, then progress to FL-HCC [21]. Further studies will be needed to determine the precise tumorigenic mechanism from the cooperation of the β-catenin/WNT signaling pathway and the DNAJB1-PRKACA chimera.
1. Current Therapies for FL-HCC
Surgical resection is a potentially curative surgical treatment option for the resectable FL-HCC. The surgical resection rate in FL-HCC is approximately 60–70% and a median tumor size of 10.5 centimeters [22]. Resection usually is the first option for young, physically fit patients and the ones without cirrhosis, even for patients with large size tumors. In patients in the advanced stage with a preserved liver function, resection is still the optimal choice for them to control the disease [23,24]. More than 70 % of the patients undergo an extended hepatectomy or hemihepatectomy, while rest of patients require a partial or minor hepatectomy [23,25]. 30% to 60% of tumor resected patients encounter lymph node involvement. Due to the fact that positive lymph node is an unsatisfactory prognostic factor, complete periportal lymphadenectomy is regularly utilized in radical resections for FL-HCC patients. Approximately 26-76 % of patients survive over five years. The medium survival ranges from 32 to 174 months [26-29] (Table 1).
Sample size |
Tumor size range (cm) |
Median tumor size (cm) |
R0/R1 /R2 Resection (%)1 |
Systemic treatment (%) |
Recurrence rate (%) |
5 yr Survival (%) |
Overall survival (month) |
Tumor free survival (month) |
References |
28 |
3-17 |
9 |
R0 (82%), R1 (18%) |
0 |
61 |
76 |
112 |
N/A |
Stipa, et al.[29] |
41 |
3-25 |
13 |
R0 (83%), R1 (10%), R2 (7%) |
39 |
65.8 |
66.2 |
126.93± 16.34 |
86.93 ± 15.24 |
Pinna, et al.[26] |
20 |
6-19 |
12.8 |
R1 (21.4%) |
0 |
66.7 |
28 |
36 |
N/A |
Herman, et al. [36] |
60 |
N/A |
N/A |
N/A |
73 |
83.7 |
N/A |
60-110.5 |
13.9 |
Kaseb, et al.[23] |
73 |
1.1-30 |
10 |
R0 (51%), R1 (11%), R2 (5.5%) |
31.5 |
77 |
N/A |
404 |
N/A |
Ang, et al.[25] |
21 |
3.5-18 |
10.5 |
R0(80.9%), R1 (9.5%), R2 (9.5%) |
52 |
57 |
51.6 |
5.3-113.5 |
15.6 |
Darcy, et al. [37] |
Table 1. Summary of Surgery Outcome in Resectable FL-HCC patients
Note: 1. A microscopically margin-negative resection is indicated by the R0 resection, where no microscopic or gross tumor survives in the primary tumor bed. The removal of all macroscopic disease, but with the positive microscopic margins for tumors is indicated by the R1 resection. Gross residual macroscopic disease with un-resectable gross residual tumor (primary tumor, macroscopic margin involvement, and regional nodes) is indicated by the R2 resection.
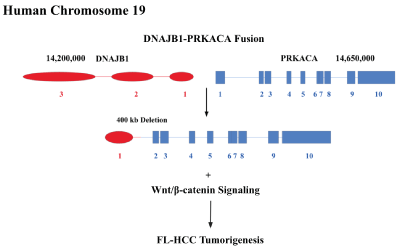
Figure 1. The fusion chimeric protein couples a segment of DNAJB1 with PRKACA in human chromosome 19
Multiple tumors, positive lymph nodes, and vascular invasion are negative prognostic factors to predict worse survival after resection [30,31]. On the other hand, absence of lymph node metastasis or vascular invasion, normal liver functions, free surgical margins, larger viability of surgical resection, and appropriate lymph node dissection predict a prolonged survival [7,32]. Nevertheless, significant number of patients after curative resection still suffer recurrent disease. The median recurrence-free survival time are 20 to 48 months [1]. The common recurrence sites include lung, peritoneal surfaces, regional and non-regional nodes [7,33]. However, metastasectomy is a viable option in this patient population. Aggressive surgical resection could potentially overall improve median survival up to 10 years compared to 3 years without surgery [34]. Because of the high recurrence rate, frequent postoperative surveillance is needed. Elevated vitamin B12 binding protein was reported as a biomarker of FL-HCC due to production or modification of the binding protein transcobalamin I by the tumor [35]. Serum vitamin B12-binding protein levels and CT scans should be performed every three to six months in the first two to three years of follow-up postoperatively. It was controversial whether adjuvant chemotherapy benefit patient survival and tumor-free interval [23,26].
2. Unresectable FL-HCC
Approximately 25% patients have un-resectable disease at the time of diagnosis due to extensive nodal spread, multifocal metastases, and/or major vessel involvement [25]. Therapies like as liver transplantation (for hepatic disease only), radiotherapy and systemic chemotherapy are usually considered.
Unlike traditional HCC, orthotopic liver transplant is often considered for the eligible patients. While the transplant data for FL-HCC patients are rather scant, transplant in patients with advanced disease is still a viable option [5]. The patient’s outcomes who underwent liver transplantation are often based on small case series. The results are rather promising [36]. Pinna et al. had indicated the outcome of 13 FL-HCC patients who underwent a liver transplant showing a 3-year overall survival rate of 45% and a 5-year survival rate of 36% [37]. Another group reported that FL-HCC patients’ overall survival at 1, 3, and 5 years was 96%, 80%, and 48% [38]. In general, liver transplantation has 29 to 55% of overall 5-year survival rate in the advanced stage of FL-HCC patients. Survival rates of transplantation are inferior to that of resection though, possibly due to more advanced stages of the disease. However, liver transplantation still remains a crucial option for those patients and it is usually considered in selected cases of unresectable FL-HCC [4].
FL-HCC may be moderately radiosensitive [35,39]. Selective internal radiotherapy (SIRT) with Yttrium-90 (Y90) is a therapy that is intra-arterial-directed for hepatic malignancy. Down stage from unresectable to resectable has been reported by Mafeld et al. [40] using Y90. Such an approach opens an opportunity for those with advanced disease. Because of the rarity of the disease, data from Y90 liver-directed therapy in FL-HCC are limited. This therapeutic modality warrants further investigation.
Systemic therapy has been evolving rapidly in conventional HCC. Conventional HCC is a vascular rich tumor. Hence, drug development has been largely focused on anti-angiogenesis pathways. Tyrosine kinase inhibitors (sorafenib, Lenvatinib and cabozantinib) and ramucirumab are all targeting vascular endothelial growth factor receptor pathways. The introduction of immune checkpoint inhibitors changed the landscaping of HCC therapy significantly. Currently, there are three immune checkpoint inhibitors approved by the US FDA. These are nivolumab, atezolizumab and ipilimumab. Although physicians generally treat FL-HCC just like conventional HCC, the outcome is poor. Early reports indicated that 5-fluorouracil (5-FU) and interferon alpha might be useful in this disease [41]. In this study, 8 FL-HCC patients treated with the regimen achieved 62.5% response with one complete response. Due to low incidence of disease, no large study has been conducted. At this point, the need for systemic therapy in FL-HCC is largely unmet.
The immune microenvironment is poorly understood. Like conventional HCC, recent studies suggest that FL-HCC microenvironment expresses multiple-immunosuppressive molecules, B7-H3, CD8, CD11c, CD68, Foxp3, IDO, LAG3, PD-1, and PDL-1 were evaluated using IHC staining on FL-HCC clinical specimens. PDL-1 expression is significantly higher in tumor tissues than that of normal hepatocytes (69% vs 17% respectively). CD8+ T cell density was noticed to be much higher at the tumor interface zone. These findings support the use of immune checkpoint inhibitors in treating this disease. It has been speculated that 5-FU could potential regulate tumor immune suppressive environment by changing CD4+ T cell and T-regulatory cell ratio, downing regulating Foxp3 and TIM-3 expression. Hence, the combination of 5-FU and immune checkpoint inhibitor, nivolumab, could potentially result in synergistic antitumor effect.
Conclusion
FL-HCC is a rare disease. The two cases described in this manuscript provide an insight of the disease clinical course and tumor biology. Our anecdotal experience showed that combination of 5-FU (or capecitabine), interferon alpha and nivolumab is a viable option in advanced FL-HCC. The regimen offers prolonged stable disease in the first case. In the second case, this regimen also demonstrated the efficacy in treating microscopic disease in the adjuvant setting. The regimen potentially can be used as standard adjuvant therapy in high-risk patients with curative surgery or liver-directed therapy as our second case demonstrated. This combination warrants further evaluation in a well-designed larger clinical study. Due to the rarity of the disease, such study requires international collaborations.
Funding
None
Conflicts of interest
None
Availability of data and material (data transparency)
We declare transparency of data
Code availability (software application or custom code)
Not applicable
Authors' contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by A Wang and L Wang. They are co-first author contributing equally and YX Jiang revises and finalizes the manuscript. All authors read and approved the final manuscript.
Acknowledgment
This is the only valid version of the paper.
References
- Eggert T, McGlynn KA, Duffy A, Manns MP, Greten TF (2013) Fibrolamellar hepatocellular carcinoma in the USA, 2000-2010: A detailed report on frequency, treatment and outcome based on the Surveillance, Epidemiology, and End Results database. United European Gastroenterol J 1: 351-357. [Crossref]
- Ramakrishna G, Rastogi A, Trehanpati N, Sen B, Khosla R (2013) From cirrhosis to hepatocellular carcinoma: new molecular insights on inflammation and cellular senescence. Liver Cancer 2: 367-383.
- Tanaka H, Hijioka S, Iwaya H, Mizuno N, Kuwahara T, et al. (2018) Fibrolamellar Hepatocellular Carcinoma with Multiple Lung Metastases Treated with Multidisciplinary Therapy. Intern Med 57: 3537-3543.
- Chaudhari VA, Khobragade K, Bhandare M, Shrikhande SV (2018) Management of fibrolamellar hepatocellular carcinoma. Chin Clin Oncol 7: 51.
- Lin CC, Yang HM (2018) Fibrolamellar Carcinoma: A Concise Review. Arch Pathol Lab Med 142: 1141-1145.
- Ibrahimi S, Mroué AA, Francois E, Jagodzinski R (2017) Jaundice in a pregnant woman. Acta Gastroenterol Belg 80: 422-424.
- Kakar S, Burgart LJ, Batts KP, Garcia J, Jain D (2005) Clinicopathologic features and survival in fibrolamellar carcinoma: comparison with conventional hepatocellular carcinoma with and without cirrhosis. Mod Pathol 18: 1417-1423. [Crossref]
- Ward SC, Waxman S (2011) Fibrolamellar carcinoma: a review with focus on genetics and comparison to other malignant primary liver tumors. Semin Liver Dis 31: 61-70.
- Honeyman JN, Simon EP, Robine N, Chiaroni-Clarke R, Darcy DG, et al. (2014) Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 343: 1010-1014.
- Graham RP, Jin L, Knutson DL, Kloft-Nelson SM, Greipp PT, et al. (2015) DNAJB1-PRKACA is specific for fibrolamellar carcinoma. Mod Pathol 28: 822-829.
- Xu L, Hazard FK, Zmoos AF, Jahchan N, Chaib H, et al. (2015) Genomic analysis of fibrolamellar hepatocellular carcinoma. Hum Mol Genet 24: 50-63.
- Darcy DG, Chiaroni-Clarke R, Murphy JM, Honeyman JN, Bhanot U, et al. (2015) The genomic landscape of fibrolamellar hepatocellular carcinoma: whole genome sequencing of ten patients. Oncotarget 6: 755-70.
- Cornella H, Alsinet C, Sayols S, Zhang Z, Hao K, et al. (2015) Unique genomic profile of fibrolamellar hepatocellular carcinoma. Gastroenterology. 148: 806-818.
- Engelholm LH, Riaz A, Serra D, Dagnæs-Hansen F, Johansen JV, et al. (2017) CRISPR/Cas9 Engineering of Adult Mouse Liver Demonstrates That the Dnajb1-Prkaca Gene Fusion Is Sufficient to Induce Tumors Resembling Fibrolamellar Hepatocellular Carcinoma. Gastroenterology 153: 1662-1673.
- Kastenhuber ER, Lalazar G, Houlihan SL, Tschaharganeh DF, Baslan T, et al. (2017) Fusion kinase interacts with β-catenin and the liver regenerative response to drive fibrolamellar hepatocellular carcinoma. Proc Natl Acad Sci U S A 114: 13076-13084. [Crossref]
- de Oliveira S, Houseright RA, Korte BG, Huttenlocher A (2020) DnaJ-PKAc fusion induces liver inflammation in a zebrafish model of fibrolamellar carcinoma. Dis Model Mech 13.
- Oikawa T, Wauthier E, Dinh TA, Selitsky SR, Reyna-Neyra A, et al. (2015) Model of fibrolamellar hepatocellular carcinomas reveals striking enrichment in cancer stem cells. Nat Commun. 6: 8070.
- Riggle KM, Riehle KJ, Kenerson HL, Turnham R, Homma MK, et al. (2016) Enhanced cAMP-stimulated protein kinase A activity in human fibrolamellar hepatocellular carcinoma. Pediatr Res 80: 110-118.
- Graham RP, Lackner C, Terracciano L, González-Cantú Y, Maleszewski JJ, et al. (2018) Fibrolamellar carcinoma in the Carney complex: PRKAR1A loss instead of the classic DNAJB1-PRKACA fusion. Hepatology 68: 1441-1447. [Crossref]
- Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP (2009) Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology 49: 821-831.
- Tao GZ, Lehwald N, Jang KY, Baek J, Xu B, et al. (2013) Wnt/β-catenin signaling protects mouse liver against oxidative stress-induced apoptosis through the inhibition of forkhead transcription factor FoxO3. J Biol Chem 288: 17214-17224.
- Mayo SC, Mavros MN, Nathan H, Cosgrove D, Herman JM, et al. (2013) Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma: a national perspective. J Am Coll Surg 218: 196-205.
- Kaseb AO, Shama M, Sahin IH, Nooka A, Hassabo HM, et al. (2013) Prognostic indicators and treatment outcome in 94 cases of fibrolamellar hepatocellular carcinoma. Oncology 85: 197-203. [Crossref]
- Meriggi F, Forni E. [Surgical therapy of hepatic fibrolamellar carcinoma]. Ann Ital Chir 78: 53-58.
- Ang CS, Kelley RK, Choti MA, Cosgrove DP, Chou JF, et al. (2013) Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest Cancer Res 6: 3-9.
- Pinna AD, Iwatsuki S, Lee RG, Todo S, Madariaga JR, et al. (1997) Treatment of fibrolamellar hepatoma with subtotal hepatectomy or transplantation. Hepatology 26: 877-883. [Crossref]
- El-Gazzaz G, Wong W, El-Hadary MK, Gunson BK, Mirza DF, et al. (2000) Outcome of liver resection and transplantation for fibrolamellar hepatocellular carcinoma. Transpl Int 13: S406-S409.
- Groeschl RT, Miura JT, Wong RK, Bloomston M, Lidsky ML, et al. (2014) Multi-institutional analysis of recurrence and survival after hepatectomy for fibrolamellar carcinoma. J Surg Oncol 110: 412-415.
- Stipa F, Yoon SS, Liau KH, Fong Y, Jarnagin WR, et al. (2006) Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer 106: 1331-1338.
- Ringe B, Wittekind C, Weimann A, Tusch G, Pichlmayr R (1992) Results of hepatic resection and transplantation for fibrolamellar carcinoma. Surg Gynecol Obstet 175: 299-305. [Crossref]
- Craig JR, Peters RL, Edmondson HA, Omata M (1980) Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer 46: 372-379.
- Liu S, Chan KW, Wang B, Qiao L (2009) Fibrolamellar hepatocellular carcinoma. Am J Gastroenterol 104: 2617-2624.
- Wojcicki M, Lubikowski J, Post M, Chmurowicz T, Wiechowska-Kozlowska A, et al. (2012) Aggressive surgical management of recurrent lymph node and pancreatic head metastases of resected fibrolamellar hepatocellular carcinoma: a case report. JOP 13: 529-532.
- Yamashita S, Vauthey JN, Kaseb AO, Aloia TA, Conrad C, et al. (2016) Prognosis of Fibrolamellar Carcinoma Compared to Non-cirrhotic Conventional Hepatocellular Carcinoma. J Gastrointest Surg 20: 1725-1731. [Crossref]
- Maniaci V, Davidson BR, Rolles K, Dhillon AP, Hackshaw A, et al. (2009) Fibrolamellar hepatocellular carcinoma: prolonged survival with multimodality therapy. Eur J Surg Oncol 35: 617-621.
- Herman P, Chagas AL, Perini MV, Coelho FF, Fonseca GM, et al. (2014) Surgical treatment of fibrolamellar hepatocellular carcinoma: an underestimated malignant tumor? Hepatobiliary Pancreat Dis Int 13: 618-621. [Crossref]
- Darcy DG, Malek MM, Kobos R, Klimstra DS, DeMatteo R (2015) Prognostic factors in fibrolamellar hepatocellular carcinoma in young people. J Pediatr Surg 50: 153-156.
- Atienza LG, Berger J, Mei X, Shah MB, Daily MF, et al. (2017) Liver transplantation for fibrolamellar hepatocellular carcinoma: A national perspective. J Surg Oncol 115: 319-323.
- Lafaro KJ, Pawlik TM (2015) Fibrolamellar hepatocellular carcinoma: current clinical perspectives. J Hepatocell Carcinoma 2: 151-157.
- Mafeld S, French J, Tiniakos D, Haugk B, Manas D (2018) Fibrolamellar Hepatocellular Carcinoma: Treatment with Yttrium-90 and Subsequent Surgical Resection. Cardiovasc Intervent Radiol 41: 816-820.
- Patt YZ, Hassan MM, Lozano RD, Brown TD, Vauthey JN, et al. (2003) Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon Alfa-2b for treatment of hepatocellular carcinoma. J Clin Oncol 21: 421-427. [Crossref]

