Chronic thromboembolic pulmonary hypertension (CTEPH) is a complication of pulmonary embolism. A deep vein thrombosis diagnosis due to uterine myoma compressing the inferior vena cava and an accompanying pulmonary artery thrombotic embolism was treated with hysterectomy and warfarin, which caused bleeding. While initially overlooked, a CTEPH diagnosis was reached.
Chronic thromboembolic pulmonary hypertension, Pulmonary embolism, Deep vein thrombosis
Pulmonary embolism is a critical disease usually occurring due to deep vein thrombosis. In Korea, the annual incidence of pulmonary embolism was 13.2 cases per 100,000 individuals in 2009 and increased to 16.6 cases per 100,000 in 2013 [1]. First-line therapy for pulmonary thromboembolism is anticoagulant administration and thrombolysis. Second-line therapy is pulmonary thromboembolectomy. However, without adequate treatment, patients have difficulty in regaining normal pulmonary circulatory function; chronic thromboembolic pulmonary hypertension (CTEPH), one of the most common causes of precapillary pulmonary hypertension, can occur following pulmonary embolism with an incidence rate of 3 to 4% [2,3]. Here, we report a case of deep vein thrombosis due to uterine myoma, slowly developed into CTEPH throughout seventeen years.
History of Presentation
A 67-year-old woman presented to the emergency department in July of 2018 due to a change in mental status. Brain computed tomography (CT) revealed a subdural hemorrhage in the left fronto-parietal area and intracerebral hemorrhage in the left occipital lobe. The patient’s INR was measured at 4.64. She underwent emergency craniectomy with hematoma removal and warfarin discontinuation. After hematoma removal, during preparation for further neurosurgery, her echocardiography indicated pulmonary thromboembolism with a large thrombus in right pulmonary artery. The systolic pulmonary artery pressure was measured at 65 mmHg. Chest CT showed an increasingly large filling defect in the right main and lobar pulmonary arteries, and a newly developed filling defect in the left inferior pulmonary artery, indicating pulmonary artery intimal sarcoma combined with pulmonary thromboembolism.
Past Medical History
The patient’s initial encounter occurred during a visit to the hospital with the chief complaint of a one-year history of dyspnea that had worsened over the previous month (February 19th, 2002). Chest CT was ordered and revealed pulmonary artery thrombosis in the right main and lower, and left upper and lower branches. Ultrasonography detected a large thrombosis in the left common and external iliac veins. For further evaluation, an abdominal CT was performed, and showed a 12x10 cm uterine mass, likely a myoma (Figure 1). The mass was suspected to be the main cause of the patient’s deep vein thrombosis (DVT) due to compression of the inferior vena cava. Mass removal was needed; however, due to the patient’s instability, surgery was delayed. She was discharged with a prescribed 6 mg/day dose of warfarin. On March 3rd, 2003, a follow-up CT showed near-complete resolution of the thromboembolism in the right pulmonary artery. With this CT-proven improvement of pulmonary thromboembolism, total abdominal hysterectomy was performed and inferior vena cava (IVC) filter insertion was performed in order to prevent DVT migration. She was discharged with a prescribed 4 mg/day dose of warfarin. In 2004, her follow-up CT showed no gross evidence of pulmonary thromboembolism compared to the previous CT. (Figure 2A). On August 3rd, 2007, the patient was presented to the emergency room with the chief complaint of cough and hemoptysis, and chest radiography and CT showed signs of pulmonary hemorrhage in the right middle lung. Warfarin was ceased immediately and her symptoms and chest X-ray improved. At that time, her INR was 1.58. She underwent regular chest CT follow-up to monitor for pulmonary thromboembolism aggravation. A chest CT in 2008 showed chronic pulmonary thromboembolism in the right pulmonary artery, and the pulmonary trunk was measured at 3.6 cm, indicating possible pulmonary hypertension (Figure 2B). Follow-up images in 2012 and 2014 showed a gradually progressing filling defect in the pulmonary artery and pulmonary trunk (Figure 2C). During which, warfarin use was continued; however, unfortunately, no other treatment was provided for pulmonary hypertension (Figure 2D).
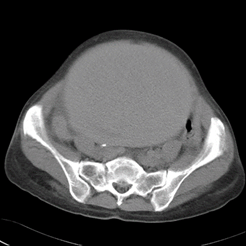
Figure 1. Abdominal computed tomography showing 12x10 cm sized uterine mass that caused deep vein thrombosis
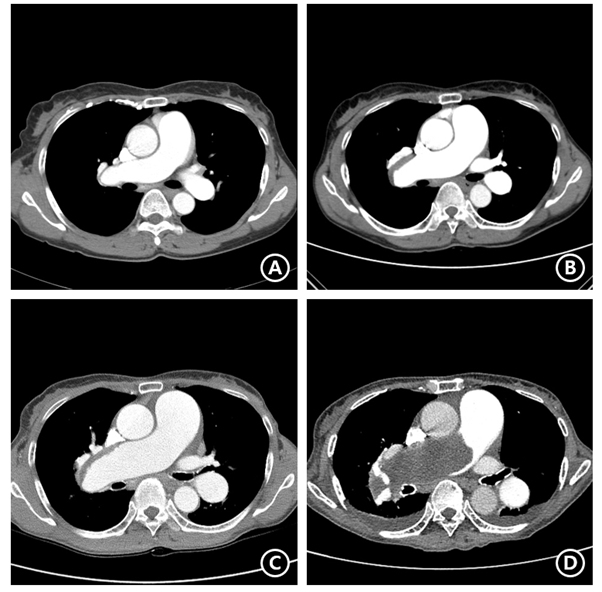
Figure 2. Chronological computed tomography (CT) findings; (A) First follow-up CT on 2004/01/12 after initiation of warfarinization to treat previously diagnosed pulmonary thromboembolism (PTE), which showed no gross evidence of PTE. (B) CT taken on 2008/01/16, when the patient visited emergency room with hemoptysis and was diagnosed with warfarin-induced pulmonary hemorrhage. Her follow-up CT showing reoccurrence of PTE in the right pulmonary artery and indicated pulmonary hypertension with pulmonary trunk’s diameter of 3.6 cm. (C) CT taken for a regular check-up on 2014/03/28 showing more prominent filling defect in the right main and lobar pulmonary arteries and aggravated dilatation of the pulmonary artery. (D) CT taken on 2018/08/20, when the patient presented with mental change due to intracranial hemorrhage, international normalized ratio was 4.64. Her CT showed increasing extent of huge filling defect in right main and lobar pulmonary artery, indicating pulmonary artery intimal sarcoma combined pulmonary thromboembolism
Investigations
In 2016, the patient was presented to the emergency room due to sudden abdominal pain. Abdominal CT and abdominal vessel sonography showed a large hematoma in the right rectus abdominis muscle with active bleeding. At that time, the patient’s INR was 3.12. After discontinuing warfarin due to the hematoma, her symptoms improved and she was discharged with a lower dose of warfarin (3 mg/day).
Management
On August 23rd, right pulmonary thromboembolectomy was performed; however, due to right ventricular dysfunction, veno-arterial extracorporeal membrane oxygenation (ECMO) was applied.
Pulmonary arterial hypertension medication including sildenafil and macitentan was administered. After right pulmonary thromboembolectomy, hypotension and tachycardia continued. Echography showed right ventricular systolic dysfunction. She had right ventricular failure, and a decision was made to apply veno-arterial (V-A) extracorporeal membrane oxygenation. After applying V-A ECMO for one week, ECMO weaning was successful; however, veno-venous (V-V) ECMO was reapplied due to respiratory failure combined with sepsis. V-V ECMO support was maintained for 78 days, and the patient was undergoing weaning. However, she succumbed to sepsis due to repetitive uncontrollable infections caused by various bacteria, such as Pseudomonas and carbapenem-resistant Acinetobacter baumannii.
In this case, the patient little by little deteriorated into chronic thromboembolic pulmonary hypertension without proper treatment. Her regular follow-up examinations showed aggravation of pulmonary thromboembolism and an increase in pulmonary artery size and pressure with time. Furthermore, several events due to warfarinization complications were reported, strongly suggesting that more active evaluation and management should have been considered. It is unfortunate that even though her symptoms and exam results consistently showed the need for more active treatment, including pulmonary arterial hypertension medication or pulmonary endarterectomy, no such interventions could be performed. Had more active measures been taken, the prognosis of this patient could have been different. The true incidence of CTEPH is difficult to determine because most patients with acute pulmonary embolism (PE) do not undergo routine follow-up assessment of pulmonary arterial pressure or repeat imaging to assess the degree of clot burden that remains [4]. In this case, however, follow-up CT results showed gradual deterioration, which finally obstructed an entire side of the pulmonary artery. Pulmonary endarterectomy (PEA) is the treatment of choice for CTEPH, and in properly selected patients usually provides significant improvements in survival, almost 99% [5,6]. Patients who are deemed inoperable by a center experienced in CTEPH and PEA may benefit from medical therapy with pulmonary vasodilator medications or pulmonary balloon angioplasty [4].
If prompt and proper treatment is provided when a patient suffers from acute pulmonary embolism, the patient’s condition can be restored. However, some patients develop CTEPH, which is usually detected late because it is rarely suspected. Therefore, we emphasize the importance of close observation in patients diagnosed with pulmonary thromboembolism, especially with respect to changes in the PE and pulmonary artery pressure. Prescribing anticoagulants is a crucial part of treating PE, but further study and measures are necessary in order to prevent deterioration into chronic thromboembolic pulmonary hypertension.
The authors have no potential conflicts of interest to disclose.
All authors contributed to the conception and interpretation of data, drafting of the manuscript, and revision and final approval of the manuscript.
Hyun Jin Kim: https://orcid.org/0000-0002-6566-310X
Hong Ju Shin: https://orcid.org/0000-0002-0731-3523
- Hong J, Lee JH, Yhim HY (2018) Incidence of venous thromboembolism in Korea from 2009 to 2013. PLoS One 13: e0191897. [Crossref]
- Pengo V, Lensing AW, Prins MH (2004) Thromboembolic pulmonary hypertension study group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 350: 2257-2264. [Crossref]
- Tapson VF, Platt DM, Xia F (2016) Monitoring for pulmonary hypertension following pulmonary embolism: the INFORM study. Am J Med 129: 978-985. [Crossref]
- Mullin CJ, Klinger JR (2018) Chronic thromboembolic pulmonary hypertension. Heart Fail Clin 14: 339-3351.
- Jamieson SW, Kapelanski DP, Sakakibara N (2003) Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg 76: 1457-1462. [Crossref]
- Madani MM, Auger WR, Pretorius V (2012) Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg 94: 97-103. [Crossref]


