Abstract
Substantial medical evidence supports the routine use of cardiac resynchronization therapy (CRT) for treating patients with chronic systolic heart failure (HF) and ventricular dyssynchrony. Through bi-ventricular pacing, CRT is able to synchronize the global ventricular depolarization and to improve contractile function and mitral regurgitation. It is the onlyHF therapy that simultaneously improves cardiac function and functional capacity as well as reduces hospitalization and prolongs survival. However, approximately a third of patients eligible for CRT fail to benefit from treatment possibly due to selection based only on New York Heart association functional class, LVEF, and QRS duration and morphology. Thus, careful patient selection, appropriate left ventricular lead implantation and optimal device programming may improve treatment benefits. This paper reviews published evidence on the CRT including its mechanism of action, indications, therapeutic benefits and challenges as well as identifies areas that would benefit from future research.
Key words
Cardiac resynchronization therapy (CRT), atrial-synchronized biventricular pacing, multisite ventricular pacing
Introduction
Heart failure (HF) was declared an emerging epidemic in 1997 [1]. Since then it has become a major clinical and public health problem affecting approximately 20 million people worldwide, and by 2030, projected to increase in prevalence by 25% [2]. Despite progressive advances in pharmacological and device therapies, unacceptably high rates of morbidity and mortality persist [3]. Ventricular conduction disturbance, most commonly the left bundle branch block (LBBB), affects an estimated 30% of HF patients and commonly results in dyssynchrony of ventricular contraction associated with increased risk of hospitalization and death [4]. Therapeutic cardiac stimulation, which targets this HF patient population, has been in use in clinical practice for the past six decades [5]. The original therapeutic use was to maintain adequate heart rates in symptomatic bradycardia patients. The initial single ventricle stimulation was effective but suboptimal from a physiologic standpoint. Its therapeutic inadequacy became apparent in patients with sustained bradyarrhythmia who require frequent ventricular stimulation [6].
At present, cardiac resynchronization therapy (CRT), developed from the concept of therapeutic cardiac stimulation, involves bi-ventricular pacing with or without atrial pacing. It has grown into an established non-pharmacological therapy for HF patients with reduced left ventricular (LV) ejection fraction (LVEF), and wide QRS complex [7,8]. Pacing using CRT has revolutionized care for patients with advanced HF whose only previous option was heart transplantation, and now a realistic option for selected patients with mild HF [7,8]. At present, CRT is the only HF therapy that simultaneously improves both cardiac function and functional capacity, as well as reduces hospitalization and prolongs survival [4]. However, there is wide variations in CRT treatment benefits ranging from complete normalization of ventricular volumes and LVEF to a complete lack of benefit. This variation has motivated continuous research to understand appropriate use of CRT – where treatment benefits should outweigh adverse treatment consequences as well as consequences of living with the device. This review paper highlights the current indications, pathobiology, treatment benefits and adverse outcomes associated with the CRT treatment.
Definition of CRT, ventricular dyssynchrony
Cardiac resynchronization therapy (CRT), at times referred to as atrial-synchronized biventricular pacing or multisite ventricular pacing is one of the most common cardiovascular implanted electronic device therapy. CRT involves simultaneous pacing of the right ventricle (RV) and the left ventricle (LV) in patients with biventricular HF. In addition to conventional RV endocardial lead with or without right atrial (RA) lead, CRT involves a third lead for LV pacing inserted in the coronary sinus [9]. The primary indication for CRT in HF patients is the treatment of ventricular dyssynchrony (VD), defined as a delay in ventricular electromechanical conduction identified by multiple imaging techniques including echocardiography [10]. CRT is effective in HF patients with VD and a wide QRS. A narrow QRS describes QRS duration of < 120 ms while a wide QRS describes ≥120 ms sometimes accompanied by LBBB, right bundle branch block (RBBB) or non-specific intraventricular conduction delay morphology [10]. Prolonged QRS complex is common in ~30% of patients with advance HF with varying degrees of ventricular electromechanical delay or dyssynchrony [10].
Pathobiology of CRT: ventricular dyssynchrony
The primary treatment goal of CRT is to reduce or eliminate ventricular dyssynchrony using bi-ventricular pacing to synchronize the global ventricular depolarization and achieve improved contractility and mitral regurgitation (MR) [11]. Thus, the knowledge of ventricular dyssynchrony is essential to understand clinical use of CRT in the treatment of eligible HF patients.
Types of ventricular dyssynchrony
Three chief presentations of ventricular dyssynchrony are: (a) atrioventricular [AV] dyssynchrony occurring between atrial and ventricular contraction; (b) interventricular [VV] dyssynchrony occurring between the left and right ventricular contractions; and (c) intraventricular dyssynchrony due to disruption in the normal activation and contraction sequence of LV wall segment [12].
Atrioventricular dyssynchrony: Atrioventricular (AV) dyssynchrony describes a delay between atrial and ventricular contraction. The delay can result in mitral valve incompetence with occurrence of late diastolic regurgitation, shortened ventricular filling time limiting net diastolic stroke volume and superimposition of atrial contraction on the early passive filling reducing LV filling [13,14]. Adjusting CRT settings can optimize AV delay and echocardiography can show hemodynamic optimization by measuring LV outflow tract and mitral inflow velocity profiles [12].
Interventricular dyssynchrony: Dyssynchronous electrical activation of the RV and LV ventricles may occur because of decreased ventricular mechanical performance due to factors such as the presence of LBBB in which the RV contraction precedes the LV contraction, different local contraction patterns, abnormal distribution of the mechanical work in the LV and deficiencies in regional perfusion [12]. Delayed onset of the LV contraction and relaxation produces VV dyssynchrony leading to abnormal (paradoxical) septal motion and its contribution to LV ejection. Earlier onset of the RV contraction superimposes the RV ejection on the LV end-diastolic. The higher pressure within the RV reverses the trans-septal pressure gradient and, thus, displaces the septum into the LV [15].
Intraventricular dyssynchrony: Intraventricular dyssynchrony describes alterations in the normal ventricular activation sequence. Since Coordinated LV contraction depends on normal ventricular activation sequence, premature activation of a portion of the LV generates regions of both early and delayed contraction leading to dis-coordinated contraction of the LV segments [16] and poor performance because early and late shortening results in wasted work [17]. Early contraction occurs when pressure is low and does not contribute to LV ejection of blood. The late contraction occurs at a higher stress causing a paradoxical stretch of the early contracting segments resulting in decreased systolic performance, increased end-systolic volume and wall stress, delayed relaxation, declined mechanical efficiency and a greater metabolic cost of LV contraction [18,19]. In addition, MR may worsen partly because of LV remodelling and pre-systolic regurgitation that may occur with VD and delayed contraction of the papillary muscle root attachments [12].
Pathophysiology of ventricular dyssynchrony
The pathophysiology of VD is complex and multifaceted [9]. Patients with LV systolic dysfunction (LVSD) and LV dilation with or without clinical signs and symptoms of HF frequently exhibit ventricular conduction delays, which mostly manifest as LBBB [20]. LBBB may lead to delayed depolarization and contraction of the lateral LV free wall but the inter-ventricular septum shows normal early contraction resulting into paradoxical septum motion (VD). This abnormal activation sequence due to spontaneous LBBB or RV pacing produces changes in regional ventricular loading conditions, and possibly lead to redistribution of myocardial blood flow and create a regional non-uniform myocardial metabolism [21,22].
The resultant effect of VD on myocardial circulation and metabolism may contribute to disease progression in LVSD patients [23]. Experimental studies on HF induced by rapid ventricular pacing reveal regional differences in the extent of ventricular hypertrophy with apicobasal and septolateral-oriented gradient [24]. Experimentally induced LBBB demonstrates a large effect on the expression of regional stress kinase and calcium-handling proteins [25]. Preliminary evidence associates the endocardium of the late-activated region with significantly increased expression of stress kinase (p38-MAPK) and significantly decreased expression of phospholamban, and in the region of early activation, decreased sarcoendoplasmic reticulum Ca 2+ ATPase [24].
Prolonged AV interval may also lead to delayed systolic contraction affecting early diastolic filling [26]. During atrial relaxation, atrial pressure falls. Delays in ventricular contraction causes LV diastolic pressures to rise exceeding atrial pressure, which results in diastolic MR. The loss of ventricular pre-load then leads to depressed LV contractile function due to the loss of the Starling mechanism. Both inter- and intra-ventricular conduction delays lead to asynchronous contraction of the LV wall regions, which impairs cardiac efficiency and decreases stroke volume and systolic blood pressure. Poor coordination of papillary muscle function may precipitate or aggravate functional systolic MR. Impaired performance promotes adverse LV remodelling [27].
However, there is limited understanding on the role of dis-coordinated activation sequence in the alterations in regional loading conditions, myocardial circulation and metabolism, and gene and protein expression. Nevertheless, it is likely the consequences of ventricular dyssynchrony result in re-arrangement of contractile and non-contractile cellular elements and possibly the extracellular matrix in the heart to stimulate ventricular remodelling [9]. Evidence from the David Trial [23] comparing RV pacing with either no pacing or atrial pacing in LVSD patients supports the concept that VD represents an important pathophysiological mechanism leading to depressed ventricular function, ventricular dilatation and ultimately HF. The DAVID trial associated RV pacing with HF progression including increased incidence of worsening HF [23].
Working mechanisms of CRT
The working mechanism of CRT is complex and partially understood. Primarily, CRT corrects electric substrate originating from conduction disorders but it exerts its effect through correcting mechanical inefficiency [4]. Electrical synchronization reduces LBBB-induced mechanical VV dyssynchrony between the RV and LV and the intraventricular dyssynchrony within the LV. Minimizing VD improved global LV function through one or more of the following mechanisms: (a) increasing LV filling time; (b) decreasing septal dyskinesia, increasing LV dp/dt; and (c) reducing mitral regurgitation [28].
Increasing LV filing time
The LV filling time describes the diastolic filling period, which commences at the beginning of E-wave (mitral flow velocity during early filling) and terminating at the end of A-wave (mitral flow velocity during atrial contraction). In the presence of interventricular conduction delay, there is a delay in LV activation whereas there is no delay in atrial activation. Passive filling and atrial activation occur simultaneously, resulting in shortened LV filling time and decreased LV preload. The related echocardiographic finding is the fusion of E and A waves. With the initiation of CRT pacing, both the LV and RV activate simultaneously resulting in LV completing the contraction and beginning relaxation earlier causing an increase in ventricular filling time. The resultant echo effect is the separation of the E and A-waves on Doppler transmitral flow measurement [29].
Decreasing septal dyskinesia, increased LV dp/dt
The effect of interventricular conduction delay also extend to the normal activation contraction sequence between the septum and free wall. Free wall contracts in time distance after the septal contraction and the resultant time mismatch causes the septum to move away from the free wall during ventricular systole diminishing the contribution of the septum to LV stroke volume. CRT pacing causes the septal and free walls to activate synchronously and allow ventricular ejection of occur prior to relaxation of the septum in turn improving the stroke volume and other systolic indices such as LV dp/dt [29].
Reducing mitral regurgitation
Normal mitral valve opening and closure depends on appropriately timed atrial and ventricular contraction. In the presence of interventricular (VV) and atrioventricular (AV) conduction delays, mitral valve closure may not complete. If the time lag is long enough, ventricular-atrial pressure gradient may cause diastolic mitral regurgitation. With CRT pacing, the AV and VV activation is resynchronized and mitral regurgitation reduced or eliminated [29].
Through these three mechanisms, CRT helps restore AV, inter- and intra-ventricular synchrony, improve LV function, reduce MR and induce LV reverse modelling through increasing LV filling time and LVEF, and decreased LV end-diastolic and LV end-systolic volumes, MR and septal dyskinesia [30-32]. However, since the mechanism of CRT benefits is very heterogeneous – treatment benefits vary from one individual to another and within the same individual over time – there is no single measure that will accurately predict treatment response to CRT. [33,34].
These acute mechanical effects of CRT may be accompanied by chronic adaptations leading to long-term benefits in HF patients. CRT can improve disturbed neurohormonal balanced seen in patients with chronic HF [35]. An early work on CRT indicate normalization of plasma norepinephrine levels [36]. There is evidence suggesting that CRT may improve the levels of serum natriuretic peptides and a variety of other neurohormones as well as restore autonomic balance in HF patients [37]. Bi-ventricular pacing has been shown to result in significant improvement in heart rate variability, suggesting a decline in cardiac adrenergic activity or an increase in parasympathetic activity or their combination [28,38]. Bi-ventricular pacing has also been associated with reversal of adverse LV remodelling in patients with chronic HF with suggestions that LV mechanical synchrony was the predominant underlying mechanism [39]. A post-hoc analysis of CARE-HF trial reported CRT induces sustained (long-term) LV reverse remodelling with the most marked effects observed within the first 3 to 9 months. There was a relationship between the extent of remodelling in response to CRT and aetiology of HF and to a lesser extent to the interventricular mechanical dyssynchrony [40].
Patient selection
Careful patient selection is essential to identify only patients who would show clinically beneficial outcomes are treatment with CRT. The current selection criteria is based on electrocardiography (ECG) markers of VV dyssynchrony [10,27]. However, emerging proposal support the inclusion of mechanical (echocardiography) markers because of their potential additive value to improve the selection of patients with a positive response to CRT. On the other hand, identification of non-responders is an ongoing research area complicated by a clear lack of standardized definition of CRT benefit and lack of uniformity in the quantification of the benefit [9].
Electrocardiographic markers
Current electrocardiographic (ECG) markers focus on HF patients with LBBB. However, with increasing numbers of RBBB patients receiving CRT with a subset indicating favourable treatment outcomes, there is a need for markers to identify RBBB patients for CRT as well.
LBBB QRS morphology: The most reliable electrocardiographic (ECG) biomarkers for patient selection for CRT are QRS duration and LBBB morphology [10]. Prolonged QRS complex (QRS duration ≥ 120 ms), which occurs in ~30% of patients with advanced HF is linked to varying degrees of electromechanical delay or dyssynchrony. In these patients, treatment by CRT modifies this delay. However patients with narrow QRS complexes (QRS duration < 120 ms) do not exhibit any proven clinical benefit of CRT pacing [11]. Recent meta-analysis also question the use of CRT in patients with QRS duration 120-149 ms [41,42]. By contrast, enrolment criteria for CRT trials have used QRS duration ≥120 ms irrespective of the imaging technique used to assess for the presence or absence of dyssynchrony.
The controversy in the role of dyssynchrony in assessing the likelihood of response to CRT has contributed the current guidelines of the ACC/AHA/HRS on appropriate use of ICD/CRT to exclude measurement of dyssynchrony prior to implantation [10]. Based on current evidence from clinical trials, clinical practice guidelines by leading cardiology societies [27,43] recommend patient eligibility for CRT should include QRS duration > 120 ms and LBBB morphology. Patients with QRS duration < 150 ms are eligible only when there is a clear diagnosis of LBBB morphology of the QRS complex (Table 1).
Table 1. Indications for CRT in Patients in Sinus Rhythm
Selection Criteria |
CRT is recommended… |
LBBB with QRS duration > 150 ms |
In chronic HF patients and LVEF ≤ 35% who remain in NYHA functional class II, III and ambulatory IV despite adequate medical treatment. |
LBBB with QRS duration 120-150 ms |
In chronic HF patients and LVEF ≤ 35% who remain in NYHA functional class II, III and ambulatory IV despite adequate medical treatment. |
Non-LBBB with QRS duration > 150 ms |
In chronic HF patients and LVEF ≤ 35% who remain in NYHA functional class II, III and ambulatory IV despite adequate medical treatment. |
Non-LBBB with QRS duration 120-150 ms |
In chronic HF patients and LVEF ≤ 35% who remain in NYHA functional class II, III and ambulatory IV despite adequate medical treatment. |
QRS duration < 120 ms |
Not recommended in chronic HF patients. |
Adapted from 2013 ESC Guidelines on cardiac pacing and CRT [27]
Despite its usefulness, some important limitations have emerged on the use of ECG for qualifying patients for treatment with CRT. Standardized ECG criteria for classifying LBBB is lacking and significant differences (in ECG findings) across scientific organizations, investigators, trials and guidelines complicate meta-analysis and comparative studies on CRT treatment outcomes. Moreover, ECG values for LBBB have not been designed to predict response to CRT [4]. The challenge in using ECG pertains to the detection of QRS slurring and notching to identify LBBB. Modern quantitative ECG lacks standard definition for QRS notch and slur partly because definitions are difficult to apply manually in clinical practice and clinicians usually rely on small amplitude and duration measurements. Additionally, the process of measuring and interpreting the QRS complex is lengthy and tedious, more so, in patients with an underlying disease such as previous myocardial infarction or myocardial hypertrophy, which may alter QRS morphology and duration [4].
Although QRS delineation and duration are easier to determine than notching and slurring, recent report suggests large inter- and intra-observer variability, and limited accuracy and precision of automated measures of QRS duration among ECGs [44,45]. The difference could exceed 10-15 ms, which is clinically significant for qualifying a patient to CRT or for providing a class of recommendation [45]. Further, there is inadequate studies examining the correlation of specific morphological markers for intraventricular conduction delays, LBBB or RBBB with clinical in vivo measurements of intra-cardiac activation times. In addition, the basis of the current threshold of abnormal QRS duration of 120 ms is pattern recognition comparing dogs with humans with no objective measurements in humans [46]. Finally, the limitations of surface ECG in accurately and precisely defining LBBB and reliable prediction of CRT response suggests the consideration of other ECG-derived indexes to assess LBBB morphology.
RBBB QRS morphology: At present, clinical guidelines and clinical trials of patients treated with CRT recommend and demonstrate respectively that only those with LBBB QRS morphology benefit from CRT. This observation stems from several key physiological features explaining significant hemodynamic improvements in LBBB HF relative to RBBB HF [47]. First, RBBB HF has significantly less dyssynchrony than LBBB HF. Second, because in pure RBBB HF the septum and not the LV free wall contracts later there is no hemodynamic improvement from LV free wall pre-excitation in RBBB HF patients. Third, the LV free wall is large without aby other structure to prevent stretch while LV septum has a smaller area supported against stretch by the pressure in the RV cavity. As a result, improvements in LV mechanical in RBBB HF with only RV pacing is significantly less than the improvement in LV mechanics in LBBB HF with LV only pacing [47].
Despite evidence discouraging the use of CRT in RBBB HF patients, a growing number of patients with RBBB QRS morphology or intraventricular conductional abnormalities have received CRT treatment since its introduction in clinical practice. In a recent review, an average of 18% (range 5% to 26%) of all treated CRT patients had RBBB [48]. These patients require adjunct therapies on top of optimal medical therapy. Although the available evidence discourages the use of CRT in RBBB patients [49], a subgroup of RBBB patients may benefit from CRT including those with QRS morphology on limb leads that resemble LBBB and show delayed LV activation especially on the LV free wall [49]. Thus, individualized treatment strategy in RBBB patients is important and should depend on the presence of LV and RV dyssynchrony demonstrated by advanced echocardiographic techniques [50] or surface ECG [48].
Mechanical markers
Rationale for mechanical markers: Two landmark clinical trials: the Multisite Stimulation in Cardiomyopathy (MUSTIC) [49] and the Multicentre InSync Randomized Clinical Evaluation (MIRACLE) [50] large contributed to the present LBBB QRS morphology criteria for selecting patients for CRT – (a) several HF despite optimal medical therapy; (b) depressed LVEF; (c) wide QRS complex with LBBB morphology [27,43]. However, both trials demonstrated that 20% to 30% of patients meeting the selection criteria but do not exhibit left ventricular dyssynchrony did not respond to CRT [49,50]. Some patients with wide QRS complex do not exhibit LV dyssynchrony while some patients with narrow QRS may demonstrate LV dyssynchrony [51-54]. These considerations show a need for additional selection criteria to identify potential responders to CRT [49,50]. Since the effect of CRT is correcting electrical substrate but exert its effect on correcting mechanical inefficiencies, markers of mechanical dyssynchrony may provide additive selection criteria for selecting patients for CRT.
Echocardiography is the principal imaging technique used for evaluating all the three forms of mechanical dyssynchrony (AV, VV and intraventricular). Several echo modalities are available including conventional 2-dimensional or M-mode, tissue Doppler, strain rate and tissue tracking imaging. Of these modalities, the most practical and predictive of CRT response and non-response to CRT remains to be determined in careful prospective clinical trials [12]. Cardiac magnetic resonance imaging has also been used to record LV dyssynchrony but limited to patients without pacemaker wires because it would be problematic following CRT effects [55].
Echocardiographic markers: Echocardiographic imaging has shown promising ability to select potential responders to CRT. Initially, simple echocardiographic markers such as apical rocking, septal flash and interventricular mechanical dyssynchrony appear valuable in identifying patients who would most likely benefit from CRT [56]. In the early 2000, tests for newer echocardiographic indexes began. However, many large randomized clinical trials evaluating different echocardiographic indexes have shown disappointing results. The EchoCRT study [57] enrolled patients with QRS < 130 ms and mechanical dyssynchrony selected based on VV mechanical delay and/or longitudinal strain. Patients on bi-ventricular pacing showed poor outcome and higher mortality compared to the control group. In a sub-group of patients having low global longitudinal strain showed unfavourable clinical outcomes, indicating deleterious effect of improper patient selection for CRT and poor myocardial contractile function [57].
Recent data suggests sophisticated strain-based indices of mechanical dyssynchrony in patients with QRS > 130 ms have shown promising results in patient selection for CRT. Several large observational studies using advanced echocardiographic indices including speckle-tracking indices reported improvements in predicting CRT response combined with QRS duration and QRS morphology [49,58,59]. Combining speckle-tracking radial strain with QRS ≥ 120 ms, non-LBBB patients with radial dyssynchrony has a more favourable event free survival than those with no dyssynchrony (Hazard ratio [HR] 2.6; 95% CI: 1.47-4.53’ p=0.0008) versus (HR: 4.9; 95% CI: 2.60-9.16; p=0.0007) [49]. The findings associated mechanical dyssynchrony and QRS morphology with favourable outcome following CRT. In a related analysis of echocardiographic dyssynchrony indices by combining patient data with multiscale computer simulations, septal systolic rebound stretch (R= -0.56) and interventricular mechanical dyssynchrony (R= -0.50) had better correlation with CRT response compared to septal-to-lateral peak shortening delay (R= -0.48) and septal-to-posterior wall motion delay (R= -0.39). Septal systolic rebound stretch and interventricular mechanical dyssynchrony better represent LV dyssynchrony amenable to CRT and better predict CRT response than indices assessing time-to-peak deformation or motion [59].
Studies using computer models to analyse patients’ data and evaluate regional differences in morphology of strain curves suggest a computer model can be helpful in understanding septal deformation and predicting cardiac resynchronization therapy response [60,61]. The studies report regional differences in morphology of strain curves provide better prediction to distinguish LBBB-like conduction abnormalities amenable to correction by CRT from ventricular conduction disturbance unlikely to respond to CRT [60,61]. The same findings apply to RBBB patients in whom the presence of regional difference of strain curves resembling LBBB-like pattern pointing to positive response to CRT [49].
Challenges in identifying non-responders
Despite therapeutic success of CRT in selected HF patient population, ~20% to 30% of patients who have received CRT appear to derive no clinical benefit from the therapy [49,50]. Since this non-response group of patients may substantially diminish the cost-benefit ratio of CRT, there is need to minimize their proportion. However, accurate identification of non-responders has been undermined by various factors, which need further investigation by clinical trials with careful patient selection for clarity.
Lack of definitional uniformity of CRT benefits: The lack of definitional uniformity of CRT benefit and non-uniformity in the quantification of benefits following CRT therapy across clinical trials complicate accurate identification of non-responders. Benefits such as changes in functional classification or walked distance are soft end-points, which spontaneous changes or placebo may also influence. Alternatively, changes in oxygen uptake at aerobic threshold during exertional activities or reduction of LV systolic and diastolic volumes represent potential harder end-points to define non-responding patients. Increasing exercise tolerance with decreasing ventricular chamber size may suggest a large improvement in cardiac and systemic hemodynamics occurring at a lower myocardial energy cost [4]. However, at present data is unavailable on the minimum change of ventricular chamber dimensions predicting a change in prognosis and symptoms.
Lack of clarity of CRT benefits: Data from the COMPANION trial [62] showed patients randomized to optimal medical therapy exhibited progressive reduction of systolic blood pressure that is consistent with the progression of the underlying cardiac disease whereas patients assigned to CRT did not exhibit a similar reduction. The findings indicate that stabilization of the patient even in the absence of subjective improvement indices such as remaining in the same NYHA functional class III rather than progressing into NYHA IV may be considered to a certain extent a therapeutic benefit of CRT.
Role of pacing site: Pacing site has been associated with improvement in ventricular mechanics. It could be postulated pacing of non-responders is at a sub-optimal site. However, recent echocardiography evidence suggests that in a substantial proportion of CRT patients, the anatomically selected pacing site does not always coincide with ventricular regions with large mechanical delay. Furthermore, identifying the most optimal pacing site by different non-invasive cardiac imaging techniques is still an area of ongoing research. Whereas 3-dimnesional electro-anatomic mapping may visualize electrical derangement along with ventricular function, the method is invasive, costly and time-consuming and rarely used [21].
Time-dependent benefits: Benefits to CRT therapy may be time-dependent based on patients’ baseline characteristics. Two prospective trials [63,64] revealed a time-dependent effect of CRT on QRS duration. Patients with QRS duration > 150 ms at baseline exhibited large and almost immediate benefits while patients with baseline QRS duration ranging between 120 ms and 150 ms exhibited a delayed response (> 6 months) in NYHA functional class and exercise capacity.
End-stage disease: Some HF patients have an advanced disease and any intervention may not change the course of the end-stage disease process [4,21].
Although several reasons have been highlighted to explain why some HF patients eligible for CRT may not derive any clinical benefit from therapy, there is need to develop better characterization of pacing site and proper patient selection to improve CRT beneficial outcomes.
Contraindications for CRT
At present, there is no well-established contraindication to CRT. Nevertheless, isolated anecdotal evidence suggests CRT may be contraindicated in patients in whom weaning from parenteral inotropic therapy has not been possible. In these patients, severe pulmonary hypertension and intractable right HF are frequent [9]. However, there is no data to indicate that patients with moderate pulmonary hypertension are contraindicated for CRT. Whether co-occurring disorders such as atrial fibrillation, previous valve replacement, chronic obstructive pulmonary disorder and peripheral artery disease present a contraindication remains unknown. The most recent guidelines for pacing [10,43] indicate CRT in patients non-responsive to optimal medical therapy (with refractory symptoms). Patients who do not tolerate HF medications such as beta-blockers or those in whom optimal dose of angiotensin converting enzymes – inhibitors (ACE-I) or beta-blockers cannot be reached may benefit from CRT.
Optimization of the CRT
Patients with chronic HF represent a heterogeneous group suggesting the importance of tailoring CRT to the individual patient. Nevertheless, current evidence on the benefit of CRT optimization is inconclusive. Observational studies associate sub-optimal programming of the AV and /or VV delays with poor response to CRT [65]. Small-scale randomized clinical trials and observation studies support these findings by showing significant improvements in HF symptoms and reduction in HF hospitalization after optimizing AV and VV delays [66-75] especially in HF patients with ischaemia. By contrast, larger multicentre trials suggest routine AV/VV delay optimization has limited effect on clinical of echocardiographic outcomes compared with fixed 100-120 ms AV deal and simultaneous bi-ventricular pacing [76-82]. However, heterogeneity in patient selection, procedural timings and methodology in individual studies complicate comparison of these outcomes. Although current evidence and the ESC guidelines [27] discourages routine AV and VV optimization in all CRT patients, in non-responders, such as HF ischaemic patients or those in need of atrial pacing, optimization of AV and VV is recommended to correct sub-optimal device setting [27]. The ESC guidelines classifies methods to optimize AV and VV into two groups based on echocardiography and non-echocardiographic methods (Table 2).
Table 2. Optimal CRT Programming
Parameter |
Standard Practice |
CRT Optimization |
LV lead position |
Posterolateral |
Avoid apical
Target latest activated area |
AV delay |
Fixed empirical AV interval 120 ms (range 100-120 ms) |
Echo-Doppler: shortest AV delay without truncation of the A-wave to change in LV systolic function |
Device-based algorithms |
VV delay |
Simultaneous bi-ventricular |
Echo: residual LV dyssynchrony |
Echo-Doppler largest stroke volume |
ECG: narrowest LV-paced QRS – difference between biventricular and pre-implantation QRS |
Device-based algorithms |
LV pacing alone |
Simultaneously biventricular |
Not applicable |
Adapted from the 2013 ESC CRT Guidelines [27]
Generally, CRT optimization for eligible individual HF patients, especially non-responders to CRT, may be achieved through pacing the RV and LV, optimizing AV delay and optimizing VV delay [29].
Pacing the RV and the LV
The initial step and the key to provide effective bi-ventricular pacing that best correct the electromechanical delay in the LV is the selection of an appropriate LV pacing site. The pacing sites of the latest activation of the LV provides the greatest improvement in pulse pressures and LV dp/dt [28,50,83]. Insertion of the LV lead tip at the latest site of activation intersects the LV electrogram signal with the latter part of the QRS on surface ECG. The position of the RV lead relevant to LV lead should be another important consideration. To obtain optimal RV pacing, the position of the RV lead should be far away as possible from the LV lead. Maximizing the RV/LV lead distance reduces the risk of far-field sensing as well as improves the effectiveness of bi-ventricular pacing. Similarly, the position of the LV lead in the lateral and posterolateral veins have shown to provide the most effective bi-ventricular pacing [29].
Optimizing the AV delay
Optimizing AV delay is important to adjust the contraction sequence between the left atrium and the LV leading to optimized LV filling without truncating atrial contribution. An optimal AV delay maximizes stroke volume and minimizes MR. several methods are available to determine optimal AV delay. The first is empirical calculation in which the optimal AV time is the function of the half of the sensed PR interval minus 20. The second technique and a more complicated formulation, is the Ritter technique [84]. This technique requires obtaining a pulsed wave Doppler view of the transmitral flow through a 4-chamber view. As the ECG E- and A-wave recordings are visualized, a short sensed AV interval (AVshort) is programmed and the corresponding QA (QAshort) is measured. Next, the long sensed AV interval (AVlong) is programmed and the corresponding QA (QAlong) is measured. The optimal AV delay (AVopt) is calculated as follows:
AVopt = AVshort + [(AVlong + QAlong) - (AVshort + QAshort)].
The third method is the iterative method in which the operator starts with an AV delay programming that causes ventricular pre-excitation. Then the programmed AV delay is increased until the A-wave begins to truncate. The AV delay is then increased until the completion of the A-wave contribution is observed. The specific period is then taken as the optimal AV delay. The fourth and the last method is the pulse pressure method, where an arterial line is utilized to measure the central aortic pressure accurately. The AV delay programming begins at a lower value and then delay is increased progressively to get an optimal value that provides maximum difference between systolic and diastolic blood pressures [29].
Optimizing the VV delay
Optimizing the pacing delay between the RV and LV helps to adjust contraction sequence between the two ventricles, and ideally, optimize the LV to produce the largest stoke volume in certain patients [27]. The optimal velocity time integral (VTI), which is a surrogate marker for stroke volume, is useful for the determination of an optimal VV delay setting. The VTI technique involves obtaining continuous or pulse wave Doppler velocities across the aortic valve using the apical long axis view, then the VTI (the area under velocity time curve) is calculated. The multiplication of the VTI with the LV outflow tract provides the stroke volume. A larger VTI thus represents greater stroke volume. To obtain different VV settings, all VTI values are calculated next without moving the sample volume on Doppler echocardiogram. The average value of two-three VTI values measured for each VV setting is then obtained. The greatest VTI with maximal stroke volume is determined and the associated setting taken as the optimal VV delay for the patient [29].
Meta-analysis of efficacy and safety of CRT
Despite marked advances in medical therapy relieving symptoms, improving QoL and survival of patients with symptomatic HF, prognosis has remained unfavourable [85]. Progressive pump failure, arrhythmias and perturbations in ventricular conduction system are frequent aetiologies of mortality in HF patients despite optimal medical therapy. With the introduction of pacing technology in clinical practice, progressive improvement in device and therapy has seen its recommendation in HF guidelines for the treatment of selected HF patient populations with malignant arrhythmias or conduction problems [86]. Current data demonstrate CRT improves cardiac function by minimizing or terminating abnormal ventricular activation and contraction in patients with LVSD [10,27,43]. Previous systematic review and meta-analyses [87-91] have established morbidity and mortality benefits with the use of CRT in randomized controlled trials. Since then, a number of newer RCTs on CRT efficacy or safety have since been published and their effect on pooled evidence is unknown. The present systematic review and meta-analysis pools available published data to determine treatment efficacy and safety in randomized trial participants of CRT compared to optimal medical therapy and/or CRT combined with ICD (CRT-D) in HF patients with LVSD.
Methods
Data sources and study search: This systematic analysis include electronic search on PubMed/MEDLINE from inception through to January 2018. The following key words were used to identify eligible studies: cardiac resynchronization therapy, bi-ventricular pacing, cardiac pacing or ventricular pacing. The search criteria was limited to studies published in English language and involved humans. The electronic literature search was supplemented by searching the website www.clinicaltrial.gov, reports from the US Food and Drugs Administration and reference lists of included studies, published meta-analyses and relevant review articles.
Eligibility criteria: Studies were included if they met the following eligibility criteria. They were (a) randomized parallel control trials or randomized crossover trials; (b) enrolled patients with impaired LV systolic function; (c) compared RCT with optimal medical therapy, inactive pacing, or CRT and ICD; (d) reported all-cause, mortality, HF hospitalization, change in LVEF or functional outcomes (NYHA functional class, six minute walk test, or QoL); and (e) included at least 25 patients and one month follow-up. Primary literature search was completed by one of the investigators using standardized inclusion/exclusion form then two investigators reviewed the full-text of all the potentially relevant studies. The final decision for study inclusion or exclusion was reached by consensus.
Data abstraction and quality assessment: Two investigators completed data abstraction in duplicate and independently using standardized data extraction forms. For crossover trials, data from the first period (prior to crossover) were extracted. The following data was extracted from the included studies: trial design, inclusion criteria, baseline characteristics, safety and efficacy outcomes, and quality assessment. Any discrepancy was resolved by consensus. Outcomes: The primary outcomes for this meta-analysis were all-cause mortality and HF hospitalization for RCT and non-RCT patients, those receiving optimal medical therapy, placebo/inactive pacing or CRT-D (dual treatment using CRT and ICD). For crossover trials, the results of the first period were considered. Secondary outcomes were functional status and functional outcomes – six-minute walk distance (6MWD), peak oxygen consumption and/or QoL.
Methodological quality: The assessment for methodological quality for all the studies included in this meta-analysis was in accordance with the Delphi criteria [92] and scored based on the Jadad Scale [93]. Factors considered in assessment of methodological quality included adequacy of randomization methods used and allocation concealment, similarity of treatment arms at baseline, specification of inclusion criteria and blinding of the patient, clinician and outcome assessor, measures of variability of outcome, and the description of withdrawals and dropouts [92,93].
Data synthesis and analysis: For categorical data such as number of patients and sex distribution, calculation used frequency and percentage. For dichotomous results such as all-cause mortality and HF hospitalization, calculation used relative risk (RR) and 95% confidence interval (CI) for pooled estimated. For continuous variables such as 6MWD, peak oxygen consumption (VO2) and QoL, calculations used standardized mean difference (SMD) and 95% CI for pooled estimates. The I2 statistics was used to quantify the percentage of total variation across studies that is due to heterogeneity rather than chance. A value of 0% indicates limited heterogeneity while larger values demonstrate increasing heterogeneity [94]. A value I2 > 50% was considered to indicate substantial heterogeneity. Fixed effect model was used when I2 ≥ 50% while random effect model was used when I2 < 50% and a p-value of < 0.05 was considered statistically significant.
Results
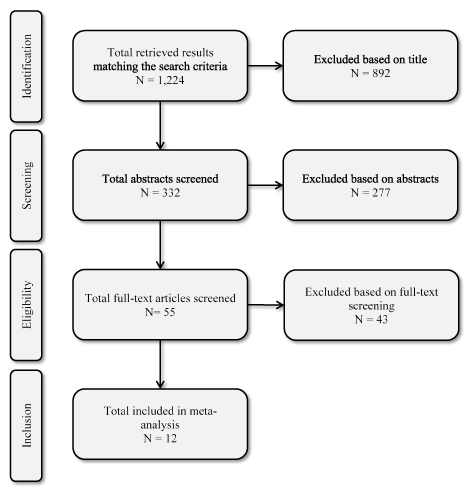
Study selection: Two investigators agreed on the selection and methodological assessment. The initial search process retrieved 1,224 studies. Of these, 892 were excluded by title search due to irrelevant content, non-human studies and non-English studies. The abstracts of the remaining 332 articles were reviewed and 277 excluded because the studies were non-randomized and retrospective analysis. Full-text screening excluded a further 43 articles because they did not report outcomes of interest. The remaining 12 clinical trials [30,31,62,63,95-102] were included in this meta-analysis (Figure 1).
Study characteristics: Table 3 provides a summary of baseline characteristics and main findings in the included studies. In all, the 12 trials included 8,288 patients randomized into CRT (treatment) arm (n=4,882) and intervention arm consisting of CRT-D, optimal medical therapy or placebo pacing (n=3,406). The patients were older (mean age = 64 years; range = 60-67 years) and a greater proportion of male patients (73%). Treatment by CRT was compared to conventional OMT in six trials [30,62,95,97-99], to inactive CRT (CRT-OFF or placebo pacing) in two trials [100,102], to CRT-D in four trials [31,62,96,101] and to LV or RV pacing [63]. The main inclusion criteria based on LEVF, QRS duration and NYHA functional class varied across studies. Three trials used LVEF ≤ 30% [31,63,101], eight trials used LVEF ≤ 35% [30,62,95-99,102] and one trial used LVEF ≤ 40% [100]. The cut-off used for wide QRS complex also varied from QRS duration (QRSd) < 120 ms in one trial [102], ≥ 120 ms in six trials [62,96,99,100,101]; ≥ 130 ms in four trials [30,31,97,98], ≥ 150 ms in two trials [63,95]. Five trials included patients with NYHA functional class III-IV [30,95,96,97,99], two trials each included NYHA II-III [101,102] and II-IV [62,63], and three trials included NYHA I-II [31,98,100]. Follow-up period for the evaluation of treatment efficacy was 6 months in five trials [30,95-98], 12 months in four trials [31,63,100,102], and one trial each for 15 months [62], 29.4 months [99] and 40 months [101].
Table 3. Summary of Included Studies
Author |
Year |
Study Design |
CRT (N) |
Mean Age |
Male (%) |
Main Inclusion Criteria |
Primary/Secondary End-points |
Summary of Main Findings |
MUSTIC-SR [95] |
2001 |
Single-blind crossover randomized CRT, OMT 6 months |
58 |
63 |
50 |
NYHA III; LVEF < 35%; QRSd > 150 |
6MWD, QoL, VO2, hospitalization, mortality |
Improves exercise tolerance and QoL |
MIRACLE [30] |
2002 |
Double-blind randomized CRT, OMT 6 months |
453 |
64 |
155 |
NYHA III-IV; LVEF: ≤ 35%, QRSd ≥ 130 |
6MWD, QoL, NYHA, VO2 |
Improves 6MWD, NYHA, QoL, hospitalization |
CONTAK-CD [96]
|
2003 |
Double-blind randomized CRT-D, ICD 6 months |
490 |
66 |
203 |
NYHA III-IV; LVEF: ≤ 35%, QRSd ≥ 120 |
All-cause mortality, hospitalization |
Improves functional status |
MIRACLE-ICD [97]
99,97,96 |
2003 |
Double-blind randomized CRT, OMT 6 months |
369 |
67 |
142 |
NYHA III-IV; LVEF: ≤ 35%, QRSd ≥ 130 |
QoL, 6MWD, NYHA, VO2, hospitalization |
Improves QoL, functional status, and exercise capacity |
PATH-CHF [63]
98,100,31 |
2003 |
Single-blind crossover randomized RV, LV, BiV, 12 months |
86 |
60 |
26 |
NYHA II-IV, LVEF: ≤30%, QRSd > 150 |
Exercise capacity (peak VO2), QoL, NYHA class |
Improves exercise tolerance and QoL |
COMPANION [62] |
2004 |
Double-blind randomized OMT, CRT, CRT-D 15 months |
1520 |
67 |
67 |
NYHA: II-IV, LVEF: ≤35%, QRSd ≥120 |
All-cause mortality, hospitalization |
Reduces all-cause mortality and hospitalization |
MIRACLE-ICD II [98] |
2004 |
Double-blind randomized CRT, OMT 6 months |
186 |
63 |
75 |
NYHA II; LVEF: ≤ 35%, QRSd ≥ 130 |
VO2, NYHA, QoL, 6MWD |
Improves cardiac structure and function but not exercise capacity |
CARE-HF [99] |
2005 |
Double-blind randomized OMT, CRT-P 29.4 months |
813 |
67 |
304 |
NYHA III-IV; LVEF: ≤ 35%, QRSd ≥ 120 |
All-cause death, unplanned hospitalization |
Improves symptoms and QoL, reduces complications/risk of death |
REVERSE [100] |
2008 |
Double-blind randomized CRT-ON, CRT-OFF 12 months |
610 |
63 |
327 |
NYHA: II, LVEF: ≤ 40%, QRSd ≥ 120 ms |
All-cause mortality, hospitalization |
Reduces HF hospitalization and improves cardiac structure and function |
MADIT-CRT [31] |
2009 |
Single-blind randomized DCRT-D, ICD 12 months |
1820 |
65 |
814 |
NYHA: I-II, LVEF: ≤ 30%, QRSd ≥ 130 ms |
Death from any cause and non-fatal HF events |
CRT combined with ICD decreased the risk of HF events |
RAFT [101] |
2010 |
Double-blind randomized CRT-D, ICD 40 months |
1798 |
66 |
758 |
NYHA: II-III, LVEF: ≤30%, QRSd >120 ms |
All-cause death, hospitalization |
CRT-D reduces rates of all-cause death and HF hospitalization |
LESSER-EARTH [102] |
2013 |
Double-blind randomized CRT-ON vs. CRT-OFF 12 months |
85 |
62 |
28 |
NYHA: II-III, LVEF: ≤35%, QRSd <120 ms |
Exercise duration, 6MWD |
CRT-On did not improve 6MWD, NYHA and LV remodelling |
6MWD: Six Minute Walk Distance: CARE-HF: Cardiac Resynchronization-Heart Failure trial; COMPANION: Comparison of Medical Therapy, Pacing, and Defibrillation in Chronic Heart Failure trial; CONTAK-CD: CONTAK-Cardiac Defibrillator trial; CRT: Cardiac Resynchronization Therapy; CRT-D: Cardiac Resynchronization Therapy-Defibrillator; LESSER-EARTH: Evaluation of Resynchronization Therapy for Heart Failure trial; LVEF: Left Ventricular Ejection Fraction; MADIT-CRT: Multicentre Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy trial; MIRACLE: Multicentre InSync Randomized Clinical Evaluation; MIRACLE-ICD: Multicentre InSync Implantable Cardioverter Defibrillator trial; MUSTIC: Multisite Stimulation in Cardiomyopathies; NYHA: New York Heart Association; OMT: Optimal Medical Therapy; QoL: Quality of Life; QRSd: QRS Duration; RAFT: Resynchronization-Defibrillation for Ambulatory Heart Failure trial; REVERSE: REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction trial
Study outcomes
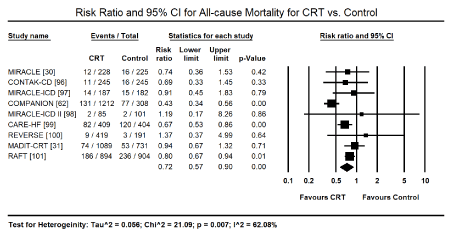
All-Cause Mortality: HF patients treated with CRT had fewer deaths (n=521; 10.9%) compared to non-CRT patients (n=538; 16.3%). Pooled data from nine trials [30,31,62,96-100] on treatment effect of CRT showed it significantly reduced the risk of all-cause mortality by 28% (Risk Ratio [RR]: 0.72; 95% CI: 0.57 to 0.90; p = 0.001). (Figure 2). Excluding studies enrolling patients with NYHA function class I and II, CRT had a comparable significant risk reduction of 30% to all-cause mortality (RR: 0.70, 95% CI: 0.56 to 0.87; p = 0.001). The pooled analysis did not discriminate between cardiac causes of death since this analysis relied on only published data, which did not provide data for specific causes of death.
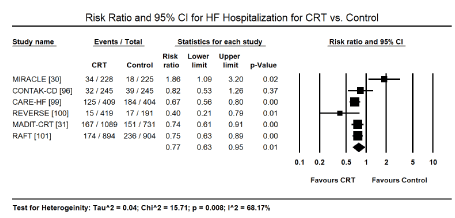
HF hospitalization: Six trials [30,31,96,99-101] provided data on patients hospitalized for cardiac causes following CRT or control treatment. Pooled analysis revealed treatment with CRT significantly reduced hospitalization frequency by 23% (RR: 0.77; 95% CI: 0.63-0.95; p=0.01). Only one trial [96] reported non-significant effect of CRT on reduction of HF hospitalization (Figure 3).
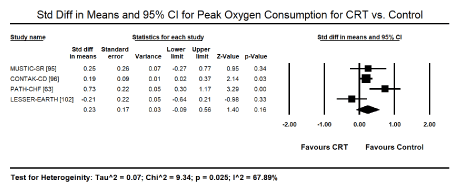
Peak oxygen consumption (VO2): Data for peak oxygen consumption after CRT or control was provided standard in four trials [63,95,96,102]. Pooled analysis showed a positive trend towards improved peak oxygen consumption (VO2) on patients treated with CRT, standard mean difference (SMD: 0.23; 95% CI: -0.09 to 0.56; p=0.16]. The effect of CRT was not clear since some of the patients included were in NYHA function class II-IV. Sub-analysis of patients with only NYHA functional class III-IV would have provided a more accurate effect of CRT on peak VO2.
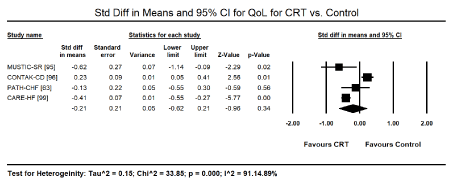
Quality of life (QoL): Comparison data on QoL between CRT and control was available in four trials [63,95,96,99]. Patients treated with CRT showed a significant increase in Minnesota Living with Heart Failure Questionnaire (MLHFQ) QoL relative to patients receiving OMT or placebo pacing (SMD: -0.21; 95% CI: -0.62 to 0.21; p=0.34). For MLHFQ, a greater decrease in the score suggests improved QoL (Figure 5). However, significant heterogeneity was found across studies I2 = 91.14% largely attributed to symptom status and NYHA functional class at baseline.
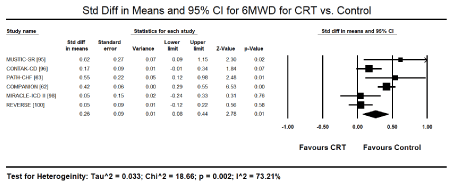
Six-minute walk test: Data on functional status measured using 6MWD was provided by six trials [62,63,95,96,98,100]. Compared to control treatment, CRT achieved significant improvement in 6MWD (SMD: 0.26; 95% CI: 0.08 to 0.44; p=0.01) (Figure 6). Significant heterogeneity (I2 = 73.21%) across studies because of the inclusion of NYHA functional class I-IV at baseline.
Discussion
The present meta-analysis involving 12 clinical trials involving 8,288 patients finds CRT is safe and efficacious in treating symptomatic or mild symptomatic HF (NYHA functional class II-IV), depressed LV systolic function, and a wide QRS complex finds CRT. Compared with optimal medical therapy or placebo pacing, the present meta-analysis associates CRT with a significant reduction in all-cause mortality (by 28%), HF hospitalization (by 23%) as well as improvement in functional capacity – 6MWD and QoL, with a positive trend towards improve peak oxygen consumption. The relative benefits on reduction of mortality is also seen in patients with NYHA class I-II. Treatment effect of CRT showed a positive trend towards improved peak oxygen consumption but the effect might have been affected by the inclusion of mild symptomatic HF patients (NYHA functional class I and II).
The present findings are consistent with those of previous systematic reviews and meta-analysis. The meta-analysis by Bradley et al. [87] including four studies (N=1634) reported CRT reduced death from progressive HF by 51% (Odds Ratio [OR]: 0.49; 95% CI: 0.25 to 0.93) and HF hospitalization by 29% (OR: 0.71; 95% CI: 0.53 to 0.96) and a trend towards reducing all-cause mortality (OR: 0.77; 95% CI: 0.51 to 1.18). Rivero-Ayerza et al [89] included five RCTs (N=2371) and found, compared to optimal medical therapy, CRT reduced all-cause mortality by 29% (OR: 0.71; 95% CI: 0.57-0.88) and mortality due to progressive HF by 38% (OR: 0.62; 95% CI: 0.45 to 0.84) but no effect on sudden cardiac death. McAlister et al. [90] included 14 trials (N=4420) and reported CRT improved LVEF (weighted mean difference [WMD] 3.0%) QoL by MLWHFQ by eight points and decreased hospitalization by 37% (95% CI: 7% to 57%) and all-cause mortality by 37% (95% CI: 9% to 33%). Finally, Adabag et al. [91] included five RCTs (N=4317) and found relative to ICD, CRT reduces all-cause mortality (RR: 0.81; 95% CI; 0.65 to 0.99) and hospitalization (RR: 0.68; 95% CI: 0.59 to 0.79) as well as improved LVEF and LV volume. The benefits were lower for mild symptomatic HF patients (NYHA II).
According to the current device-based therapy guidelines [10,43] selection for patients for CRT should largely depend on prolonged QRS duration (≥120 ms), NYHA functional class III-IV, and the extent of LV systolic dysfunction (LVEF ≤35%). The RCTs included in the present meta-analysis, LVEF, QRS duration and NYHA functional class were major determinants for patient inclusion, with some variations. It has been indicated that a widened QRS complex in 12-lead ECG represents delayed ventricular depolarization, and particularly in patients with LBBB, may result in LV dyssynchrony and increased myocardial strain [103]. In HF patients with myocardial disease, these findings are associated with abnormal ventricular remodelling resulting in increased LV volume and depressed LVEF. Treatment using CRT creates a more synchronous contraction with partial restoration of the LV systolic activation and conduction [104]. Improvement in contractility with CRT reverses abnormal ventricular remodelling resulting on a reduction in myocardial energy cost and oxygen consumption [105-107]. In severe symptomatic patients (NYHA functional class III-IV) with depressed LVEF and widened QRS interval, CRT causes reverse remodelling of the LV dimensions and volume and reduced mitral regurgitation [107-109]. Theses morphological changes have been associated with improvement in symptoms, QoL and exercise tolerance as well as reduction in hospitalization and mortality due to cardiac causes. The effect of CRT on cardiac functional status, hospitalization and mortality may extend to mild symptomatic HF patients (NYHA I-II) [91].
Despite the demonstrated treatment benefits of CRT on mortality, hospitalization, and functional status (6MWD, QoL and peak oxygen consumption), the present meta-analysis had some valid limitations. First, all the included trials were randomized controlled parallel or crossover, which limits the generalizability of findings to clinical practice in respect to response rates, clinical effects and safety when used in routine practice outside of clinical trial centres. Thus, a meta-analysis of observational cohort studies is important to determine whether the present findings can be extended to actual clinical settings. Second, the inclusion criteria used in the individual trials differed slightly from each other. Although the difference were not large enough to prevent pooling of these trial populations, it did not show the treatment effect on different sub-population of HF patients such as those with mild symptomatic HF (NYHA I-II) or severe symptomatic HF (NYHA III-IV). No data was available to evaluate treatment effect on sub-groups such as sex, patients with QRS duration > 150 ms and LBBB patients who benefited more from CRT in individual trials. Finally, while additive value of CRT on ICD has been suggested, the data was not sufficient to demonstrate this suggestion. Additional randomized clinical trials or observation studies with carefully selected patient population suitable for ICD who are not eligible for CRT. Such studies may provide important data on the value of adding CRT on patients who currently only satisfy the criteria for ICD but unclear whether a dual therapy would offer any survival benefits.
Conclusion
Cardiac resynchronization therapy (CRT) is a well-established device-based treatment for patients with advanced systolic heart failure (HF) and ventricular dyssynchrony, which may present as atrioventricular (AV), interventricular (VV) or intraventricular dyssynchrony. CRT works by correcting electric substrate originating from conduction disorders and exerts its effect via reducing mechanical inefficiency resulting in increased LV filling time, decreased septal dyskinesia and reduced mitral regurgitation. The most reliable markers for CRT eligibility include ECG-based – wide QRS duration > 120 ms and LBBB morphology, depressed systolic function (LVEF), and NYHA functional class III-IV. Since about a third of HF patients with these markers do not benefit from RCT, research to improve patient selection by identifying complementary echocardiographic markers are ongoing. Identifying potential non-responders is also challenging because of the lack of definitional uniformity and quantification for CRT benefits, the effect of pacing site on treatment outcomes, time-dependent benefits and end-stage disease (non-responsive to treatment). Defines contraindications to CRT are also lacking. Although current pacing guidelines discourage CRT optimization, in non-responding HF patients such as those with ischaemia or in need of atrial pacing, optimization may be achieved through pacing the RV or LV, optimizing AV delay and/or VV delay. The established treatment benefits of CRT include the reduction of mortality and hospitalization, and improved quality of life, and functional capacity. Additional clinical trials should focus on improving patient selection including a sub-group of RBBB HF patients who will benefit from CRT as well as determining the therapeutic benefit of adding CRT to ICD patients to improve treatment efficiency as well as expand clinical utility CRT.
References
- Braunwald E (1997) Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med 337: 1360-1369. [Crossref]
- Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, et al. (2013) Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 6: 606-619. [Crossref].
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. (2015) Heart disease and stroke statistics: 2016 update: a report from the American Heart Association. Circulation 133: e38-e360 [Crossref]
- Auricchio A, Prinzen FW (2017) Enhancing response in the cardiac resynchronization therapy patient: the 3B perspective-bench, bits, and bedside. JACC Clin Electrophysiol 3: 1203-1219. [Crossref]
- Zoll PM, Linenthal AJ, Lucas JE (1957) Resuscitation from cardiac arrest due to digitalis by external electric stimulation. Am J Med 23: 832-837. [Crossref]
- Leclercq C, Hare JM (2004) Ventricular resynchronization: current state of the art. Circulation 109: 296-299. [Crossref]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, et al. (2016) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 18: 891-975. [Crossref]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, et al. (2013) 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol 62: e147-239. [Crossref]
- Auricchio A, Abraham WT (2004) Cardiac resynchronization therapy: current state of the art: cost versus benefit. Circulation 109: 300-307. [Crossref]
- Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, et al. (2013) ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy. J Am Coll Cardiol 61: 1318-1368. [Crossref]
- Beshai JF, Grimm RA, Nagueh SF, Baker JH, Beau SL, et al. (2007) Cardiac-resynchronization therapy in heart failure with narrow QRS complexes N Engl J Med 357: 2461-2471. [Crossref]
- Bax JJ, Ansalone G, Breithardt OA, Derumeaux G, Leclercq C, et al. (2004) Echocardiographic evaluation of cardiac resynchronization therapy: ready for routine clinical use? A critical appraisal. J Am Coll Cardiol 44: 1-9. [Crossref]
- Brecker SJ, Xiao HB, Sparrow J, Gibson DG (1992) Effects of dual-chamber pacing with short atrioventricular delay in dilated cardiomyopathy. Lancet 340: 1308-1312. [Crossref]
- Kass DA (2002) Ventricular dyssynchrony and mechanisms of resynchronization therapy. Eur Heart J 4: D23-D30.
- Grines CL, Bashore TM, Boudoulas H, Olson S, Shafer P, et al. (1989) Functional abnormalities in isolated left bundle branch block. The effect of interventricular asynchrony. Circulation 79: 845-853. [Crossref]
- Prinzen FW, Augustijn CH, Arts TH, Allessie MA, Reneman RS (1990) Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol 259:H300-H308. [Crossref]
- Nelson GS, Curry CW, Wyman BT, Kramer A, Declerck J, et al. (2000) Predictors of systolic augmentation from left ventricular preexcitation in patients with dilated cardiomyopathy and intraventricular conduction delay. Circulation 101: 2703-2709. [Crossref]
- Park RC, Little WC, O'rourke RA (1985) Effect of alteration of left ventricular activation sequence on the left ventricular end-systolic pressure-volume relation in closed-chest dogs. Circ Res 57: 706-717. [Crossref]
- Heyndrickx GR, Vantrimpont PJ, Rousseau MF, Pouleur HU (1988) Effects of asynchrony on myocardial relaxation at rest and during exercise in conscious dogs. Am J Physiol 254: H817-H822. [Crossref]
- Baldasseroni S, Opasich C, Gorini M, Lucci D, Marchionni N, et al. (2002) Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J 143: 398-405. [Crossref]
- Prinzen FW, Hunter WC, Wyman BT, McVeigh ER (1999) Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol 33: 1735-1742. [Crossref]
- Ukkonen H, Beanlands RS, Burwash IG, de Kemp RA, Nahmias C, et al. (2003) Effect of cardiac resynchronization on myocardial efficiency and regional oxidative metabolism. Circulation 107: 28-31. [Crossref]
- Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, et al. (2002) Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. Jama 288: 3115-3123. [Crossref]
- Kajstura J, Zhang X, Liu Y, Szoke E, Cheng W, et al. (1995) The cellular basis of pacing-induced dilated cardiomyopathy: myocyte cell loss and myocyte cellular reactive hypertrophy. Circulation. 92: 2306-2317. [Crossref]
- Spragg DD, Leclercq C, Loghmani M, Faris OP, Tunin RS, et al. (2003) Regional alterations in protein expression in the dyssynchronous failing heart. Circulation 108: 929-932. [Crossref]
- Brignole M, Gammage M, Puggioni E, Alboni P, Raviele A, et al. (2005) Comparative assessment of right, left, and biventricular pacing in patients with permanent atrial fibrillation. Eur Heart J 26: 712-722. [Crossref]
- Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, et al. (2013) 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 15: 1070-1118. [Crossref]
- Auricchio A, Stellbrink C, Sack S, Block M, ürgen Vogt J, et al. (2002) Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol 39: 2026-2033. [Crossref]
- Ermis C (2007) Optimal programming in cardiac resynchronization therapy. Anatolian Journal of Cardiology 7: 50-52. [Crossref]
- Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, et al. (2002) Cardiac resynchronization in chronic heart failure. N Engl J Med 346: 1845-1853. [Crossref]
- Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, et al. (2009) Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 361: 1329-1338. [Crossref]
- Brignole M, Botto GL, Mont L, Oddone D, Iacopino S, et al. (2012) Predictors of clinical efficacy of ‘Ablate and Pace’therapy in patients with permanent atrial fibrillation. Heart 98: 297-302. [Crossref]
- Leclercq C, Cazeau S, Lellouche D, Fossati F, Anselme F, et al. (2007) Upgrading from single chamber right ventricular to biventricular pacing in permanently paced patients with worsening heart failure: The RD‐CHF Study.Pacing Clin Electrophysiol 30: S23-S30. [Crossref]
- Delnoy PP, Ottervanger JP, Vos DH, Elvan A, Misier AR, et al. (2011) Upgrading to biventricular pacing guided by pressure–volume loop analysis during implantation. J Cardiovasc Electrophysiol 22: 677-683. [Crossref]
- Francis GS, Cohn JN, Johnson G, Rector TS, Goldman S, et al. (1993) Plasma norepinephrine, plasma renin activity, and congestive heart failure. Relations to survival and the effects of therapy in V-HeFT II. The V-HeFT VA Cooperative Studies Group. Circulation 87: VI40-V148. [Crossref]
- Saxon LA, De Marco T, Schafer J, Chatterjee K, Kumar UN, et al. (2002) Effects of long-term biventricular stimulation for resynchronization on echocardiographic measures of remodelling. Circulation 105: 1304-1310. [Crossref]
- Abraham WT, Hayes DL (2003) Cardiac resynchronization therapy for heart failure. Circulation 108: 2596-2603. [Crossref]
- Adamson PB, Kleckner KJ, VanHout WL, Srinivasan S, Abraham WT (2003) Cardiac resynchronization therapy improves heart rate variability in patients with symptomatic heart failure. Circulation 108: 266-269. [Crossref]
- Yu CM, Chau E, Sanderson JE, Fan K, Tang MO et al, (2002) Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation 105: 438-445. [Crossref]
- Ghio S, Freemantle N, Scelsi L, Serio A, Magrini G, et al. (2009) Long‐term left ventricular reverse remodelling with cardiac resynchronization therapy: results from the CARE‐HF trial. Eur J Heart Fail 11: 480-488. [Crossref]
- Sipahi I, Carrigan TP, Rowland DY, Stambler BS, Fang JC (2011) Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Arch Intern Med 171: 1454-1462. [Crossref]
- Stavrakis S, Lazzara R, Thadani U (2012) The benefit of cardiac resynchronization therapy and QRS duration: A meta‐analysis. J Cardiovasc Electrophysiol 23: 163-168. [Crossref]
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NM, Freedman RA, et al. (2008) ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines J Am Coll Cardiol 51: e1-62. [Crossref]
- Tomlinson DR, Bashir Y, Betts TR, Rajappan K (2009) Accuracy of manual QRS duration assessment: its importance in patient selection for cardiac resynchronization and implantable cardioverter defibrillator therapy. Europace 11: 638-642. [Crossref]
- Vancura V, Wichterle D, Ulc I, Smíd J, Brabec M, et al. (2017) The variability of automated QRS duration measurement. Europace 19: 636-643. [Crossref]
- Auricchio A, Lumens J, Prinzen FW (2014) Does cardiac resynchronization therapy benefit patients with right bundle branch block: cardiac resynchronization therapy has a role in patients with right bundle branch block. Circ Arrhythm Electrophysiol 7: 532-542. [Crossref]
- Bilchick KC (2014) Does cardiac resynchronization therapy benefit patients with right bundle branch block: left ventricular free wall pacing: seldom right for right bundle branch block Circ Arrhythm Electrophysiol 7: 543-552. [Crossref]
- Fantoni C, Kawabata M, Massaro R, Regoli F, Raffa S, et al. (2005) Right and left ventricular activation sequence in patients with heart failure and right bundle branch block: a detailed analysis using three‐dimensional non‐fluoroscopic electroanatomic mapping system. J Cardiovasc Electrophysiol 16: 112-119. [Crossref]
- Hara H, Oyenuga OA, Tanaka H, Adelstein EC, Onishi T, et al. (2012) The relationship of QRS morphology and mechanical dyssynchrony to long-term outcome following cardiac resynchronization therapy. Eur Heart J 33: 2680-2691. [Crossref]
- Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, et al. (2001) Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 344: 873-880. [Crossref]
- Yu CM, Lin H, Zhang Q, Sanderson JE (2003) High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart 89: 54-60. [Crossref]
- Bleeker GB, Schalij MJ, Molhoek SG, Verwey HF, Holman ER, et al. (2004) Relationship between QRS duration and left ventricular dyssynchrony in patients with end‐stage heart failure. J Cardiovasc Electrophysiol 15: 544-549. [Crossref]
- Achilli A, Sassara M, Ficili S, Pontillo D, Achilli P, et al. (2003) Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardio 42: 2117-2124. [Crossref]
- Stankovic I, Prinz C, Ciarka A, Daraban AM, Mo Y, et al. (2017) Long-term outcome after CRT in the presence of mechanical dyssynchrony seen with chronic RV pacing or intrinsic LBBB. JACC: Cardiovasc Imaging 10: 1091-1099. [Crossref]
- Nelson GS, Curry CW, Wyman BT, Kramer A, Declerck J, et al. (2000) Predictors of systolic augmentation from left ventricular pre-excitation in patients with dilated cardiomyopathy and intraventricular conduction delay. Circulation 101: 2703-2709. [Crossref]
- Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, et al. (2013) Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 369: 1395-1405. [Crossref]
- Bax JJ, Delgado V, Sogaard P, Singh JP, Abraham, et al. (2016) Prognostic implications of left ventricular global longitudinal strain in heart failure patients with narrow QRS complex treated with cardiac resynchronization therapy: a subanalysis of the randomized EchoCRT trial. Eur Heart J 38: 720-726. [Crossref]
- Risum N, Tayal B, Hansen TF, Bruun NE, Jensen MT, et al. (2015) Identification of typical left bundle branch block contraction by strain echocardiography is additive to electrocardiography in prediction of long-term outcome after cardiac resynchronization therapy. J Am Coll Cardiol 66: 631-641. [Crossref]
- Lumens J, Leenders GE, Cramer MJ, De Boeck BW, Doevendans PA, et al. (2012) Mechanistic evaluation of echocardiographic dyssynchrony indices: patient data combined with multiscale computer simulations. Circulation: Cardiovasc Imaging 5: 491-499 [Crossref]
- Leenders GE, Lumens J, Cramer MJ, De Boeck BW, Doevendans PA, et al. (2012) Septal deformation patterns delineate mechanical dyssynchrony and regional differences in contractility: analysis of patient data using a computer model. Circulation: Heart Fail 5: 87-96. [Crossref]
- Lumens J, Tayal B, Walmsley J, Delgado-Montero A, Huntjens PR, et al. (2015) Differentiating the electromechanical substrate responsive to cardiac resynchronisation therapy from non-electrical dyssynchrony substrates by computer-assisted regional strain analysis. Eur Heart J 36: 889-889. [Crossref]
- Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, et al. (2004) Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 350: 2140-2150. [Crossref]
- Auricchio A, Stellbrink C, Butter C, Sack S, Vogt J, et al. (2003) Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. J Am Coll Cardiol 42: 2109-2116. [Crossref]
- Butler C, Auricchio A, Stellbrink C, Kirkels H, Sack S, et al. (2003) Clinical efficacy of one year cardiac resynchronization therapy in heart failure patients stratified by QRS duration: results of the PATH-CHF II trial. Eur Heart J 5: 363.
- Behar S, Zissman E, Zion M, Hod H, Goldbourt U, et al. (1993) Prognostic significance of second-degree atrioventricular block in inferior wall acute myocardial infarction. Am J Cardiol 72: 831-834. [Crossref]
- Sawhney NS, Waggoner AD, Garhwal S, Chawla MK, Osborn J, et al. (2004) Randomized prospective trial of atrioventricular delay programming for cardiac resynchronization therapy. Heart rhythm 1: 562-567. [Crossref]
- Vidal B, Tamborero D, Mont L, Sitges M, Delgado V, et al. (2007) Electrocardiographic optimization of interventricular delay in cardiac resynchronization therapy: a simple method to optimize the device. J Cardiovasc Electrophysiol 18: 1252-1257. [Crossref]
- Col JJ, Weinberg SL (1972) The incidence and mortality of intraventricular conduction defects in acute myocardial infarction. Am J Cardiol 29: 344-350. [Crossref]
- Gross GJ, Chiu CC, Hamilton RM, Kirsh JA, Stephenson EA (2006) Natural history of postoperative heart block in congenital heart disease: implications for pacing intervention. Heart Rhythm 3: 601-604. [Crossref]
- Krongrad E (1978) Prognosis for patients with congenital heart disease and postoperative intraventricular conduction defects. Circulation 57: 867-870. [Crossref]
- Murphy DA, Tynan M, Graham G, Bonham-Carter RE (1970) Prognosis of complete atrioventricular dissociation in children after open-heart surgery. Lancet 295: 750-752. [Crossref]
- Squarcia U, Merideth J, McGoon DC, Weidman WH (1971) Prognosis of transient atrioventricular conduction disturbances complicating open heart surgery for congenital heart defects. Am J Cardiol 28: 648-652. [Crossref]
- Weindling SN, Saul JP, Gamble WJ, Mayer JE, Wessel D, et al. (1998) Duration of complete atrioventricular block after congenital heart disease surgery. Am J Cardiol 82: 525-527. [Crossref]
- Chang SM, Nagueh SF, Spencer WH, Lakkis NM (2003) Complete heart block: determinants and clinical impact in patients with hypertrophic obstructive cardiomyopathy undergoing nonsurgical septal reduction therapy J Am Coll Cardiol 42: 296-300. [Crossref]
- Topilski I, Sherez J, Keren G, Copperman I (2006) Long-term effects of dual-chamber pacing with periodic echocardiographic evaluation of optimal atrioventricular delay in patients with hypertrophic cardiomyopathy> 50 years of age. Am J Cardiol 97: 1769-1775. [Crossref]
- Abraham WT, CaloL IN, Klein N, Alawwa A, Exner D, et al. (2010) Randomized controlled trial of frequent optimization of cardiac resynchronization therapy: results of the Frequent Optimization Study Using the QuickOptTM Method (FREEDOM) Trial. Heart Rhythm 7: 2-3.
- Abraham WT, León AR, Sutton MG, Keteyian SJ, Fieberg AM, et al. (2012) Randomized controlled trial comparing simultaneous versus optimized sequential interventricular stimulation during cardiac resynchronization therapy. Am Heart J 164: 735-741. [Crossref]
- Boriani G, Müller CP, Seidl KH, Grove R, Vogt J, et al. (2006) Randomized comparison of simultaneous biventricular stimulation versus optimized interventricular delay in cardiac resynchronization therapy: the Resynchronization for the Hemodynamic Treatment for Heart Failure Management II Implantable Cardioverter Defibrillator (RHYTHM II ICD) study. Am Heart J 151: 1050-1058. [Crossref]
- Martin DO, Lemke B, Birnie D, Krum H, Lee KL, et al. (2012) Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm 9: 1807-1814. [Crossref]
- Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, et al. (2010) Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation 122: 2660-2668. [Crossref]
- León AR, Abraham WT, Brozena S, Daubert JP, Fisher WG, et al. (2005) Cardiac resynchronization with sequential biventricular pacing for the treatment of moderate-to-severe heart failure. J Am Coll Cardiol 46: 2298-2304. [Crossref]
- Ritter P, Delnoy PP, Padeletti L, Lunati M, Naegele H, et al. (2012) A randomized pilot study of optimization of cardiac resynchronization therapy in sinus rhythm patients using a peak endocardial acceleration sensor vs. standard methods. 14: 1324-1333. [Crossref]
- Blanc JJ, Etienne Y, Gilard M, Mansourati J, Munier S, et al. (1997) Evaluation of different ventricular pacing sites in patients with severe heart failure: results of an acute hemodynamic study. Circulation 96: 3273-3277. [Crossref]
- Ritter PH, Dib JC, Lelievre T (1994) Quick determination of the optimal AV delay at rest in patients paced in DDD mode for complete AV block. Eur J Cardiac Pacing Electrophysiol. 4: A163.
- McMurray JJ, Pfeffer MA (2005) Heart failure. Lancet 365: 1877-1889. [Crossref]
- Chow AW, Lane RE, Cowie MR (2003) Science, medicine, and the future: New pacing technologies for heart failure. BMJ: British Medical Journal 326: 1073-1077. [Crossref]
- Bradley DJ, Bradley EA, Baughman KL, Berger RD, Calkins H, et al. (2003) Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. Jama 289: 730-740. [Crossref]
- McAlister FA, Ezekowitz JA, Wiebe N, Rowe B, Spooner C, et al. (2004) Systematic review: cardiac resynchronization in patients with symptomatic heart failure. Ann Intern Med 141: 381-390. [Crossref]
- Rivero-Ayerza M, Theuns DA, Garcia-Garcia HM, Boersma E, Simoons M, et al. (2006) Effects of cardiac resynchronization therapy on overall mortality and mode of death: a meta-analysis of randomized controlled trials. Eur Heart J 27: 2682-2688. [Crossref]
- McAlister FA, Ezekowitz J, Hooton N, Vandermeer B, Spooner C, et al. (2007) Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. Jama 297: 2502-2514. [Crossref]
- Adabag S, Roukoz H, Anand IS, Moss AJ (2011) Cardiac resynchronization therapy in patients with minimal heart failure: a systematic review and meta-analysis. J Am Coll Cardiol 58: 935-941. [Crossref]
- Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, et al. (1998) The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 51: 1235-1241. [Crossref]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1-2. [Crossref]
- Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta‐analysis. Stat Med 21: 1539-1558. [Crossref]
- Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, et al. (2001) Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 344: 873-880. [Crossref]
- Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, et al. (2003) Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol 42: 1454-1459. [Crossref]
- Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, et al. (2003) Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 289: 2685-2694. [Crossref]
- Abraham WT, Young JB, Leon AR, Adler S, Bank AJ, et al. (2004) Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation 110: 2864-2868. [Crossref]
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, et al. (2005) The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352: 1539-1549. [Crossref]
- Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, et al. (2008) Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol 52: 1834-1843. [Crossref]
- Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, et al. (2010) Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 363: 2385-2395. [Crossref]
- Thibault B, Harel F, Ducharme A, White M, Ellenbogen KA, et al. (2013) Cardiac Resynchronization Therapy in Patients With Heart Failure and a QRS Complex ,120 Milliseconds: The Evaluation of Resynchronization Therapy for Heart Failure (LESSER-EARTH) Trial. Circulation 127: 873-881. [Crossref]
- Moss AJ (2010) Preventing heart failure and improving survival.N Engl J Med 363:2456-2457. [Crossref]
- Bertini M, Marsan NA, Delgado V, van Bommel RJ, Nucifora G, et al. (2009) Effects of cardiac resynchronization therapy on left ventricular twist. J Am Coll Cardiol 54: 1317-1325. [Crossref]
- St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, et al. (2003) Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 107: 1985-1990. [Crossref]
- Solomon SD, Foster E, Bourgoun M, Shah A, Viloria E, et al. (2010) Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation 122: 985-992. [Crossref]
- Nelson GS, Berger RD, Fetics BJ, Talbot M, Spinelli JC, et al. (2000) Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation 102: 3053-3059. [Crossref]
- Chan KL, Tang AS, Achilli A, Sassara M, Bocchiardo M, et al. (2003) Functional and echocardiographic improvement following multisite biventricular pacing for congestive heart failure. Can J Cardiol 19: 387-390. [Crossref]
- Ypenburg C, Lancellotti P, Tops LF, Boersma E, Bleeker GB, et al. (2008) Mechanism of improvement in mitral regurgitation after cardiac resynchronization therapy. Eur Heart J 29: 757-765. [Crossref]