Abstract
Background: Nonischemic Cardiomyopathies including Myocarditis and Primary Cardiomyopathies as hypertrophic CMP, dilated CMP, restrictive CMP, noncompation CMP...
Echocardiography is the intial evaluative method for CMP diagnosis, but it is limited by poor acoustic windows. Cardiac magnetic resonance imaging allows accurate assessment of myocardial anatomy, function and tissue characterization.
End point of study: to review the ability of cardiac MRI in the diagnosis of nonischemic cardiomyopathies.
Result: 185 patients with nonischemic cardiomyopathies of 243 patients underwent cardiac MRI with 1,5 Tesla GE Explorer at Medic HCMC, since October 2020 to April 2023. In wich, 88% of primary CMP and 12% of Myocarditid were notified.
Patients had CMR using the 1.5 Tesla GE Explorer and CVI 42 version 5.13.10, with the following protocol:Axial FIESTA,SA cine, 4C and 2C cine, T1 mapping with MOLLI, Triple IR FSE T2, myocardial perfusion imaging TI, LGE-MRI.
Conclusion: Cardiac MRI has been useful imaging modality for assessment nonischemic cardiomyopathies with the highaccuracy..Cardiac MRI is not only the establishing diagnosis but also prognosis.
Keywords
cardiac magnetic resonance, non-ischemic cardiomyopathy, native T1 mapping, extracellular volume, T2 mapping, late gadolinium enhancement
Background
Cardiomyopathies (CMPs) are disorders of the heart that show specific morphological and physiological characteristics related to heart dysfunction [1].The American Heart Association (AHA) currently distinguishes between two groups of CMPs: primary, which primarily affects the heart, and secondary, which covers a much larger group and in which cardiac involvement is a component of a systemic generalized disease process [2].Echocardiography is still the initial evaluation for CMPs diagnosis; however, it has some potential pitfalls due to poor acoustic windows [3].Conversely, CMR imaging offers a practical benefit over echocardiography in identifying NICM not easily visible by ultrasound, providing exact left ventricular (LV) wall thickness measures, and providing rigorous quantification of LV and right ventricular (RV) chamber size, LV mass, and LV function performance [4,5].CMR is now a standard diagnostic innovation in cardiovascular medicine [4,6,7]. In recent years, CMR has seen incredible growth, attracting the attention of clinical and scientific communities across the globe [7].
Objective
This article aims to overview the role of CMR imaging in the diagnosis of nonischemic cardiomyopathies ( NICM).
Method
Retrospective study
We used cardiac MRI machine 1,5 Tesla GE Explorer with the following protocol
Axial FIESTA: fast imaging employing stedy state acquisition.
Native and postcontrast T1 mapping, MOLLI ( Modified Look-Lock inversion recovery for high resolution T1 mapping of the heart ) measuring ECV ( extracellular volume).
Triple IR T2: detecting myocardial edema, studying the ratio between myocardium and skeletal muscle in T2W before gadolinium.
LGE-MRI ( Late dodolinium enhancement ): visualization the scar of myocardium, the location and distribution of LGE allows to distinguish nonischemic cardiomyopathies and ischemic heart diseases.
The images were analyzed by the software CVI 42 version 5.13.10
Results
185 patients with suspected nonischemic cardiomyopathies of 243 patients underwent cardiac MRI with 1,5 Tesla GE Explorer at Medic HCMC, since October 2020 to April 2023. In wich, 88% of primary CMP and 12% of Myocarditid were notified.
Patients had CMR using the 1.5 Tesla GE Explorer and CVI 42 version 5.13.10, with the following protocol: Axial FIESTA, SA cine, 4C and 2C cine, T1 mapping with MOLLI, Triple IR FSE T2, myocardial perfusion imaging TI, LGE-MRI.
There were 87 patients with hypertrophic cardiomyopathy, 42 patients with dilated cardiomyopathy, 22 patients with myocarditis, 12 patients with noncompaction cardiomyopathy 8 patients with amyloid cardiomyopathy, 6 patients with tachycardia-induced cardiomyopathy, 5 patient with arrhythmogenic right ventricular cardiomyopathy, one patient with Fabry's disease, and one patient with lipomatous infiltration of the right ventricle. So, CMR is an important imaging tool for accurately assessing NICM (Chart 1) [4,6].
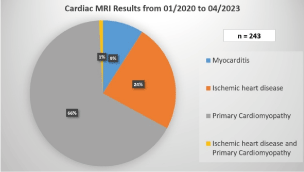
Chart 1. Distribution of nonischaemic and ischemic heat disease
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a hereditary cardiac disease affecting many people worldwide [8]. It is defined by the existence of unexplained LV hypertrophy in the absence of LV dilation without the presence of any other cardiac or systemic disorder [3].According to the 2020 ACC/AHA guideline, in the absence of any cause of hypertrophy in adults, CMR demonstrating a maximum end-diastolic wall thickness of 15 mm anywhere in the LV may establish a clinical diagnosis of HCM in individuals (Figure 1). In patients with a positive genetic test or family history of HCM, even mild hypertrophy (13–14 mm) may be diagnostic [5]. In children, diagnostic criteria must be adjusted for growth and body size. Therefore, the diagnosis of HCM in children should consider the screening status and the pretest probability of disease: a threshold of z-score > 2.5 may be appropriate for identifying a family history of early HCM in asymptomatic individuals. Among children who test positive, a threshold of z-score > 2 may be sufficient for early detection [5]. CMR can help classify HCM based on hemodynamic features (e.g., obstructed versus non-obstructed) or areas of hypertrophy (e.g., septum, mid-ventricular involvement, and apex) [9]. Assessing the flow dynamics and dynamic obstruction of the left ventricular outflow tract (LVOT) in HCM patients is possible using cine CMR with flow velocity encoding [10].Besides, CMR also provides another parameter, LGE, which can be useful in predicting serious arrhythmias. A range of LGE ≥ 15% of LV mass was related to an increased risk of sudden cardiac death [10-12]. Native T1 and ECV also increase significantly in HCM [12,13].
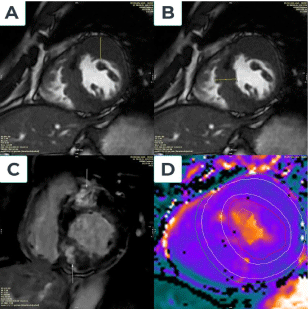
Figure 1. Hypertrophic type without LVOT obstruction cardiac disease. A Fifty-five-year-old male patient admitted palpitations and angina; CMR imaging showed marked the basal and median anterior wall thickness of 22 mm (Figure 1A), basal and septal thickening of 22 mm (Figure 1B), and median inferior wall thickness of 21 mm. LV mass = 181 g, LVEF = 68% and septal anterior motion (SAM) of the mitral valve (+). The location of late gadolinium enhancement is anteroseptal and inferoseptal, LGE.mass is 49 grams, and LGE.mass/LV.mass is 27% (Figure 1C). Increased myocardial native T1=1117ms and Increased ECV= 46% (Figure 1D)
Left ventricular non-compaction (LVNC) is a congenital morphological disorder characterized by prominent left ventricular trabeculae and deep inter-trabecular recesses [1,2]. CMR can provide morphological evaluation and non-invasive tissue characterization, so it should be used to confirm the diagnosis of LVNC and exclude other cardiovascular diseases [14,15]. Diagnostic criteria for LVNC with a non-compacted (NC) to a compacted (C) ratio in diastole greater than 2.3, indicating pathogenic involvement with reported sensitivity, specificity, and positive and negative predictions of 86%, 99%, 75%, and 99%, respectively [16]. Trabeculated LV mass more significant than 20% of the global LV mass had a sensitivity and specificity of 93.7% and 93.7%, respectively, when diagnosing LVNC [17].Matthias Grothoff,et al.proposed highly specific and sensitive quantifiable criteria for LVNC diagnosis: a total NC mass index of 15 g/m2 global LV mass with NC mass greater than 25% of total LV mass [18].
Myocarditis
Myocarditis is an inflammation of the myocardium that may result from several causes [10]. CMR findings include myocardial edema, patchy myocardial LGE, wall motion abnormalities, and pericardial effusion [19,20]. LGE in myocarditis is typically patchy and involves the subepicardial regions. During the acute phase of myocarditis, LGE is caused by edema, not scarring, so the area of LGE usually decreases with time [21]. For over a decade, people have used the Lake Louise Criteria 2009 to diagnose myocarditis [19]. Recent technology developments and several additional pieces of evidence led to the 2018 revision of the Lake Louise Criteria [20] (Figure 2).
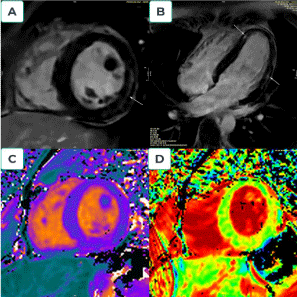
Figure 2. Myocarditis. A 19-year-old man who admitted to the emergency department with fever, fatigue and chest pain. Troponin T = 400 ng/l and C-reactive protein = 17 mg/L.ECG shows regular sinus rhythm, and negative T wave with ST depression at V4, V5, and V6.No stenosis of the coronary artery was detected on coronary angiography.CMR after day five following admission show: Region signal intensity increase in T2W; Increased ratio between myocardium and skeletal muscle in T2W before gadolinium =184/60 (>3).Four chamber and short view: showed sub-epicardial fibrosis with late gadolinium enhancement (LGE) in the apical septal and mid anterolateral; LGE.mass is 10 grams, and LGE.mass/LV.mass is 11% (Figures 2A and B). Increased myocardial Native T1:1079ms (Figure 2C); Native T2: 56ms and Increased ECV: 29%(Figure 2D)
Amyloidosis
Amyloidosis is a systemic disorder characterized by amyloid fibril infiltration in several tissues, including the heart [22].The diagnosis of cardiac amyloidosis (CA) requires a combination of different modalities and remains a challenge [23,24]. CMR is a noninvasive diagnostic technique that assists in evaluating and characterizing amyloidosis-specific tissues [25]. The main finding was myocardial thickening associated with cardiac amyloid infiltration [26]. LGE is also a good indicator of amyloid accumulation [27]. Following the progression of the disease, typical LGE characteristics (independent of amyloid type) include the absence, global subendocardial, and transmural areas [27]. Native T1 and extracellular volume (ECV) are other major parameters of significant interstitial expansion, markedly elevated in the light chain and transthyretin amyloidosis [28,29].However, there is currently no consensus between the ESC and AHA/ACC diagnostic approaches for CA [23,24] (Figure 3).
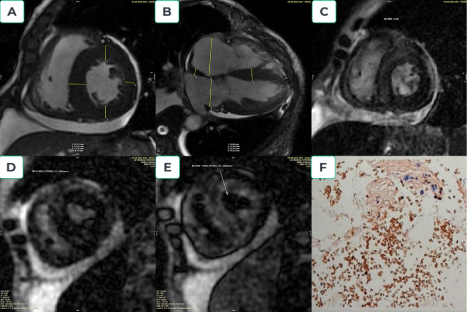
Figure 3.Amyloidosis cardiomyopathy. A 66-year-old male presented with shortness of breath, chest pain, and lower limb edema. The patient was scanned CMR by suspected restrictive cardiomyopathy; short-axis CINE images showed symmetric left ventricular hypertrophy and pericardial effusion (Figure 3A); long-axis images showed two atria enlargement (RA=55mm, LA-39mm), interatrial septal thickness (9mm), the increased left ventricle mass (173gram), and also pericardial effusion (Figure 3B). Myocardial delayed enhancement sequence demonstrates diffuse and irregular hyperenhancement (circumferential hyperenhancement) (Figure 3C). The myocardium (Myocardial null-point 206msec) reaches the null point before the blood pool (Blood null-point 260msec) (Figures 3D and 3E). Abdominal fat biopsy: low amyloid deposition in adipose fibrous connective tissue, consistent AL amyloid from Kappa light chain (Figure 3F)
Arrhythmogenic cardiomyopathy
Arrhythmogenic cardiomyopathy (ACM) is a rare heart disease characterized by fibro-fatty tissue gradually replacing the ventricular myocardium. Whether it affects the RV, the LV, or both, its most notable characteristic is a myocardial scar (fibro or fibrofatty myocardial replacement), which clinically results in life-threatening arrhythmias and sudden cardiac death [30,31].
Initially, people believed the ACM disorder only affected the right ventricle [32]. A group of experienced cardiomyopathy specialists released the first task force guidelines for arrhythmogenic right ventricular cardiomyopathy (ARVC) in 1994, which contain several limitations [33]. In 2010, a revised International Task Force standard was introduced, including quantitative imaging reference values to identify normal RV and varying degrees of structural and functional problems and quantitative histomorphological definitions of myocardial fibrofatty replacement in endomyocardial biopsy to increase diagnostic accuracy [34]. Since then, several pathological and clinical studies have provided a better understanding of the phenotypic expression of the disease, leading to the current concept of cardiomyopathy, which can be biventricular involvement [35,36].Subsequently, the original term ARVC was replaced with the broader definition of ACM [37].In 2020, international experts released the Padua criteria of the ACM, which depend significantly on the CMR. CMR is currently required to describe the ACM phenotype and rule out other diagnoses [31].
Anderson–Fabry disease
Anderson-Fabry disease is a rare X-linked genetic disorder caused by a lack of α-galactosidase, leading to excessive accumulation of sphingolipids in the lysosomes [38]. The cardiac manifestations of Anderson-Fabry disease are characterized by increased LV wall thickness and should be evaluated along with any other cause of LV hypertrophy [38]. When clinical signs and symptoms are consistent, CMR is an essential tool for assessing cardiac morphology and diagnosing Anderson-Fabry disease early [38]. Because of the intra-myocyte accumulation of lysosomal sphingolipids, Anderson-Fabry disease is associated with a low native T1 value despite normal ECV values [39-41].In the later stages of Anderson-Fabry disease, a significant number of necrotic myocytes result in fibrosis; LGE can be seen, usually located in the basal and inferolateral basal segments of the LV with a mid-wall distribution [42,43]. On the other hand, this LGE pattern is not unique to Anderson-Fabry disease since it is also present in other diseases like sarcoidosis, ACM, myocarditis, etc [44].
Fatty infiltration of the myocardium occurs when adipose tissue replaces muscular fibers, primarily on the heart’s right side [45,46].However, fat can be present between the fibers of the RV myocardial fibers without associated signs of fibrosis or inflammation [46].Most cases are asymptomatic and discovered incidentally at autopsy [45]. CMR imaging showed a lipomatous infiltration with an RV thickness of less than 6 mm but without regional or global RV dysfunction, which seemed different from a fatty RV related to arrhythmogenic right ventricular dysplasia [46] (Figure 4).
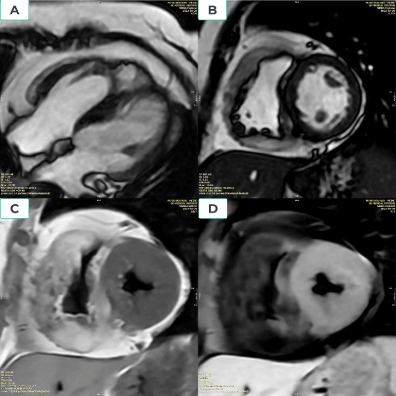
Figure 4.Lipomatous infiltration in right ventricle. A 52-year-old woman was admitted of shortness of breath and palpitations; CMR imaging showed: The right ventriclemyocardium is heavily thickened, the free wall of the right ventricle is 15mm thick, the lower border of the right ventricle is 12mm, the right ventricle septum is 6-8mm (Figures 4A and 4B). The myocardium of the right ventricular walls is hyperintense on T1-weighted and non-defatting T2-weighted images and loses signal on T1-weighted and T2-weighted defatting. (Figures 4C and 4D)
Tuberous sclerosis complex
Tuberous sclerosis complex (TSC) is a rare autosomal dominant genetic syndrome caused by mutations in the TSC1 or TSC2 suppressor genes [47-49]. These mutations lead to hyperactivity of the protein involved in cellular proliferation, the mechanistic target of rapamycin [47].Diagnosing TSC in a clinical setting might be challenging [47]. Rhabdomyomas, a benign tumor of striated muscle, are common cardiac manifestations of TSC [48]. However, myocardial fatty foci deposition with or without rhabdomyoma has been reported incidentally in some patients with TSC [47,48].Experts created a set of diagnostic standards for TSC in 1998 [50], which were updated in 2012 [51] (Figure 5).
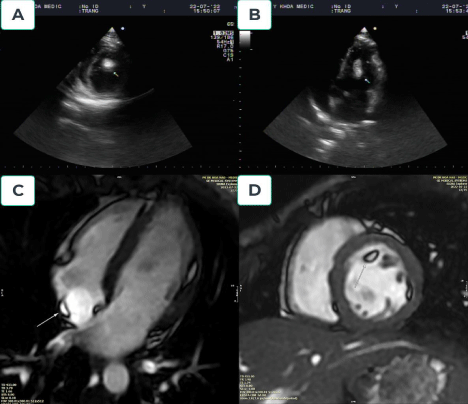
Figure 5.Tuberous sclerosis complex. A 24-year-old female presented to our hospital with seizures. The patient had been diagnosed with TSC at five years old. Echocardiography demonstrated normal global left ventricular wall motion, ejection fraction, and an intracardiac left ventricular mass (Figures 5A and 5B). A multiplanar contrast-enhanced CMR demonstrated normal cardiac anatomy. Three definitive areas of myocardial fatty foci were identified within the subendocardial region of the mid anterolateral (d=3x16mm), lateral wall of the right atrium (d=3x9mm), and trabeculated basal anterior of left ventricular (d=6x10x16mm) (Figures 5C and 5D)
Discussion
Hypertrophic cardiomyopathy is the most frequent type of nonischemic cardiomyopathies.The most serious complications of HCM are sudden cardiac death induced by ventricular arrhythmia and heart failure. CMR imaging is a valuable imaging method for detecting HCM because of its accurate measurement of wall thickness and myocardial mass without limited window and late gadolinium enhancement (LGE) to quantify myocardial fibrosis (fibrosis more than 15% of myocardium related to the worse prognosis of HCM).
Dilated cardiomyopathy: Late gadolinium enhancement (LGE), commonly found at cardiac magnetic resonance imaging in idiopathic dilated cardiomyopathy (DCM), in most cases shows a nonischemic pattern of LGE, midmyocardial or epicardial. In order to our experience, dilated cardiomyopathies usually the cause of heart failure at young patients without the evident etiology of cardiac failure. LGE related midmyocardial was seen usually.
Myocarditis has often arrived post-virus infection, especially in young patients that sommetimes only presented by chest pain. Serum hs Troponin maybe verry high but normal coronary angiography.CMR showed subepidocardial late gadolinium enhancement.
Noncompaction cardiomyopathy is not routine cardiomyopathy, Patient often presented by severe and refractory heart failure. Cardiac ultrasound, CTA and CMR may confirm the diagnosis of the disease by the following criteria (1) the presence of 2 distinct layers of myocardium, including a compacted epicardial layer and a noncompacted endocardial layer, (2) the presence of trabeculations and deep intratrabecular recesses within the noncompacted endocardial layer, and (3) a noncompacted to compacted ratio of ≥2.3:1 in end diastole.
Cardiac amyloidosis: CMR is cornestone of the diagnosis because biopsies are not routine and easy procedures. MRI showed LVH, IAS hypertrophy, pericardial effusion and especially the pattern of myocardial nulling is an accurate tool to detect cardiac amyloidosis: myocardium had null-point early than cardiac cavities.
Conclusion
Cardiac magnetic resonance imaging has been a helpful non-invasive imaging modality for assessing nonischemic cardiomyopathies with high accuracy. CMR may distinguish the tissue characteristic of myocardium. CMR should be a standard step in the diagnosis process for various cardiomyopathies.
Conflicts of Interest
None declared.
Funding
None.






